Back to Journals » Journal of Pain Research » Volume 14
Antinociceptive Effect of Magnolol in a Neuropathic Pain Model of Mouse
Authors Zhang X, Wang J, Sui A , Zhang N, Lv Q, Liu Z
Received 23 April 2021
Accepted for publication 23 June 2021
Published 8 July 2021 Volume 2021:14 Pages 2083—2093
DOI https://doi.org/10.2147/JPR.S317204
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Xiao Zhang,1,* Juntao Wang,1,* Aihua Sui,2 Nannan Zhang,1 Qiulan Lv,2 Zhenfang Liu3
1Department of Anesthesiology, The Affiliated Hospital of Qingdao University, Qingdao, 266000, People’s Republic of China; 2Medical Research Center, The Affiliated Hospital of Qingdao University, Qingdao, 266000, People’s Republic of China; 3Department of Emergency, The Affiliated Hospital of Qingdao University, Qingdao, 266000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhenfang Liu Email [email protected]
Background and Objective: Neuropathic pain remains a clinical challenge with limited effective treatments. Previous studies have found that magnolol (Mag), an ingredient existing in some herbs, showed neuroprotective effect. However, it remains unclear whether Mag can alleviate neuropathic pain.
Methods: Chronic constriction injury (CCI) is used as the neuropathic pain model. Mice were randomly divided into 5 groups: Sham, CCI, CCI + 5, 10, 30 mg/kg Mag groups. Thermal and mechanical paw withdrawal threshold were performed at baseline and on the 3rd, 5th, 7th, 14th days post-surgery. Lumbar spinal cord and blood samples were collected on the 14th day. Blood lipid profile, kidney and liver functions, as well as the activation of microglia were evaluated, along with the related signal pathway examined using multiple methods including immunohistochemistry, RT-PCR and Western blot.
Results: Mag alleviated thermal and mechanical hypersensitivity in CCI mice. CCI activated microglia and upregulated the expression of P2Y12, while Mag inhibited microglial activation, and downregulated the expression of P2Y12. Mag also blocked the activation of p38 mitogen-activated protein kinase (MAPK) and other pain-related cytokines such as IL-6, TNF-α and IL-1β.
Conclusion: The findings indicate that Mag has antinociceptive effect on neuropathic pain, probably mediated through P2Y12 receptors and p38 MAPK mediated pathways. With its relatively safe profile, Mag may be a potential therapeutic agent for neuropathic pain.
Keywords: magnolol, neuropathic pain, P2Y12, CCI, MAPK, cytokines
Introduction
Neuropathic pain (NP) is a chronic pain state resulting from injury or disease in somatosensory pathways at the peripheral or central level.1 Typically, NP is characterized by hyper-excitability of the primary afferent sensory neurons,2 along with increased release of transmitters or mediators such as glutamate, ATP, substance P, brain derived neurotrophic factor (BDNF) etc.2–5 These substances accordingly drive the hyperactivity of postsynaptic neurons in the spinal dorsal horn (SDH), causing central sensitization, which is widely believed to be one of the main reasons for the persistent pain of the patients.6
While all these changes are mainly mediated by neurons, recent studies provide overwhelming evidence which confirms the importance of glial cells in the maintenance of these plastic changes in SDH.7 There are mainly two types of glial cells in SDH: astrocytes and microglia; and both contribute to the pathophysiology of the NP.8
Microglia are the resident immune cells of the central nervous system (CNS) with numerous surface receptors and molecules; and they also have the capability of rapid activation in response to the extracellular changes to maintain CNS homeostasis.9 Among these surface receptors and molecules, some are expressed in microglia selectively, such as P2Y12.10,11 Previous reports revealed that P2Y12 was actively involved in neuropathic pain;12,13 therefore, targeting P2Y12 in the microglia can be promising for inhibiting pain and potentially avoiding the side effects of acting on neurons.
In the context of NP, microglia in SDH are widely activated by the signals released from primary afferent terminals, followed by releasing of inflammatory cytokines such as IL-1β, IL-6 and TNF-α from microglia.14 All these cytokines could substantially modulate the central sensitization through neuron-glial interactions.15 This whole process is also subject to complicated modulation by a couple of signal pathways, especially mitogen-activated protein kinase (MAPK) pathways.16
Therefore, microglia provide an interesting therapeutic target for NP and numerous reports have revealed that inhibiting microglial activation could attenuate the pain following nerve injury.8,17 Among various kinds of resources for drug candidates, natural resources such as herbs have been proven to be successful in providing new potential candidates for analgesia.18
Magnolol (Mag), an extract from the herb “Hou Pu”, has demonstrated powerful effects of anti-inflammation19 and neuroprotection.20 Importantly, Mag also inhibited the activation of microglial cells and decreased the release of inflammatory factors to protect neurons from damage both in vitro and in vivo.20 Furthermore, it was also proved to be effective for inflammatory pain.21 However, it remains elusive whether Mag has similar anti-nociceptive effect in NP.
In this experiment, therefore, we employed a chronic constriction injury (CCI) in mice as the pain model, to examine the effect of Mag on neuropathic pain. We also investigated the underlying cellular and molecular mechanisms, focusing specifically on the microglia as well as the related cytokines. Our results revealed that Mag successfully alleviated the mechanical and thermal hypersensitivity, and blocked spinal microglial activation through P2Y12, thus blocking the upregulation of cytokines, probably mediated by P38-MAPK.
Materials and Methods
Animals and Groups
All animal protocols were approved by the Animal Care Committee of Affiliated Hospital of Qingdao University (Qingdao, China) and were carried out in accordance with the guidelines of the NIH regulations on animal use and care (Publication 85–23, Revised 1996). Adult male C57BL/6J mice (aged 6–8 weeks, weight 18–20 g) were purchased from Shandong Laboratory Animal Center (Permit: SCXK 20190003). Mice were housed in a specific pathogen-free (SPF) environment with 12-hour light/12-hour dark lighting cycle, and were given ad libitum access to food and water. All animals were allowed at least 5 days for acclimation before all experiments. Animals were randomly divided into the following 5 groups (n = 6 for each group): (1) Chronic constriction injury (CCI) + vehicle group; (2) Sham group; (3) CCI + 5 mg/kg Mag; (4) CCI + 15 mg/kg Mag; and (5) CCI + 30 mg/kg Mag. The concentrations of Mag were selected based on previous reports.21,22
Surgery and Drug Administration
CCI was performed as previously reported (Bennett and Xie, 1988). Briefly, sciatic nerve on the left side was exposed under sevoflurane anesthesia, then three 4–0 loose chromic gut ligatures (Jinhuan Medical Co., Shanghai, China) were loosely tied proximal to the trifurcation of the sciatic nerve followed by closure of the wound. Mag was dissolved into DMSO, reaching a stocking concentration of 135 mg/mL, and further diluted in corn oil to reach the final concentration (5, 15 and 30 mg/kg in 0.2 mL). Mag, DMSO and corn oil were purchased from Solarbio (Beijing, China). All drugs were injected intraperitoneally (i.p). A mixture of corn oil and DMSO (1:4) was used as vehicle. For Sham group: sciatic nerve on the left side was exposed without ligation. For CCI + Mag group, mice were treated with daily dosing of 5, 15 and 30 mg/kg Mag from 7 to 14 days after CCI surgery.
Behavioral Tests
Behavioral studies were conducted by an investigator blind to the treatment groups. Animals were tested at baseline, 3, 5, 7 and 14 days after surgery. Paw Thermal and Mechanical withdrawal tests (PTWT and PMWT) were carried out on the left hind paws (ipsilateral side) at the same time of each day. All measurements were repeated three times with a 10 min interval between each test during which animals were returned to home cages. The average value of the 3 measurements was used as threshold.
Thermal pain threshold was measured by intelligent hot-plate apparatus (Anhui Zhenghua Biologic Facilities, ZH-YLS-6BS, China). All mice were placed on the surface of the hot plate (53°C ± 0.5°C) to assess the thermal withdrawal latency of the left hindpaw (Tansley and Macintyre et al., 2019). The latency was recorded until mice showed one of the following responses: jumping, shaking or licking the left hind paw. The cut-off time is 20 seconds in order to avoid tissue injury.
Mechanical pain threshold was measured with an electronic pressure analgesy-meter (Anhui Zhenghua Biologic Facilities, YLS-3E, China), a modified device of Randall-Selitto test (Randall and Selitto, 1957; Whiteside and Harrison et al., 2004). Briefly, the mice were placed in a special cage and allowed to acclimate with the environment for 10 min. Then a blunt probe was applied to the left hind paw with an increased pressure, until the animals started to retract the hind limb. Then the pressure was recorded as the mechanical withdrawal threshold.
During the whole experiment, for animals with CCI surgery, all thermal and mechanical pain thresholds were < 70% of baseline at 7 days after the surgery, indicating the success of the CCI model.
Sample Preparation
Mice were anesthetized with 10% chloral hydrate (Affiliated Hospital of Qingdao University, China). Blood was collected with cardiac puncture, followed by centrifuge at 5000 g for 15 min at 4°C. Serum was collected for biochemistry analysis. After cardiac puncture, mice were perfused with phosphorylated saline buffer and the lumbar (L3-5) segment of spinal cord was dissected for further analysis.
qRT-PCR
Total RNA from spinal cord samples was extracted by Trizol (Accurate Biology, China), and reverse transcription was performed using SPARKscript reverse transcription (SparkJade, China). Quantitative real time PCR (qRT-PCR) was carried out using a quantitative SPARKscript PCR Kit (SparkJade, China). Two microliters of cDNA were subjected to amplification in a total volume of 20 µL containing 10X buffer, 1.5 mM MgCl2, 0.2 mM of each dNTP, 1 U Taq polymerase, and a pair of primers (0.2 mM each). The program was running at 95°C for 15 s, 60°C for 1 min, 95°C for 15 s, and 40 cycles were repeated. Primers were designed with software Primer 5 and the sequences were as follows: P2RY12 forward primer, CCTGCCTTGATCCATTCATCTA and reverse primer GTCCTTTCTTCTTGTTTGTCCC, GAPDH (mice) forward primer, AAATGGTGAAGGTCGGTGTGAACG, reverse primer, ATCTCCACTTTGCCACTGC. GAPDH was used as an internal reference. The quantitative analysis was carried out using the comparative CT (2−∆∆CT) method.
Western Blot
Lumbar spinal cord was homogenized at 4°C in radioimmunoprecipitation assay (RNPA) buffer with 1% Phenylmethanesulfonyl fluoride (PMSF) and centrifuged at 14,000 g for 15 min at 4°C for the collection of cytosolic fractions. Protein concentration within each sample was determined using a BCA kit (Pierce, Rockford, Ill., USA) before equivalently loaded into wells of 10% SDS-PAGE gels for electrophoresis.
Proteins were then transferred electrophoretically to a nitrocellulose membrane (Schleicher and Schell, USA). The membrane was washed for 10 min with TTBS (20 mM Tris-Cl, pH 7.5, containing 0.15 M NaCl, and 0.05% Tween-20) followed by the blocking solution with 10% nonfat milk in TTBS. The blocked membrane was incubated overnight at 4°C with the following primary antibodies: rabbit anti-iba-1 (1:1000, Abcam), rabbit anti-IL6 (1:1000, Abcam), rabbit anti-phosphorylated p38 (1:1000, Cell signaling), rabbit anti-p38 (1:1000, Cell signaling), rabbit anti-TNF-α (1:1000, Abcam), rabbit anti-IL-1β (1:1000, Abcam), rabbit anti-P2Y12 (1:500, Novus) and mouse anti-β-actin (1:5000, Abcam). The membranes were then incubated with Anti-mouse IgG, HRP-linked Antibody (1:6000, CST, USA) or Anti-rabbit IgG, HRP-linked Antibody (1:6000, CST, USA) respectively at 37°C for 1 h. The protein bands were probed with an ECL Plus chemiluminescence regent Kit (Amersham Biosciences, Arlington Heights, PA, USA), visualized using the image system (Versa Doc, model 4000, Bio-Rad, Hercules, CA, USA) and analyzed by Image software (Image J 1.4, NIH, Bethesda, MD, USA).
Immunohistochemistry
The lumbar spinal cord segments (L3-5) samples of Sham, CCI, CCI + Mag 30 mg/kg groups were dissected out and fixed in 4% paraformaldehyde. The specimens were embedded in paraffin, sectioned at 3 µm. Then the sections were dewaxed, hydrated and received antigen repairing with saline sodium citrate. H2O2 solution (3%) was used to block endogenous peroxidase activity for 10 min. After being washed 2 times with distilled water and 3 times with PBS, the sections were incubated with anti-Iba-1 antibody (1:800, Abcam) at 37°C for 1 h, then washed 4 times with PBS and incubated with the secondary antibody (PV-6001, Goat Anti-Rabbit IgG, ZSGB-BIO, China) at 37°C for 30 min. After being washed 4 times with PBS, diaminobenzidine (DAB) was applied for 1 min to develop color. After this, cresyl violet was used for Nissl staining. At the end, the sections were sealed with neutral resins after dehydration. All the samples were observed under a microscope (Olympus, CX31).
Examination of Liver and Kidney Enzymes and Lipid Profile in Blood Plasma Sample
To explore the possible toxicity of Mag, liver and kidney enzyme activities were examined in the blood by analyzing aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea (UA) and creatinine (CREA). Lipid profile was measured by cholesterol (TC) and triglyceride (TG). All samples were tested by an automatic animal biochemical analyzer (Mindray, China).
Molecular Docking
Molecular docking was carried out to examine the binding affinity (ΔG in kcal/mol) between the target proteins (P2Y12, P2X4, P2RX7 receptors) and ligand (Mag) using Schrödinger 2015 software. The chemical structure of Mag was drawn by Chemdraw 2012 software. Ligand structure was optimized by Ligprep tool. The structure of PDB ID: 4PXZ protein was imported and optimized by Protein Preparation Wizard. Energy was minimized, and 2MeSADP in 4PXZ was selected as center of 15Å x 15Å x 15Å docking grid. The target protein and ligand were docked by Glide docking in extra precision (XP) mode.
Statistical Analysis
All statistics were carried out with SPSS17.0 (IBM, America). Data are presented as the mean ± standard error (x ± SEM). Behavioral tests were analyzed using one-way repeated-measures analysis of variance (ANOVA), followed by the Tukey-Kramer test for multiple comparisons or Student’s t-test for two comparisons. Other results were also analyzed with ANOVA followed by Bonferroni’s post hoc analysis. Differences of p < 0.05 were considered statistically significant.
Results
Mag Attenuated Paw Thermal and Mechanical Hypersensitivity Following CCI Surgery
At 3 days after CCI surgery, the paw thermal withdrawal threshold (PTWT) and paw mechanical withdrawal threshold (PMWT) was reduced from 14.51 ± 0.40 s to 10.05 ± 0.47 s and from 142.56 ± 25.93 g to 85.79 ± 16.59 g respectively (p < 0.01, n = 6), indicating the establishment of neuropathic pain. Also, the hypersensitivity was further aggravated from day 3 to the end of the study (D14). In contrast, the PTWT and PMWT in Sham group remained stable during the whole study (Figure 1A and B).
Daily injection of Mag for 8 days (D7-14) successfully attenuated the PTWT and PMWT at all 3 doses (5, 15 and 30 mg/kg). At a dose of 5 mg/kg, Mag started to show analgesic effect, which increased the PTWT from 5.91 ± 0.14 s to 7.04 ± 0.26 s (p < 0.01, n = 6), and PMWT from 48.86 ± 4.81 g to 57.29 ± 3.73 g (p < 0.05, n = 6). Importantly, Mag demonstrated a dose dependent analgesic effect in both PTWT and PMWT. For example, Mag with 30 mg/kg showed the biggest analgesic effect, which increased the PTWT from 5.91 ± 0.14 to 8.95 ± 0.38 s (p < 0.01, compared with CCI group, n = 6, Figure 1C), and PMWT from 48.86 ± 4.81 to 76.79 ± 5.84 g (p < 0.01, compared with CCI group, n = 6, Figure 1C). We also compared the area under the curve (AUC) for both PTWT and PMWT. Analysis of AUC revealed a more drastic significant difference. For PTWT, the AUC values for Sham, CCI, CCI + Mag 5, 15 and 30 mg/kg at 14 days are 195.7 ± 41.5, 107.5 ± 38.1, 111.1 ± 29.1, 113.8 ± 34.9 and 117.2 ± 27.1 respectively (p < 0.001). For PMWT, the AUC values for Sham, CCI, CCI + Mag 5, 15 and 30 mg/kg at 14 days are 1901.5 ± 99.6, 940.4 ± 48.8, 948.3 ± 51.2, 987.8 ± 64.2, and 1020.6 ± 84.1 respectively (p < 0.001). All these results demonstrated that Mag had an analgesic effect on CCI induced pain behaviors.
Mag also demonstrated good tolerance with mice. After 8 days of dosing, even the highest dose of Mag (30 mg/kg) did not cause any visible sign of toxicity, as shown in Table 1. There was no statistically significant difference between groups in index of blood lipid (TC, TG), renal function (UREA, CREA-S) and liver function (AST, ALT) (p > 0.05, n = 6). Accordingly, the dose of 30 mg/kg was selected for the following experiments.
 |
Table 1 The Effect of Magnolol (30 Mg/Kg) on the Markers for Liver (ALT and AST), Blood Lipid Profile (TG and TC), and Kidney Functions (CREA and UREA) |
Mag Has Binding Preference with P2Y12
Previously it has been reported that the therapeutic effects of Mag were probably mediated by certain receptors. To identify which receptors are responsible for the analgesic effect of Mag, we took advantage of computation simulation and chose molecular docking to map which receptor(s) has the best potential. Our molecular docking results showed that P2Y12 has the best score (Figure 2A), compared with other receptors such as P2X4 receptors (data not shown). Benzene ring in Mag has π-π interaction with HIS187/ARG256, and the phenolic hydroxyl of Mag forms a hydrogen bond with ASN191/ASN159.
Since the molecular docking indicated that P2Y12 was the most promising candidate, we went further to check the effect of Mag on the expression of P2Y12 in the spinal cord. At day 14 following CCI, there was the significant upregulation of P2Y12 mRNA compared with Sham group (1.62 ± 0.28 vs 0.97 ± 0.13, p < 0.01, Figure 2B). Application of Mag (30 mg/kg) blocked the upregulation of mRNA which was reduced to 1.09 ± 0.15. Mag also showed blocking effect on the protein level of P2Y12 (Figure 2C). The protein level was increased to 2.00 ± 0.31 following CCI surgery, while Mag greatly reduced the upregulation of P2Y12 from 2.00 ± 0.31 to 1.3 ± 0.14 (p < 0.01, n = 6).
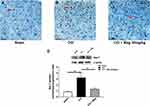
Mag Inhibited Microglial Activation in CCI Mice
P2Y12 is shown to selectively express in the microglia in the spinal cord (Tozaki-Saitoh et al, 2008). To confirm the effect of Mag on microglia, we examined the activation of microglia using both immunohistochemistry and western blot. Figure 3A–C shows the sections from the ipsilateral side of spinal cord dorsal horn, where neurons were stained blue with Nissl staining, and microglia are shown as dark by the marker Iba-1. Only scarce Iba-1 immuno-like (IL) cells were detected in sections from Sham group (Figure 3A). But at 2 weeks following CCI surgery, Iba-1 IL cells were increased greatly, with more processes extending, indicating the activation of glial cells. Application of Mag (30 mg/kg) for 8 days (days 7–14 after CCI surgery) apparently inhibited the activation of microglia, as shown in Figure 3C, with fewer Iba-IL cells. Protein expression of Iba-1 also showed similar trends as shown in Figure 3D; CCI surgery upregulated the relative expression ratio of Iba-1 to more than 3 times of Sham group. With the dose of 30 mg/kg, Mag showed a drastic blocking effect, which almost reduced the expression of Iba-1 to the level of Sham group (p < 0.01, n = 6).
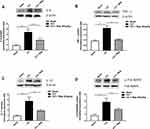
Mag Decreased Pain-Related Cytokines
Since we proved that Mag has an inhibitory effect on the microglia, we suspected that the antinociceptive effect of Mag might be mediated by inhibiting the inflammation cytokines released from the microglia. We further examined the expression of IL-6, which is believed to be a main inflammatory cytokine from microglia. Following CCI surgery, there was a significant increase in IL-6, which was increased to 3.43 ± 0.56 compared with Sham group (Figure 4A). Mag showed a drastic inhibitory effect which reversed the IL-6 by half, with the expression level similar to Sham group (p < 0.01, n = 6 per group).
IL-6 can induce a cascade of inflammation cytokines, so we further detected proinflammation factors such as TNF-α and IL-1β (Figure 4B and C). Compared with control group, the expression of TNF-α in CCI group was more than doubled to 2.13 ± 0.20; and IL-1β was increased to 3.72 ± 0.75 (Figure 4B and C, p < 0.05 for TNF-α and p < 0.01 for IL-1β vs Sham group, n = 6). For both cytokines, Mag showed a significant inhibitory effect, which was reduced to 1.05 ± 0.08 and 1.91 ± 0.19 (Figure 4B, p < 0.01 vs CCI group, n = 6). Furthermore, p38-MAPK is considered to be upstream in the activation pathway of cytokines, so the changes of p38-MAPK were compared among the 3 groups as well. At 2 weeks following CCI surgery, phosphorylation levels of p38-MAPK were increased by about 2 times compared with the control group, while Mag blocked the increase (Figure 4D, p < 0.01, n = 6). Among all 3 groups, expression of p38-MAPK was not affected. Overall, Mag showed an overwhelming anti-inflammation effect in these cytokines, and this could explain its significant anti-nociceptive effect in the current CCI model.
Discussion
Mag has been proven to be effective in multiple neurological diseases including epilepsy,23 age-related memory deficit,24 depression,25 etc. However, compared with the diseases mentioned above, the function of Mag in pain has been less studied and the related mechanism remains largely elusive. Currently, there are only few reports about the effect of Mag on pain, which covers inflammation pain and other pain models,22 but not a NP model. Therefore, to the best of our knowledge, our report is the first one to evaluate the therapeutic effect of Mag on neuropathic pain.
Our current research used one of the most well recognized NP models, the CCI model.26 The establishment of the model was proven by the robust thermal and mechanical hypersensitivity following surgery, which is consistent with previous reports.27 In this model, Mag showed a significant attenuation in both mechanical and thermal hypersensitivity of the ipsilateral side (Figure 1). Previously it was shown that Mag has an analgesic effect in the inflammatory pain induced by formalin and substance P,21,22 indicating the analgesic effect of Mag is not model dependent, which definitely raises the interest of Mag as a potential antinociceptive drug candidate.
The doses we used in the current report ranged from 5 to 30 mg/kg; and all these doses exhibited significant inhibition in both mechanical and thermal hypersensitivity, though with different extents. Previous literature reported an analgesic effect starting at 10 mg/kg,22 which is slightly higher than our effective concentration (starting at 5 mg/kg); this discrepancy could be explained by the duration of dosing and the employment of different animal models, where the authors used glutamate to induce pain. In a previous paper,22 Mag showed a trend for analgesia starting at 5 mg but without statistical significance. In our report, the animals were dosed for longer (8 days). There could be a chance that the antinociceptive effect take effect earlier and we only measured the response at 14 days; however, the current study mainly focuses on testing whether Mag showed any antinociceptive effects instead of focusing on complete pharmacokinetic studies, which will be in our future research. Lastly, different dosing paradigms were used. The current study used post drug application instead of pre-administration which is more clinically relevant. The difference in the study approach could have influenced the effective dose range or the timing.
During the whole period of the current study (8 days), Mag showed a great safety profile. The panel of metabolite profile for liver and blood showed no significant difference even after 8 days of dosing, suggesting the good tolerance of Mag in the current animal model. While a longer and more detailed toxicology study is required to conclude the safety of the drug, current data suggest that Mag potentially is a safe therapeutic. The safety of Mag was also confirmed in the previous literature. Mag has been estimated to be safe in humans at up to 1.64 mg/kg,28 which is 20 mg/kg when translated to mice.29 In our report, data from the panel of metabolite profile of liver and blood showed that the metabolite profile was not significantly changed even with the upper dose of 30 mg/kg. Therefore, to the best of our knowledge, our report provided the first line of evidence showing Mag can be safe at a higher dose when injected intraperitoneally (30 mg/kg).
Previous reports about Mag suggested that Mag has multiple functions such as anxiolytic, analgesic, antidepressant and neuroprotective effects.21,22 Apparently these effects were mediated by a wide range of different cells and receptors, as well as being signal pathway related.
Mag is reported to have affinity with GABA receptor and NMDA receptor;22,30 which are widely expressed in the nervous system, and also well established for their functions in the modulation of pain. Previously it has been shown that Mag can block NMDA induced Ca2+ influx or NMDA mediated mitochondrial dysfunction,31,32 indicating that Mag can act on NMDA receptors. Interestingly, NMDA receptors have been confirmed to be upregulated in CCI induced NP.2 Therefore, it is very possible that in our study, the analgesic effect of Mag is mediated by NMDA receptors. This hypothesis is also well supported by previous studies showing that Mag can inhibit NMDA induced pain in a dose-dependent manner.22 In the current study, we used molecular docking to identify the extra potential candidate receptors; and P2Y12 stood out among all the receptors (Figure 2). P2Y12 is an ADP-preferring subtype of purinergic P2Y receptors. Previously it was reported that P2Y12 regulates microglial activation and is engaged in excitatory synaptic transmission between microglia and neurons in spinal cord.13 Further studies revealed that P2Y12 contributes to microglial activation and alterations after peripheral nerve injury.33 Therefore, P2Y12 is actively involved in the development of neuropathic pain, and this is consistent with our current data showing that the expression of P2Y12 on both mRNA and protein level increased following nerve injury. This confirmed previous findings established by Kobayasi et al.34,35 Using these findings we continued to show that Mag inhibited the increase of P2Y12 following nerve injury, suggesting that P2Y12 is a new target of Mag in nerve injury induced by pain, while this has never been reported before.
Since P2Y12 is mainly distributed in microglia in the spinal cord and it is well established that spinal microglia are involved in neuropathic pain,34,36 Mag may exert a beneficial effect through microglia. As our data show in Figure 3, Mag inhibited the activation of microglia in both morphology and quantity, as well as the expression level of Iba-1, the specific microglial marker. Microglia have been regarded as the immune cells in the nervous system and it has been clearly demonstrated that microglia are involved in neuropathic pain, by releasing multiple cytokines.37 After peripheral nerve injury, multiple cells including both neurons and glia will release proinflammatory cytokines (IL-1β, IL-6 and TNF-α, etc.), which in turn activate microglia; once activated, microglia can further release these cytokines, forming a feedback loop.38 Our results showed that Mag not only inhibited microglia, but also multiple cytokines (Figure 4). These mechanisms might contribute to the analgesic effect of Mag in our neuropathic pain model.
Our study also proved that nerve injury induced p38 MAPK was blocked by Mag, and this could come from two different signal pathways, the direct and indirect pathway. Firstly, Mag might have a direct inhibitory effect on P38 MAPK. Previous findings have revealed that Mag can inhibit MAPK activation in tumor cells,39 colitis,40 and bacterial-induced infection.41 Our results provided the first report that blocking MAPK activation could be one of the mechanisms for Mag alleviation of NP. Secondly, the inhibition of MAPK can be mediated by P2Y12 receptors for which Mag has a binding affinity (Figure 3). In the partial sciatic nerve ligation (PSNL) model, the phosphorylation of p38 MAPK and pain behaviors were suppressed by P2Y12 antagonist and were elevated by P2Y12 agonist, leading to the conclusion that the increased expression of P2Y12 in activated microglia worked as a gateway of p38 signaling pathway in neuropathic pain.34 In contrast, administration of intrathecal p38 MAPK inhibitor significantly decreased P2Y14, P2Y13, and P2Y6, but not P2Y12 mRNAs; therefore, P2Y12 may be upstream of MAPK in the signal pathway. This is also supported in a pain model induced by bone cancer, where blocking of spinal P2Y12 was followed by decreased p38 MAPK and IL-6.16
While we showed the effect of Mag in CCI-induced neuropathic pain and provide possible related mechanisms, many questions remain to be answered due to the limitations of current research. As shown in the current report, while glia in the spinal cord were clearly inhibited by Mag, the extent to which the Mag analgesic effect was mediated by neurons in the current model remains elusive. These mechanisms are interesting and definitely deserve further studies since recent research showed that Mag can inhibit the sodium currents in DRG neurons,42 and synergize the function of GABA mediated by the GABA receptors.43 While we showed Mag has minimal effect on the lipid and metabolite profile shown by the biochemistry analysis (Table 1), more detailed safety tests are still needed, which is unfortunately beyond the scope of the current paper. Since we only explored the short term (8 days) analgesic effect, a longer time course study may provide more insights on the long-term effect. Considering Mag was applied after the CCI model was created, it would be interesting to observe whether Mag application before nerve injury can demonstrate similar analgesic effect.
Overall, by using the widely accepted CCI model as a neuropathic pain model, we showed that Mag significantly inhibited the mechanical and thermal hyperalgesia following nerve jury. These analgesic effects are probably mediated by P2Y12 receptors, which accordingly inhibited the activation of microglia and increased multiple cytokines. By combining all these findings with the relatively safe profile of Mag, we believe that Mag has great potential as a therapeutic candidate for neuropathic pain.
Acknowledgment
We would like to thank Mr Isaac Vallalpando for help with the English editing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi:10.1146/annurev.neuro.051508.135531
2. Gong K, Kung LH, Magni G, Bhargava A, Jasmin L. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS One. 2014;9(8):e106979.
3. Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB, ATPP2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci. 1997;17(14):5297–5304. doi:10.1523/JNEUROSCI.17-14-05297.1997
4. Wu Y, Shen Z, Xu H, et al. BDNF participates in chronic constriction injury-induced neuropathic pain via transcriptionally activating P2X7 in primary sensory neurons. Mol Neurobiol. 2021. doi:10.1007/s12035-021-02410-0
5. Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55(1):1–10. doi:10.1016/j.neures.2006.01.005
6. Woolf CJ, Salter MW. Neuroscience - neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1768. doi:10.1126/science.288.5472.1765
7. Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16(5):519–531. doi:10.1177/1073858409360822
8. Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–548. doi:10.1038/nrd4334
9. Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–643. doi:10.1146/annurev-physiol-022516-034406
10. Haynes SE, Hollopeter G, Yang G, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi:10.1038/nn1805
11. Sasaki Y, Hoshi M, Akazawa C, et al. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44(3):242–250. doi:10.1002/glia.10293
12. Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28(19):4949–4956. doi:10.1523/JNEUROSCI.0323-08.2008
13. Yu TT, Zhang X, Shi HS, et al. P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents. Cell Death Dis. 2019;10. doi:10.1038/s41419-019-1425-4
14. Zhao H, Alam A, Chen Q, et al. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Brit J Anaesth. 2017;118(4):504–516. doi:10.1093/bja/aex006
15. Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292–1311. doi:10.1016/j.neuron.2018.11.009
16. Liu M, Yao M, Wang H, et al. P2Y12 receptor-mediated activation of spinal microglia and p38MAPK pathway contribute to cancer-induced bone pain. J Pain Res. 2017;10:417–426. doi:10.2147/JPR.S124326
17. Shinoda M, Fujita S, Sugawara S, et al. Suppression of superficial microglial activation by spinal cord stimulation attenuates neuropathic pain following sciatic nerve injury in rats. Int J Mol Sci. 2020;21(7):2390. doi:10.3390/ijms21072390
18. Jahromi B, Pirvulescu I, Candido KD, Knezevic NN. Herbal medicine for pain management: efficacy and drug interactions. Pharmaceutics. 2021;13(2):251. doi:10.3390/pharmaceutics13020251
19. Steinhubl SR, Badimon JJ, Bhatt DL, Herbert JM, Luscher TF. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vasc Med. 2007;12(2):113–122. doi:10.1177/1358863X07077462
20. Zhou FF, Jiang Z, Yang BB, Hu ZP. Magnolol exhibits anti-inflammatory and neuroprotective effects in a rat model of intracerebral haemorrhage. Brain Behav Immun. 2019;77:161–167. doi:10.1016/j.bbi.2018.12.018
21. Lin YR, Chen HH, Ko CH, Chan MH. Effects of honokiol and magnolol on acute and inflammatory pain models in mice. Life Sci. 2007;81(13):1071–1078. doi:10.1016/j.lfs.2007.08.014
22. Lin YR, Chen HH, Lin YC, Ko CH, Chan MH. Antinociceptive actions of honokiol and magnolol on glutamatergic and inflammatory pain. J Biomed Sci. 2009;16:94. doi:10.1186/1423-0127-16-94
23. Li J, Copmans D, Partoens M, Hunyadi B, Luyten W, de Witte P. Zebrafish-based screening of antiseizure plants used in Traditional Chinese Medicine: Magnolia officinalis extract and its constituents Magnolol and Honokiol exhibit potent anticonvulsant activity in a therapy-resistant epilepsy model. Acs Chem Neurosci. 2020;11(5):730–742. doi:10.1021/acschemneuro.9b00610
24. Matsui N, Takahashi K, Takeichi M, et al. Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Res. 2009;1305:108–117. doi:10.1016/j.brainres.2009.09.107
25. Li LF, Yang J, Ma SP, Qu R. Magnolol treatment reversed the glial pathology in an unpredictable chronic mild stress-induced rat model depression. Eur J Pharmacol. 2013;711(1–3):42–49. doi:10.1016/j.ejphar.2013.04.008
26. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi:10.1016/0304-3959(88)90209-6
27. Zhao X, Xu Y, Zhao Q, Chen CR, Liu AM, Huang ZL. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: descending monoamine system and opioid receptors are differentially involved. Neuropharmacology. 2012;62(2):843–854. doi:10.1016/j.neuropharm.2011.08.050
28. Zhang JH, Chen ZX, Huang XH, et al. Insights on the multifunctional activities of magnolol. Biomed Res Int. 2019;2019. doi:10.1155/2019/2920169
29. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi:10.4103/0976-0105.177703
30. Chen CR, Zhou XZ, Luo YJ, Huang ZL, Urade Y, Qu WM. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, induces sleep via the benzodiazepine site of GABA(A) receptor in mice. Neuropharmacology. 2012;63(6):1191–1199. doi:10.1016/j.neuropharm.2012.06.031
31. Lin YR, Chen HH, Ko CH, Chan MH. Differential inhibitory effects of honokiol and magnolol on excitatory amino acid-evoked cation signals and NMDA-induced seizures. Neuropharmacology. 2005;49(4):542–550. doi:10.1016/j.neuropharm.2005.04.009
32. Lin YR, Chen HH, Ko CH, Chan MH. Neuroprotective activity of honokiol and magnolol in cerebellar granule cell damage. Eur J Pharmacol. 2006;537(1–3):64–69. doi:10.1016/j.ejphar.2006.03.035
33. Gu N, Eyo UB, Murugan M, et al. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun. 2016;55:82–92. doi:10.1016/j.bbi.2015.11.007
34. Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y(12) receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28(11):2892–2902. doi:10.1523/JNEUROSCI.5589-07.2008
35. Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury.. Glia. 2012;60(10):1529–1539. doi:10.1002/glia.22373
36. Kobayashi K, Fukuoka T, Yamanaka H, et al. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498(4):443–454.
37. Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19(3):138–152. doi:10.1038/nrn.2018.2
38. Zhou YQ, Liu Z, Liu ZH, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflamm. 2016;13. doi:10.1186/s12974-016-0607-6
39. Gao T, Xu HW, Jia SH, et al. Magnolol induces human Ewing sarcoma SK-ES-1 cell apoptosis via the mitochondrial and death receptor pathways. Am J Transl Res. 2020;12(5):1672–1682.
40. Shen P, Zhang ZC, He Y, et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018;196:69–76. doi:10.1016/j.lfs.2018.01.016
41. Zhang L, Wang J, Xu W, et al. Magnolol inhibits Streptococcus suis-induced inflammation and ROS formation via TLR2/MAPK/NF-kappa B signaling in RAW264.7 cells. Pol J Vet Sci. 2018;21(1):111–118. doi:10.24425/119028
42. Qiu J, Zhang LL, Hong JR, et al. Magnolol inhibits sodium currents in freshly isolated mouse dorsal root ganglion neurons. Clin Exp Pharmacol P. 2021;48(3):347–354. doi:10.1111/1440-1681.13422
43. Alexeev M, Grosenbaugh DK, Mott DD, Fisher JL. The natural products magnolol and honokiol are positive allosteric modulators of both synaptic and extra-synaptic GABA(A) receptors. Neuropharmacology. 2012;62(8):2507–2514. doi:10.1016/j.neuropharm.2012.03.002
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.