Back to Journals » Infection and Drug Resistance » Volume 12
Antimicrobial resistance and risk factors for mortality of pneumonia caused by Klebsiella pneumoniae among diabetics: a retrospective study conducted in Shanghai, China
Authors Liu B, Yi H , Fang J, Han L, Zhou M, Guo Y
Received 4 January 2019
Accepted for publication 23 March 2019
Published 7 May 2019 Volume 2019:12 Pages 1089—1098
DOI https://doi.org/10.2147/IDR.S199642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Bing Liu,1,2 Huahua Yi,1,2 Jie Fang,3 Lizhong Han,3 Min Zhou,1,2 Yi Guo1,2
1Department of Respiratory and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Institute of Respiratory Diseases, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Clinical Microbiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Purpose: To investigate antimicrobial resistance and risk factors for mortality of Klebsiella pneumoniae (KP) pneumonia in diabetics and nondiabetics.
Patients and methods: A retrospective study was conducted among inpatients of KP pneumonia via electronic medical records in a territory hospital between January 2016 and June 2018. Antimicrobial resistance in KP pneumonia was compared between diabetics and nondiabetics. Independent risk factors for mortality in KP pneumonia were identified by univariate and multivariate logistic regression among diabetics and nondiabetics separately.
Results: In this study, 456 patients with KP pneumonia were included. There were 156 cases with diabetes and 300 without diabetes. KP showed a lower antimicrobial resistance to a multitude of antimicrobials in pneumonia among diabetics than nondiabetics, namely aztreonam, cefotetan, sulperazone, meropenem, amikacin, tobramycin, sulfamethoxazole, and fosfomycin. In addition, carbapenem-resistant Klebsiella pneumoniae (CRKP) was more prevalent among nondiabetics than diabetics who were admitted to intensive care unit (ICU) (63.0% vs 45.1%, P = 0.038). Multivariable analysis showed that independent risk factors for in-hospital mortality (IHM) in KP pneumonia among diabetics differed from that among nondiabetics as well. Independent predictors for IHM of KP pneumonia among diabetics were male (OR: 5.89, 95% CI: 1.34–25.93, P = 0.019), albumin (ALB) < 35 g/L (OR: 7.00, 95% CI: 2.02–24.28, P = 0.002), bloodstream infection (BSI) (OR: 21.14, 95% CI: 3.18–140.72, P = 0.002), and invasive ventilation during hospitalization (OR: 8.00, 95% CI: 2.99–21.42, P < 0.001). In nondiabetics, independent predictors were higher CURB-65 score (OR: 1.92, 95% CI: 1.29–2.86, P = 0.001), CRKP (OR: 2.72, 95% CI: 1.07–6.90, P = 0.035), BSI (OR: 4.98, 95% CI: 1.34–18.50, P = 0.017), and ICU admission (OR: 4.06, 95% CI: 1.57–10.47, P = 0.004).
Conclusion: In KP pneumonia, diabetics showed lower antimicrobial resistance and different independent risk factors for mortality compared with nondiabetics, in line with previous studies. Importantly, further attention should be paid on rational and effective antibiotic and supportive treatments in order to reduce mortality without aggravating antimicrobial resistance and metabolic damage among diabetics.
Keywords: Klebsiella pneumoniae, pneumonia, diabetics, antimicrobial resistance, risk factor, mortality
Introduction
Pneumonia is a leading cause of death in infectious diseases according to the report released by the World Health Organization (WHO).1 In addition, it was reported by China Antimicrobial Surveillance Network (CHINET) that Klebsiella pneumoniae (KP) ranked as the most frequently isolated pathogen in respiratory tract.2 KP is a scary gram-negative bacterium with high lethality owing to constantly emerging traits of either multi-resistance or hypervirulence.3 As an opportunistic pathogen, it is more likely to cause infections in individuals with impaired immune functions.3 Diabetes, with a global prevalence of 425 million in 2017 and 629 million predicted in 2045,4 are definitely one of the largest immunocompromised groups.5 Moreover, elevated glucose concentration of airway surface liquid (ASL) can provide abundant nutrients for bacteria, complicating the clinical picture of KP pneumonia in diabetics.6
However, information regarding the antimicrobial resistance of KP in pneumonia or other infections is limited and varied.7–12 To our knowledge, only one study has explored the difference in the risk factors for mortality of pneumonia between diabetics and nondiabetics.13 Considering the harmful effects of antibiotics on metabolism and the high susceptibility to acquire KP pneumonia in diabetics,5,14 it is important to have a better understanding of antimicrobial resistance and risk factors for mortality in this risk group. Our study was designed to make a relatively comprehensive exploration on antimicrobial resistance and risk factors for in-hospital mortality (IHM) of KP pneumonia with and without diabetes.
Material and methods
Study design and data collection
A retrospective study was conducted between January 2016 and June 2018 among inpatients of KP pneumonia with and without diabetes in Ruijin Hospital, Shanghai, China. Data were extracted and collected from medical records. We collected information on baseline characteristics, laboratory tests, treatment, procedures, and outcomes. Only the first positive KP culture in sputum or blood sample of each patient was included in our analysis. Readmission was excluded and only the first hospitalization of each patient was herein included. Only patients with antimicrobial tests on imipenem, meropenem, and ertapenem were included in the analysis of carbapenem-resistant Klebsiella pneumoniae (CRKP). In addition, patients with automatic discharges were excluded on the analysis of the outcome.
Definitions
Pneumonia was defined according to Centers for Disease Control and Prevention (CDC) (Atlanta, GA, USA).15 Besides, KP pneumonia was confirmed by KP identification in a sputum culture.
The diagnosis of diabetes was based on (i) history of diabetes or hypoglycemic drug consumption or (ii) symptoms of diabetes and casual blood glucose concentrations ≥11.1 mmol/L or (iii) fasting plasma glucose ≥7 mmol/L or (iv) 2-h plasma glucose in an oral glucose tolerance test (OGTT) ≥11.1 mmol/L.16
IHM referred to overall IHM during hospitalization.
Microbiology
KP isolates were identified by Vitek 2 system (bioMérieux, Marcy l’Etoile, France). Antimicrobial susceptibility tests were conducted with disk diffusion method or Vitek 2 system.17 Extended-spectrum beta-lactamase (ESBL) screening was carried out with the aid of clavulanic acid synergy test.17 Escherichia coli ATCC 25922 was used as a quality control reference strain. The results were interpreted in accordance with the recommendations of the Clinical and Laboratory Standards Institute (CLSI2018).17 KP isolates resistant to imipenem, meropenem, or ertapenem was classified as CRKP.17
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range). Categorical variables were shown as counts or counts/total (percentages). Statistical comparisons were performed using the Student’s t-test or Mann–Whitney U-test, chi-square test or fisher’s exact test, as appropriate. Risk factors for IHM were explored using univariate and multivariate logistic regression analyses in a Forward stepwise (likelihood ratio) manner, and the results were listed as odds ratios (95% confidence interval). Statistics were analyzed using SPSS 24.0 software (IBM, Armonk, NY, USA). Two-sided significance level of 0.05 was selected.
Results
Sociodemographic and clinical characteristic of KP pneumonia patients with and without diabetes
In this study, 456 patients with KP pneumonia were included. There were 156 cases with diabetes and 300 without diabetes (Table 1). Diabetics, compared with nondiabetics, were older (66.5 vs 62.7 years), together with a higher body mass index (BMI) (24.2 vs 22.9) and more coexisting diseases such as chronic heart diseases (42.9% vs 24.0%), chronic renal diseases (12.8% vs 6.7%), stroke (25.0% vs 15.0%), and hypertension (62.2% vs 39.0%). Moreover, diabetics suffered from much severe KP pneumonia with a higher intensive care unit (ICU) admission rate (48.1% vs 37.0%) and CURB-65 score (1.7 vs 1.4).
 | Table 1 Sociodemographic and clinical characteristic of KP pneumonia patients with and without diabetes |
Antimicrobial resistance of KP in sputum among diabetics and nondiabetics
All the 456 patients had a positive KP culture in sputum. In 38 (8.3%) patients, KP was also isolated from blood (Table 2). ESBLs tests were conducted in 417 (91.4%) patients. Besides, 357 (78.3%) patients experienced antimicrobial tests on imipenem, meropenem, and ertapenem. The level of HbA1c was available in 83 (53.2%) diabetics.
 | Table 2 Antimicrobial resistance rate of Klebsiella pneumoniae cultured from sputum in pneumonia patients with and without diabetes |
Among 22 drugs commonly used clinically, KP showed lower resistance to sulfamethoxazole (SMZ) (22.7% vs 32.5%) and fosfomycin (26.7% vs 37.6%) in pneumonia among diabetics. In ICU, resistance rates of KP to aztreonam (53.4% vs 69.5%), cefotetan (45.2% vs 63.2%), meropenem (37.7% vs 59.8%), amikacin (37.8% vs 52.8%), tobramycin (42.5% vs 61.0%), and fosfomycin (42.6% vs 62.6%) were significantly lower in diabetics than that in nondiabetics. Besides, diabetics were shown to have a lower prevalence of CRKP (45.1% vs 63.0%). In non-ICU, only SMZ displayed a lower resistance degree (13.8% vs 25.6%). Compared with diabetics with HbA1c < 6.5%, those with a higher HbA1c level showed significantly lower resistance to sulperazone (11.7% vs 40.0%) and fosfomycin (14.3% vs 66.7%). No significant difference in ESBLs distribution was found between diabetics and nondiabetics.
IHM in KP pneumonia and bloodstream infection (BSI) among diabetics and nondiabetics
Here, 43 (9.4%) patients were of auto-discharge and excluded in the analysis of the outcome. IHM of KP pneumonia was higher in diabetics than nondiabetics (25.5% vs 16.4%). No significant difference in IHM was found in ESBL-KP pneumonia, CRKP pneumonia, or CRKP-BSI between diabetics and nondiabetics as well (Table 3).
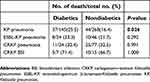 | Table 3 In-hospital mortality of Klebsiella pneumoniae pneumonia and bloodstream infection with and without diabetes |
Risk factors for IHM in KP pneumonia patients with and without diabetes
We conducted univariate and multivariate logistic regression analyses in KP pneumonia patients with and without diabetes, respectively (Tables 4 and 5). In diabetics, risk factors for IHM in KP pneumonia were male (OR: 5.89, 95% CI: 1.34–25.93), ALB < 35 g/L (OR: 7.00, 95% CI: 2.02–24.28), KP-BSI (OR: 21.14, 95% CI: 3.18–140.72), and invasive ventilation during hospitalization (OR: 8.00, 95% CI: 2.99–21.42). In nondiabetics, independent predictors for IHM in KP pneumonia were higher CURB-65 score (OR: 1.92, 95% CI: 1.29–2.86), CRKP (OR: 2.72, 95% CI: 1.07–6.90), KP-BSI (OR: 4.98, 95% CI: 1.34–18.50), and ICU admission (OR: 4.06, 95% CI: 1.57–10.47).
 | Table 4 Univariate and multivariate analyses of independent predictors for in-hospital mortality in Klebsiella pneumoniae pneumonia among diabetics |
 | Table 5 Univariate and multivariate analyses of independent predictors for in-hospital mortality in Klebsiella pneumoniae pneumonia among nondiabetics |
Discussion
In this study, a comprehensive exploration on antimicrobial resistance and risk factors for IHM in KP pneumonia patients with and without diabetes was carried out. We found that: (1) Antimicrobial resistance of KP to several commonly used drugs in pneumonia was lower in diabetics than in nondiabetics. Furthermore, in diabetics, antimicrobial resistance was lower in those HbA1c ≥6.5% than that in those HbA1c < 6.5%; (2) Diabetics showed a lower prevalence of CRKP in KP pneumonia among patients admitted to ICU; (3) Independent risk factors for IHM in diabetics and nondiabetics were different: male, ALB < 35 g/L, KP-BSI, and invasive ventilation during hospitalization in diabetics, compared with higher CURB-65 score, CRKP, KP-BSI, and ICU admission in nondiabetics.
Diabetics had higher BMI and more commodities, and were more advanced in age compared with nondiabetics, which was also found in previous studies.13,18 Additionally, pneumonia in diabetics were shown to be of higher severity in our study and previous studies.9,13,18
The overall antimicrobial resistance rate in our study was higher than that presented in a report published by CHINET,2 showing the severe condition of drug resistance, particularly in tertiary hospitals.11 Out of our expectation, we found that diabetics were of lower resistance to antimicrobials than nondiabetics. It was reported that glucose could stimulate the uptake of aminoglycoside antimicrobials by promoting the TCA cycle and thus restore the susceptibility of bacteria, including KP.19 However, a slight similar effect was found regarding β-lactams and quinolones.19 According to a study, KP possessed orthologs of mammalian 3-mercaptopyruvate sulfurtransferase (3MST), which could provide resistance to a multitude of antimicrobials by catalyzing the production of hydrogen sulfide (H2S).20 Furthermore, hyperglycemia and diabetes were demonstrated to impair the 3-MP/3-MST/H2S pathway, leading to a decreased level of H2S.21 We found a higher prevalence of CRKP in pneumonia among diabetics admitted to ICU, in accordance with a previous study on CRKP-BSI.7 A previous study has shown that diabetes was an independent risk factor for hypervirulent (hypermucoviscous) KP (HvKP or HMKP) infection.22 In another study, it has been reported that high glucose levels could stimulate biosynthesis of capsular polysaccharide (CPS), and that could be helpful in evading phagocytosis, as well as killing, leading to increase of invasiveness and virulence.23 Hypervirulent and multidrug-resistant KP were generally separated and HvKP often presented a low antimicrobial resistance.22,24 However, it was shown in a previous study that KP possessed a higher resistance to some commonly used drugs in liver abscess.9 Since no statistic on previous antibiotics exposure of patients was present in the former study, we could not figure out whether diabetics and nondiabetics had a comparably previous antibiotics use, which could act as a force of natural selection.9 In another study on urinary tract infection in women, antimicrobial resistances of KP and other uropathogens to first-line treatment antibiotics were comparable in Type 2 diabetics and nondiabetics.25 Only 53 KP isolates were included in the analysis of resistance, which is a relatively small sample size.25 No association was found between ESBL-KP pneumonia and diabetes, in line with a previous study on KP-BSI.10
Although HvKP and CRKP were usually separated,22,24 hypervirulent CRKP and carbapenem-resistant HvKP have occurred by the acquisition of virulent or resistant plasmid, which was easy to transfer among bacteria.24,26 Even worse, it was reported that the use of antimicrobials might be detrimental to metabolism, elevating the risk of development of diabetes.14 Therefore, there could be a tendency that diabetics may become the target of super bacteria with combination of hypervirulence and multi-resistance in the future. As a result, the prospect of the war against KP would be much tougher in diabetics. Although the drug resistance rate in diabetics observed in our study was obviously low, the application of antimicrobials in the treatment of KP pneumonia was similar. Further attention should be paid to the appropriate use of antimicrobials in diabetes clinically.
A guideline in China reported that the mean all-cause mortality in hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) was 22.3% and 34.5%, respectively.27 However, the IHM of KP pneumonia in our study was relatively low, which might be explained by a difference in pathogens and the exclusion of auto-discharge in the analysis of outcome. And, patients with auto-discharge were likely to end-up with bad outcome. Tian et al reported a 28-day mortality of 33.3% in CRKP-BSI.7 A previous study, which considered discharge to hospice as death, reported an IHM of 39% both in CRKP pneumonia and CRKP-BSI.28 In this study, IHM in CRKP pneumonia was similar with the previous study but mortality in CRKP-BSI was much higher,7,28 which could be justified by the fact that CRKP-BSI in our study was mainly developed from lung infections, which was more likely to die.7
Both fasting blood glucose (FBG) and HbA1c levels, regardless of diabetic conditions, did not seem to influence the outcome in our study. It might be related to the fact that hospital glycemia was often influenced by not only diabetic conditions but also stress status.29 It was suggested that chronic hyperglycemia and severe acute hyperglycemia might be detrimental, but mild to moderate stress hyperglycemia was protective.30 However, it remained unclear at what threshold stress hyperglycemia became detrimental.30 HbA1c was only obtained from 149 patients, so the sample size might be insufficient to estimate the long-term blood glucose control levels. As a result, the impact of FBG and HbA1c levels on mortality in KP pneumonia needs to be further studied.
BSI was identified as a powerful risk factor for mortality both in diabetics and nondiabetics, which was in line with previous studies.18 The effect of gender on mortality for patients with infectious diseases in diabetics and nondiabetics was controversial.13,18,31 In our study, males showed to be an independent risk factor for IHM in diabetics alone. It was reported that X chromosome carries several genes involved in innate and adaptive immunity, and sex hormones play an important role in modulating immune molecules.32 Consequently, females possessed less susceptibility and mortality to various infections, and the double immunosuppressive effect from gene and hyperglycemia may determine higher risk of death in male diabetics.33 KP pneumonia among diabetics with ALB<35 g/L was poor, which is consistent with previous studies in community-acquired KP-BSI with type 2 diabetes.34 Compared with nondiabetics, diabetics are of elevated glycation, glycoxidation, and excretion, and decreased synthesis of ALB.35 Glycated albumin (GA) and HbA1c could in turn exacerbate the existent deleterious effects of diabetes on ALB.35 And, decreased ALB level in plasma or serum serves as a risk factor for development of complications.35 As a result, diabetic patients might suffer much more than nondiabetics owing to ALB deficiency. In a previous study, mechanical ventilation was shown to be an independent predictor for IHM of KP infections.36 Invasive mechanical ventilation was selected as an independent risk factor in diabetics, but not in nondiabetics in our analysis. This is probably due to the fact that diabetics had more diffusion impairment in lung function, as well as difficulties on wound healing caused by the invasive manipulation. Furthermore, CURB-65 score and ICU admission are two important indicators of severity in pneumonia. In our study, both of them were independent predictors of IHM in KP pneumonia among nondiabetics, but not among diabetics. Diabetics tended to be older, with more kidney and cardiovascular impairment. As a result, diabetics might be affected by a similar higher severity when they suffer from KP pneumonia, and less difference in severity might lead to less negative influences on IHM compared with other risk factors or nondiabetics. Ben-David et al indicated that CRKP was independently associated with increased mortality in BSI.36 In the present study, CRKP was found to be independently associated with increased mortality in nondiabetics. As mentioned above, hyperglycemia could reduce or reverse drug resistance to some extent, and thus alleviate the harmful impact of drug-resistant bacteria.19–21
There are certain limitations in the present study. First, this was a retrospective study and data were retrieved from previous electronic medical records. Therefore, some information, including some antimicrobial susceptibility results, was incomplete. Second, patients with potential KP pneumonia but without a positive sputum culture were not included in our study. Third, there might have been certain misclassification of infection and colonization to some degrees, as patients who suffer from major operations or heart failure might also have pulmonary infiltration. Fourth, our findings in antimicrobial resistance and its mechanism need further validation in metabolomics, genomics, and a larger population, which is also the emphases of our future study.
At the same time, we contrast the antimicrobial resistance of pneumonia-causing KP, which is a hot topic recently, in different subgroups to make the results more reliable. Moreover, we made an exploration on difference on risk factors for IHM between diabetics and nondiabetics, which was a relatively poor investigated approach.
Conclusion
In conclusion, we demonstrated that KP was associated with lower antimicrobial resistance in pneumonia among diabetics clinically. In addition, prevalence of CRKP in KP pneumonia was lower among diabetics who stayed in ICU than nondiabetics. Independent risk factors for IHM of KP pneumonia in diabetics and nondiabetics were not the same. Importantly, further attention should be paid on rational and effective antibiotic and supportive treatments in order to reduce mortality without aggravating antimicrobial resistance and metabolic damage.
Ethics approval and informed consent
The Ethics Committee of Ruijin Hospital gave approval for this study, and all patients provided written informed consent, in compliance with the Declaration of Helsinki.
Abbreviation list
ALB, albumin; BMI, body mass index; BSI, bloodstream infection; CRKP, carbapenem-resistant Klebsiella pneumoniae; CVC, central venous catheter; ESBL, extended-spectrum β-lactamase; ESBL-KP, extended-spectrum β-lactamase-Klebsiella pneumoniae; FBG, fasting blood glucose; HAP, hospital-acquired pneumonia; HbA1c, hemoglobin A1c; ICU, intensive care unit; KP, Klebsiella pneumoniae; LOS, length of stay; PTZ, piperacillin-tazobactam; SMZ, sulfamethoxazole.
Acknowledgments
The authors greatly appreciate all the patients involved in the study. National Key R&D Program of China (No. 2017YFC1309700), National Natural Science Foundation of China (No.81570029), Shanghai Key Discipline for Respiratory Diseases (No. 2017ZZ02014), and Innovative Research Team of High-level Local Universities in Shanghai.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1.
2.
3. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80:(3:629–661. doi:10.1128/MMBR.00078-15
4.
5. Dryden M, Baguneid M, Eckmann C, et al. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21(Suppl 2):S27–32. doi:10.1016/j.cmi.2015.03.024
6. Baker EH, Baines DL. Airway glucose homeostasis: a new target in the prevention and treatment of pulmonary infection. Chest. 2018;1; 53(2):507–514. doi:10.1016/0006-2944(75)90147-7
7. Tian L, Tan R, Chen Y, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5:48. doi:10.1186/s13756-016-0145-0
8. Lee DS, Choe HS, Lee SJ, et al. Antimicrobial susceptibility pattern and epidemiology of female urinary tract infections in South Korea, 2010-2011. Antimicrob Agents Chemother. 2013;57(11):5384–5393. doi:10.1128/AAC.00065-13
9. Tian LT, Yao K, Zhang XY, et al. Liver abscesses in adult patients with and without diabetes mellitus: an analysis of the clinical characteristics, features of the causative pathogens, outcomes and predictors of fatality: a report based on a large population, retrospective study in China. Clin Microbiol Infect. 2012;18(9):E314–330. doi:10.1111/j.1469-0691.2012.03912.x
10. Man MY, Shum HP, Chan YH, et al. Clinical predictors and outcomes of Klebsiella pneumoniae bacteraemia in a regional hospital in Hong Kong. J Hosp Infect. 2017;97(1):35–41. doi:10.1016/j.jhin.2017.06.007
11. Ripabelli G, Tamburro M, Guerrizio G, et al. Tracking multidrug-resistant Klebsiella pneumoniae from an Italian hospital: molecular epidemiology and surveillance by PFGE, RAPD and PCR-based resistance genes prevalence. Curr Microbiol. 2018;75(8):977–987. doi:10.1007/s00284-018-1475-3
12. Durdu B, Meric Koc M, Hakyemez IN, et al. Risk factors affecting patterns of antibiotic resistance and treatment efficacy in extreme drug resistance in intensive care unit-acquired Klebsiella pneumoniae infections: a 5-year analysis. Med Sci Monit. 2019;25:174–183. doi:10.12659/MSM.911338
13. Jimenez-Trujillo I, Jimenez-Garcia R, de Miguel-Diez J, et al. Incidence, characteristic and outcomes of ventilator-associated pneumonia among type 2 diabetes patients: an observational population-based study in Spain. Eur J Intern Med. 2017;40:72–78. doi:10.1016/j.ejim.2017.01.019
14. Mikkelsen KH, Knop FK, Frost M, et al. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab. 2015;100(10):3633–3640. doi:10.1210/jc.2015-2696
15.
16. Expert committee on the diagnosis and classification of diabetes mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(9):1183–1197.
17.
18. Di Yacovo S, Garcia-Vidal C, Viasus D, et al. Clinical features, etiology, and outcomes of community-acquired pneumonia in patients with diabetes mellitus. Medicine (Baltimore). 2013;92(1):42–50. doi:10.1097/MD.0b013e31827f602a
19. Peng B, Su YB, Li H, et al. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015;21(2):249–262. doi:10.1016/j.cmet.2015.01.008
20. Shatalin K, Shatalina E, Mironov A, et al. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334(6058):986–990. doi:10.1126/science.1209855
21. Coletta C, Modis K. Szczesny B et al. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by DL-alpha-lipoic acid. Mol Med. 2015;21:1–14. doi:10.2119/molmed.2015.00035
22. Zhang Y, Zhao C, Wang Q, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. 2016;60(10):6115–6120. doi:10.1128/AAC.01127-16
23. Lee CH, Chen IL, Chuah SK, et al. Impact of glycemic control on capsular polysaccharide biosynthesis and opsonophagocytosis of Klebsiella pneumoniae: implications for invasive syndrome in patients with diabetes mellitus. Virulence. 2016;7(7):770–778. doi:10.1080/21505594.2016.1186315
24. Bialek-Davenet S, Criscuolo A, Ailloud F, et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis. 2014;20(11):1812–1820. doi:10.3201/eid2011.140206
25. Vinken JEM, Mol HE, Verheij TJM, et al. Antimicrobial resistance in women with urinary tract infection in primary care: no relation with type 2 diabetes mellitus. Prim Care Diabetes. 2018;12(1):80–86. doi:10.1016/j.pcd.2017.08.003
26. Feng Y, Lu Y, Yao Z, et al. Carbapenem-resistant hypervirulent Klebsiella pneumoniae of sequence type 36. Antimicrob Agents Chemother. 2018;62:7. doi:10.1128/AAC.02644-17
27.
28. Hauck C, Cober E, Richter SS, et al. Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin Microbiol Infect. 2016;22(6):513–519. doi:10.1016/j.cmi.2016.01.023
29. Russo MP, Elizondo CM, Giunta DH, et al. Prevalence of hyperglycemia and incidence of stress hyperglycemia in hospitalized patients: a retrospective cohort. Eur J Intern Med. 2017;43:e15–e7. doi:10.1016/j.ejim.2017.04.012
30. Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;6;17(2):305.
31. Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21(7):1138–1145.
32. Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2017. doi:10.1007/s12016-017-8648-x
33. Kang YM, Kim YJ, Park JY, et al. Mortality and causes of death in a national sample of type 2 diabetic patients in Korea from 2002 to 2013. Cardiovasc Diabetol. 2016;15(1):131. doi:10.1186/s12933-016-0451-0
34. Huang CH, Tsai JS, Chen IW, et al. Risk factors for in-hospital mortality in patients with type 2 diabetes complicated by community-acquired Klebsiella pneumoniae bacteremia. J Formos Med Assoc. 2015;114(10):916–922. doi:10.1016/j.jfma.2015.07.011
35. Bhat S, Jagadeeshaprasad MG, Venkatasubramani V, et al. Abundance matters: role of albumin in diabetes, a proteomics perspective. Expert Rev Proteomics. 2017;14(8):677–689. doi:10.1080/14789450.2017.1352473
36. Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi:10.1111/j.1469-0691.2011.03478.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
