Back to Journals » Infection and Drug Resistance » Volume 15
Antimicrobial Activity of Selected Ethnoveterinary Medicinal Plants of Southern Region, Ethiopia
Authors Dilbato Dinbiso T , Deressa FB, Legesse DT, Shumi Gebisa E, Choramo Diko A , Tolosa Fulasa T
Received 12 May 2022
Accepted for publication 28 September 2022
Published 27 October 2022 Volume 2022:15 Pages 6225—6235
DOI https://doi.org/10.2147/IDR.S366063
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Tegegn Dilbato Dinbiso,1 Feyissa Begna Deressa,2 Diriba Tadesse Legesse,2 Eshetu Shumi Gebisa,2 Alemayehu Choramo Diko,3 Tadele Tolosa Fulasa2
1School of Veterinary Medicine, Department of Veterinary Science, Ambo University, Guder Mamo Mezemir Campus, Ambo, Ethiopia; 2School of Veterinary Medicine, Jimma University College of Agriculture and Veterinary Medicine, Jimma, Ethiopia; 3School of Veterinary Medicine, Bonga University College of Agriculture and Natural Resource, Bonga, Ethiopia
Correspondence: Alemayehu Choramo Diko, Tel +251917106238 ; +251 966889055, Email [email protected]
Background: For decades, Ethiopians have employed ethnoveterinary medicinal plants to cure both human and livestock ailments. Currently, few studies have been conducted on antimicrobial activity evaluation in Ethiopia. This study, therefore, is designed to evaluate the antibacterial activities of selected ethnoveterinary medicinal plants used in treating livestock ailments in the study area.
Methods: Ethanol extracts of plants obtained by maceration of roots and leaves of four medicinal plant species were studied for potential antimicrobial activity using a disc diffusion method against S. aureus and E. coli. Data obtained from experiments were analyzed using ANOVA and the significant test was set to P < 0.05.
Results: The antibacterial properties of four ethanol extracts of leaves of Withania somnifera L., Becium obovatum, Ageratum conyzoides L., and root of Pentas lanceolata (Forssk.) Defiers were evaluated in vitro and found to be effective against S. aureus but not E. coli. There was no significant difference between the studied plant species and concentrations (p > 0.05), according to the results. The four test extracts had minimum inhibitory concentrations (MICs) ranging from 6.25 to 25 mg/mL, with inhibitory potential ranging from 12.5 to 100 mg/mL. Pentas lanceolata (Forssk.) Defiers’s antibacterial activity at a concentration of 100 mg/mL (18.67 3.78 mm) was comparable to the standard antibiotic (Gentamicin 20g per disc), which had a measurement of 23.08 ± 0.9 mm.
Conclusion: This finding on the selected medicinal plants of Dawuro Zone supports the traditional claims of effective antimicrobial activity in the treatment of livestock health management. Hence, the study suggests further investigations need to be conducted to isolate and elucidate active ingredients in the plant materials tested.
Keywords: antibacterial activity, Dawuro zone, extraction, medicinal plant, maceration
Introduction
Traditional medicine is defined as the totality of knowledge and practices that can be formally explained or used in the prevention and treatment of physical, mental, and social imbalances, based solely on practical experience and observation passed down verbally or in writing from generation to generation.1,2 Medicinal plants have been used as a source of medicine in practically all societies from the dawn of humanity. Traditional medicine and medicinal plants are widely used as a normative basis for the preservation of good health in most underdeveloped countries.3 Farmers have acknowledged the indigenous knowledge of ethnoveterinary medicine and its implications through a course of experience spanning hundreds of years. For addressing veterinary illnesses, livestock rearers in rural areas still rely heavily on folk wisdom practices of plants and household medicines.4
Antimicrobial resistance is currently having a significant impact due to treatment failures linked with multidrug-resistant bacteria, and it has become a global public health concern.5,6 Numerous bacteria, especially Staphylococcus aureus, and E. coli, among others, have an innate resistance to numerous antibiotic substances.9,42,48 As a result, discovering new antibiotics is a singularly essential goal. Natural products continue to be a major source of novel medicinal compounds. Biological activity has been documented in extracts obtained from a variety of plants, including antibacterial and anti-inflammatory properties.5,6
The Solanaceae family Withania somnifera L, has played a significant role in the Ayurvedic and indigenous medical systems. Numerous investigations on this plant revealed that it has antiserotogenic, immunomodulatory, hemopoietic, and rejuvenating qualities in addition to having a favorable impact on the endocrine, cardiac, and central neurological systems reported;35,36 and for treatment of blackleg, bloat, and bloody diarrhea in animals.8 Withania somnifera L. is utilized for its aphrodisiac, liver tonic, astringent, senile dementia, emaciation, sleeplessness, analgesic effects, memory-improving effects, antibacterial, and anti-fungal properties.36
Billy goat weeds, also known as Ageratum conyzoides Linn. (Family Asteraceae), are annual herbs having a long history of traditional medical usage in tropical and subtropical regions of the world. The plant’s stems and leaves are completely covered in tiny white hairs.32 Ageratum conyzoides Linn. Leaves, roots, stems, and flowers can all be used medicinally. The styptic properties of leaves help heal wounds, treat boils, and prevent tetanus. Eye lotion can also be made from leaf juice.42
Becium obovatum (E. Mey. ex Benth. in E. Mey) N.E. Br is a species belonging to the Lamiaceae family. Children with gastrointestinal problems and pain are treated with enemas, which are warm water infusions of crushed roots and leaves of Becium obovatum (E. Mey. ex Benth. in E. Mey) N.E. Br in 1996 by Hutchings.29
The Rubiaceae genus of plants is one of the families. It has roughly 40 species, several of which are widely used as medicinal plants by African natives 61. Out of 40 species, Pentas lanceolata (Forssk.) Defiers. was reported to be used as a folk treatment by African indigenous people including Ethiopia in treating some diseases such as lymphadenitis, abdominal cramps, ascariasis, snake poisoning, retained placenta;49 and veterinary diseases such as anthrax, lice infestations, parasitism, and lumpy skin disease.8
Unlike conventional drugs, the units of measurement used to determine dosage are not standardized, and there are differences in the unit of measurement, duration, and time at which traditional healers take and prescribe remedies for the same types of health problems; lack of precision in dose determination has been noted in many studies.9–13 While there have been few investigations on the therapeutic efficacy of herbal medicines in ethnoveterinary practices, there have been many studies have been found in the literature relating to the use of plants and plant materials in large animals.9,10,14 Scientifically validating the efficacy of ethno veterinary medicinal plants can influence the preservation, conservation, and sustainable utilization of these species in livestock health management.
To our knowledge, in vitro, antimicrobial activity tests on ethnoveterinary herbs are uncommon in the Southern region. As a result, the purpose of this study was to evaluate the antibacterial activity of four different browsing species found in the study area: Withania somnifera L., Becium obovatum (E. Mey. ex Benth. in E. Mey) N.E. Br, Ageratum conyzoides L., and Pentas lanceolata (Forssk.) were chosen and tested for antibacterial activity on Gram-positive S. aureus and Gram-negative E. coli from the Dawuro Zone, Southern Ethiopia.
Materials and Methods
Geographical Location of the Plants
The present study was conducted in the Dawuro zone, which is one of the 14 zones of Southern Nation Nationalities and Peoples Regional State (SNNPRS). The zone is located between 6° 59’- 7° 34’ N of latitude and 36° 68’-37° 52’ E of longitude. The total surface area of the zone is estimated to be 4436 square km which shares 4.07% of the total area of the region.
The average lowest and maximum temperatures are 15.1 and 27.5 degree Celsius, respectively, at altitudes ranging from 1001 to 3000 meters above sea level. The average lowest and maximum temperatures are 15.1 and 27.5 degree Celsius, respectively, at altitudes ranging from 1001 to 3000 meters above sea level. The zone’s annual rainfall ranges between 1201 and 1800 mm.15 The map of the study area is presented in (Figure 1).
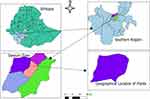 |
Figure 1 Map of the study area. |
Plant Sample Collection and Preparation
The claimed medicinal plants reported for the treatment of livestock diseases were collected from Dawuro zone Tocha district, from November 2017 to September 2018. Field trips were made with local herbalists for the collection of the selected medicinal plants. Voucher specimens of medicinal plants were collected in the field by Dr. Tegegn Dilbato with the help of traditional healers and field assistants. Collected medicinal plants were dried, numbered, pressed, and labeled and were brought to the Ethiopian Herbarium for botanical classification. Specimen identification and confirmation were undertaken by using Flora of Ethiopia and Mr. Melaku Wondafrash who is a botanical taxonomist identified the ethnoveterinary medicinal plants. Finally, the specimens were deposited at the National Herbarium (ETH) at Addis Ababa University.
To eliminate soil particles and other debris, the fresh plant leaves and root samples were gathered and cleaned individually under running water. The leaves and roots were air dried for 15 days at room temperature in the laboratory. The dried samples were ground well into a fine powder in a pestle and mortar. Before maceration, the material was sieved and weighed. For further processing, the powder was kept in airtight polythene bags in the refrigerator at 4°C.
Preparations of Crude Extracts
The crude extracts were prepared according to the procedure described in.9,16 About 25g of each test plant was weighed using a delicate balance and placed in flasks with 250 mL of 95% ethanol, which was added and mixed at maximum speed shaking for 30 minutes to aid in mixing and sufficient maceration of the plant components. For three days, the mixed substance was allowed to sit at room temperature. After 3 days, each sample was filtered using a qualitative filter paper (Whatman No 1 filter paper, Whatman Ltd., England) in separate flasks to obtain a solids-free solution. To remove the solvent, the solution is concentrated using a vacuum rotary evaporator at 50°C.
The percentage yields of each plant extract were calculated according to Kalayou et al and Tura et al.9,17 The resulting concentrated extracts of each plant material were transferred to bottle bijou which had tight fitting cups and then labeled with respective plant name before being refrigerated at 4°C until tested for antimicrobial activity. The percentage yields of each plant extract were calculated as:
A list of selected medicinal plants and their scientific name, ethnoveterinary use, preparation, route of administration; amount macerated, and yield obtained from maceration is summarized in Tables 1 and 2.
 |
Table 1 List of Selected Ethnoveterinary Medicinal Plants Used by Traditional Healers for Livestock Health Management in the Dawuro Zone |
 |
Table 2 Scientific Names, Plant Parts, Amount Macerated, and Percentage Yields of 95% Ethanolic Extracted from Test Plants |
Test Microorganisms
In this study, two species of bacteria were used for in vitro evaluation of the antibacterial activity of the claimed medicinal plants. One Gram-positive, Staphylococcus aureus (ATCC 25923) and Gram-negative, Escherichia coli (ATCC 25922) strains were used. The standard strains were obtained from the Ethiopian Biodiversity Institute (EBI), Ethiopia, in May 2018. The bacteria strains were reactivated by sub-culturing in nutrient broth at 37°C and maintained on nutrient agar slant at 4°C for further use in Veterinary Microbiology Laboratory, Jimma University College of Agriculture and Veterinary Medicine. In addition, Gentamicin (20 µg per disk) was used to compare their efficacy with herbal preparations as a positive control, while DMSO-impregnated disc was used as a negative control.
Antimicrobial Susceptibility Test
Disc Diffusion Assay
The antimicrobial test was conducted using the disc diffusion method.17–19 Muller-Hinton agar (38 gm) (Biotech UK) medium was used for the antimicrobial sensitivity test. The Agar media was prepared by mixing with one litter of distilled water, boiled to dissolve completely, and autoclaved at 121°C for 15 minutes. The medium was later dispensed into 90 mm sterile agar plates and left to set. The agar plates were incubated for 24 hrs at 37°C to confirm their sterility. When no growths occur after 24 hrs, the plates were considered sterile and used for antimicrobial sensitivity tests.
The sterile filter paper discs (Whatman No. 1, diameter 6 mm) were soaked in 30 μL of plant extract for 30 minutes. The extract-soaked filter paper discs were then placed on the inoculated MHA plates, allowing prior incubation to stand for 30 minutes at room temperature to permit proper diffusion of the extract.17,19 The top of 4–5 well-isolated colonies of the same morphology was scooped using a wire loop from the nutrient agar and mixed using sterile normal saline and agitate with a vortex mixer. The turbidity of the bacterial suspension is adjusted by comparing it with 0.5 McFarland turbidity standards (1.5 × 108 CFU/mL) and diffused on the Mueller Hinton agar (MHA) media with sterile swabs. McFarland turbidity standard is prepared by mixing 0.05 mL of 1.175% aqueous solution of barium chloride (0.048NBCL2H2O) with 9.95 mL of 1% sulfuric acid (0.036NH2SO4). The standard and the test suspension are placed in 10 mL sized test tubes and compared against a white background with contrasting black lines until the turbidity of the test suspension equates to that of the turbidity standard. Adjustments of the turbidity are made by adding saline or colonies depending on the degree of turbidity for S. aureus and E. coli.17,18,20 The appropriate crude extract-impregnated discs and conventional antibiotic discs will be applied at spaces 24 mm apart from center to center and 15 mm away from the edge of the plates. The plates turn upside down, labeled, and incubated at 37°C for 24 hr. After 24 hr incubation period diameter of the zone of inhibition (mm) was measured by using a caliper in millimeters. The experiment was done in triplicate.
Determination of in vitro Minimum Inhibitory Concentration (MIC)
The plants’ Minimum Inhibitory Concentration (MIC) was determined using standard procedures described.9,17,21,22 As a result, a stock solution containing 200 mg of plant extracts in 1 mL of DMSO was diluted using a two-fold serial dilution. This yields a series of test concentrations of 100, 50, 25, 12.5, and 6.25 mg/mL, respectively. The plant’s antibacterial activity was tested by incubating each concentration of the extract for 24 hours with an inoculum containing 100µL of the microbial cell at 37°C. The MIC of the plants was defined as the smallest concentration that inhibited the growth of the test bacteria.23,24 According to Bussmann et al,25 strong activity was defined as MIC less than 5 mg/mL.
Inhibitory Potential of Plant Extract
This test is used to assess the in vitro killing ability of ethanolic plant extracts after exposure to test organisms. Bacteria for this test were cultured at logarithmic/exponential phase for 12 hrs of age at 37°C and serial dilutions of plant extract on the 10 mL test tubes were performed. Then after, add a single colony of test bacteria into the test tubes of plant extracts of each concentration and wait for 5 minutes. Aliquots (100 µL) of the cultures were taken after 5 minutes interval plated on freshly prepared Muller Hinton agar and thoroughly mixed and incubated at 37°C for 24 hrs. Following 24 hrs of incubation, the number of colonies was counted to determine the total viable bacteria number. Cell culture with DMSO was assayed as the control.
Data Analysis
An excel spreadsheet was used to keep track of the zone of inhibition caused by each plant extract, as well as the MIC and killing capabilities of two bacteria. The statistical program SPSS version 20 was used to calculate the mean inhibitory zone of inhibition. The mean inhibitory effects of the extracts were compared using ANOVA, with a significant test set at P < 0.05.
Results
The Extract Yields of the Plants
The leaves and roots of each plant were extracted using the same volume of ethanol. The method used for the extraction achieved varying extract yields ranging from 0.668 gm of Pentas lanceolata to 4.823 gm of Withania somnifera. The highest percent extract yield was obtained from Withania somnifera leaf (19.292%) and the lowest (2.672%) was from Pentas lanceolata root. The list of selected ethnoveterinary medicinal plants for the treatment of livestock disease and yield obtained from maceration is summarized in Tables 1 and 2.
Antibacterial Activity of the Plant Extracts
All plant extracts showed good antibacterial activity by inhibiting S. aureus. Among these plants, Becium obovatum, Ageratum conyzoides, and Pentas lanceolata showed activity against S. aureus of the common microorganisms of veterinary importance at all the concentrations tested. The antibacterial activity of Pentas lanceolata at a concentration of 100 mg/mL (18.67±3.78 mm) was comparable with that of the standard antibiotic (Gentamicin 20 µg per disc), 23.08 ±0.9 mm. Generally, bacterial growth inhibition was seen to increase as the concentration of the extracts increased. Results of the mean inhibition zone and minimum inhibitory concentration (MIC) of plant extracts against the test bacteria are also depicted in Figures 2 and 3.
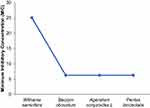 |
Figure 2 Zone of inhibition of plant extracts on S. aureus. |
 |
Figure 3 Minimum inhibitory concentration (MIC) of plant extracts. |
The results of ANOVA comparison of the zone of inhibition between plant species, different concentrations, and positive control (Gentamicin) were recorded. The results showed that there was a significant difference between plant extracts*Gentamicin (p = 0.00), but the difference between plant species* positive control was statistically non-significant (p = 0.191).
The inhibitory potential of the plant extracts was tested by culturing a colony of bacteria for different concentration of the plant extracts. A colony of the bacteria was treated in a solution of the extracts for 5 minutes after which it had been cultured on nutrient agar to recover viable bacterial cells. It showed that the bactericidal activity of the control, solvent DMSO, revealed that the colonies remained viable within the treated time. The test for the inhibitory ability of all extracts revealed a significant effect, in which the strains of S. aureus ATCC 25923 were completely inhibited in 5 minutes at 12.5 mg/mL to 100 mg/mL concentration for Ageratum conyzoides and Pentas lanceolata, 25 mg/mL to 100 mg/mL for Becium obovatum and 50 mg/mL to 100 mg/mL to Withania somnifera, respectively. For the inhibitory ability test against E. coli, all extracts even after 24 hrs of treatment, had not shown inhibitory potential.
Discussion
Natural products contain a wide range of lead compounds, which might be exploited to develop new antimicrobial drugs when standard antimicrobial treatments become ineffective owing to multidrug resistance. Secondary metabolites identified in medicinal plants may have antibacterial properties that help in resistance prevention.9,10 The type and level of different biological activities exhibited by any plant extract, on the other hand, is dependent on a variety of factors such as geographical area, time of collection, soil conditions, harvesting time, moisture content, drying method, storage conditions, and post-harvest process. Furthermore, relatively high temperatures and humidity can be created during tissue grinding, which can denature chemical contents; the extractive solvent can also impact the quantity and composition of secondary active metabolites extracted, as stated by Lumpu et al.27
According to Gupta et al,28 among modern methods of extraction, maceration is effective in extracting bioactive compounds at room temperature. These compounds contain broad-spectrum antibacterial agents acting against different species of bacteria.29–32
The present finding indicated that the highest percentage extract yield was obtained from Withania somnifera leaf and the lowest was from the root of Pentas lanceolata. The variations could be related to the concentration of the phytochemical constituents of the plant parts and species.
The extracts were tested to determine their MIC values. Becium obovatum, Ageratum conyzoides L, and Pentas lanceolata all had MICs of 6.25 mg/mL. This finding is consistent with the findings of,25,27 who found that the plants tested had strong antibacterial activity, with MIC values of less than 16 mg/mL. The relatively high values in Withania somnifera extract (25 mg/mL), imply only modest antibacterial effectiveness.
The antibacterial activity of Pentas lanceolata against S. aureus in all concentrations from highest to lowest is a promising example (9.33 mm to 18.67 mm). This plant has the largest inhibition zone (18.67 mm) against S. aureus ATCC 25923 and no inhibition against E. coli ATCC 25922. Similarly, no plant extracts demonstrated antibacterial efficacy against gram-negative E. coli species. Failure to demonstrate antibacterial action in this study, however, does not imply that the plant is devoid of active components responsible for antibacterial therapeutic efficacy on E. coli bacteria.6,9 The variance might be attributed to the bacteria E. coli, which is previously known to be multi-resistant in nature, extraction techniques, solvent selection, and ecological variation of plant extracts examined.33,34
The antibacterial activity of Withania somnifera leaf extract against S. aureus was shown to be comparably good in vitro (5 mm to 15.67 mm). This result agrees with earlier results elsewhere35,36 and also according to the report of Bokaeian et al,44 the ethanol leaf extract of W. somnifera has a great potential as an antibacterial agent. Especially, the leaf extract of W. somnifera was to be more effective in inhibiting the antibiotic-resistant S. aureus strains as stated by Bokaeian et al.44 But, the finding of this study disagrees with the conclusion of Jamal et al,37 who stated that the ethanolic extracts of Withania somnifera leaf did not demonstrate action against S. aureus. In this study, the ethanol leaf extract of W. somnifera did not show any activities against E. coli and this agrees with the discovery of Jamal et al,37 who concluded that the leaf extract of W. somnifera had no impact on E. coli.
Pentas lanceolata ethanol extracts had the largest growth inhibition zone against Gram-positive bacteria, S. aureus, with inhibition zones of 15.67 and 18.67mm at concentrations of 50 mg/mL and 100 mg/mL, respectively. This discovery is consistent with Matu et al38’s findings that ethyl acetate and methanolic root extracts of Pentas lanceolata were effective against S. aureus species. However, the current findings disagree with the findings of Matu et al,38 who found that ethyl acetate and methanolic root extracts of Pentas lanceolata were effective against E. coli species. The discrepancy might be attributed to the extractive solvent and the resistance of E. coli. The available data from indigenous practices in various places support the current study. Decoction of roots is taken as a remedy for gonorrhea, syphilis, and dysentery.39
At all dosages examined, ethanolic leaf extracts of Ageratum conyzoides demonstrated a significant growth inhibitory effect against Gram-positive bacteria, S. aureus. At a dosage of 100 mg/mL, the antibacterial activity was rather strong (16.33 mm). This result is consistent with the previous research findings40,41 and even slightly higher diameter zone of inhibition (20.3 mm ± 1.3) Ageratum conyzoides extracts had been reported45 from Indonesia and again Harjanti et al46 reported that Ageratum conyzoides extracts have promising antibacterial activities against staphylococcus species.46 However, this finding is not in line with previous findings by Lumpu et al,27 Mitra42, and Trinh et al47 who found that A. conyzoides had high antibacterial action against E. coli. The difference might be due to the bacteria E. coli, which is recognized for its multi-resistant characteristics, extraction procedures, and solvent selection. The difference may be attributed to a variety of factors such as environmental factors like geographical area, time of collection, moisture content, drying method, storage conditions, and post-harvest process, as stated by Lumpu et al.27
Becium obovatum is traditionally used to treat children with stomach problems and abdominal pain as enemas made from warm water infusions of pounded roots and leaves.43 At all doses tested, the leaf extract of Becium obovatum exhibits antibacterial action against S. aureus. This finding agrees with Fawole et al's29 findings, which reported that the extraction of Becium obovatum has good antibacterial activity against S. aureus. However, in the current study, Becium obovatum did not show any activity against E. coli. So, the antimicrobial of Becium obovatum against E. coli in this finding disagrees with the previous finding of Fawole et al29 who reported that Becium obovatum has a good antimicrobial activity against gram-negative E. coli (40).
In general, the results showed that as the concentrations of the extractions increased, so did the zones of inhibition. The inhibitory zones formed by test bacteria exposed to Withania somnifera leaf extract varied from 5 to 15.67 mm against Staphylococcus aureus (ATCC 25923) at concentrations ranging from 25 mg/mL to 100 mg/mL. Staphylococcus aureus inhibition zones varied from 4 to 14 mm for Becium obovatum, 6 to 16.33 mm for Ageratum conyzoides, and 9.33 to 18.67 mm for Pentas lanceolata leaf and root extracts, respectively. This result is also consistent with findings from other regions of the world,20,25 which discovered that the apparent antibacterial activity of the respective extracts may be affected by both concentration and nature of the extraction solvent used.
When tested against the same bacterial species, S. aureus ATCC 25923, the diversity within the various plant extracts and Gentamicin was considerable in this investigation. There was a significant difference across plant species at dosages ranging from 6.25 to 100 mg/mL for all investigated extracts with Gentamicin (p < 0.05). Ageratum conyzoides leaf extract displayed comparable antibacterial activity to Becium obovatum leaf extract, which was judged to be statistically insignificant (p > 0.05). Furthermore, there was no statistically significant difference across plant species and concentrations (p > 0.05). This might be owing to the existence of the same secondary metabolites, as well as the clinical strains’ susceptibilities.
Furthermore, the in vitro findings of the plant extracts’ inhibitory activity demonstrated that the control, solvent DMSO, revealed that the colonies remained viable during the treatment period. The inhibitory potential of all extracts was tested, and the strains of S. aureus ATCC 25923 were completely inhibited in 5 minutes at concentrations ranging from 12.5 mg/mL to 100 mg/mL for Ageratum conyzoides and Pentas lanceolata, 25 mg/mL to 100 mg/mL for Becium obovatum, and 50 mg/mL to 100 mg/mL for Withania somnifera.
Conclusion
The current study found that ethanol extracts of Withania somnifera L., Ageratum conyzoides L., and Becium obovatum (E. Mey. ex Benth. in E. Mey) N.E. Br leaves, as well as Pentas lanceolata root, have antibacterial action against Gram-positive bacteria, S. aureus, but not E. coli. The inhibitory potential of all extracts was tested, and the strains of S. aureus ATCC 25923 were inhibited in 5 minutes at concentrations ranging from 12.5 mg/mL to 100 mg/mL. This discovery of chosen medicinal herbs supports some of the traditional claims of effective antibacterial activity in animal health management therapy. However, further investigations needed to be conducted to validate the biological ingredients and test these traditional medicinal plants’ safety, efficacy, toxicity, and clinical evaluation.
Abbreviations
ANOVA, Analysis of Variance; ATCC, American Type Culture Collection; DMSO, Di-methyl-sulfoxide; DZFEDD, Dawuro Zone Finance Economic Development Department; FMD, Foot and Mouth Disease; SNNPRS, Nation Nationalities and Peoples Regional State; SPSS, Statistical Package for the Social Sciences.
Ethics Approval and Consent to Participate
Before conducting the study, verbal consent was obtained from all participants. No additional ethics approval was required.
Acknowledgments
We would like to thank the traditional healers of Dawuro Zone for their support and for sharing their invaluable knowledge on ethnoveterinary practices during data collection. We are also greatly indebted to Tocha, Mareka, and Loma districts, Development Assistants (DAs) of each district and Jimma University College of Agriculture and Veterinary Medicine, for their collaboration in supporting the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Authors state no funding involved.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bodeker G. Planning for cost-effective traditional health services, WHO. Traditional medicine, better science, policy, and services for health development; 2000: 31–70.
2. World Health Organization. WHO global report on traditional and complementary medicine 2019. World Health Organization; 2019. Available from: https://apps.who.int/iris/handle/10665/312342.
3. United Nations Environmental and Science Community Organization (UNESCO). Health, orientation texts–World decade for cultural development 1988–1999. Document CLT/DEC/PRO–1996, Paris, France; 1996: 129.
4. Kale RB, Gadge SS, Jayaswall K, Patole AO, Mahajan V, Singh M. Ethno-veterinary medicinal uses of garlic (Allium sativum) by livestock rearer. Indian J Tradit Knowl. 2021;20(2):426–435.
5. Dilbato T, Begna F, Joshi RK. Reviews on challenges, opportunities and future prospects of antimicrobial activities of medicinal plants: alternative solutions to combat antimicrobial resistance. Int J Herb Med. 2019;7:10–18.
6. Kokoska L, Kloucek P, Leuner O, Novy P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr Med Chem. 2019;26(29):5501–5541. doi:10.2174/0929867325666180831144344
7. Yirga G, Teferi M, Gidey G, Zerabruk S. An ethnoveterinary survey of medicinal plants used to treat livestock diseases in Seharti-Samre district, Northern Ethiopia. Afr J Plant Sci. 2012;6(3):113–119.
8. Lulekal E, Asfaw Z, Kelbessa E, Van Damme P. Ethnoveterinary plants of Ankober District, North Shewa Zone, Amhara Region, Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):1–19. doi:10.1186/1746-4269-10-21
9. Kalayou S, Haileselassie M, Gebre-egziabher G, et al. In–vitro antimicrobial activity screening of some ethnoveterinary medicinal plants traditionally used against mastitis, wound and gastrointestinal tract complication in Tigray Region, Ethiopia. Asian Pac J Trop Biomed. 2012;2(7):516–522. doi:10.1016/S2221-1691(12)60088-4
10. Romha G, Admasu B, Hiwot Gebrekidan T, Aleme H, Gebru G. Antibacterial activities of five medicinal plants in Ethiopia against some human and animal pathogens. Evid Based Complement Altern Med. 2018;2018:1–10. doi:10.1155/2018/2950758
11. Bakhiet AO, Adam S. Therapeutic utility, constituents and toxicity of some medicinal plants: a review. Vet Hum Toxicol. 1995;37(3):255.
12. Geyid A, Abebe D, Debella A, et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J Ethnopharmacol. 2005;97(3):421–427. doi:10.1016/j.jep.2004.08.021
13. Nasir M, Tafess K, Abate D. Antimicrobial potential of the Ethiopian Thymus schimperi essential oil in comparison with others against certain fungal and bacterial species. BMC Complement Altern Med. 2015;15(1):260. doi:10.1186/s12906-015-0784-3
14. Severino L, Ambrosio L. Herbal drugs used for domestic animals. In: Medicinal Plants: Biodiversity and Drugs. 2012:334.
15. Dawuro Zone Finance and Economic Development Department (DZFEDD). Dawro Zone Finance and Economic Development Department, Dawro Zone Annual Statistical Abstract /2016–2017. Unpublished Data; 2017.
16. Zhang L, Ravipati AS, Koyyalamudi SR, et al. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med. 2013;6(9):673–681. doi:10.1016/S1995-7645(13)60117-0
17. Tura GT, Eshete WB, Tucho GT. Antibacterial efficacy of local plants and their contribution to public health in rural Ethiopia. Antimicrob Resist Infect Control. 2017;6(1):76. doi:10.1186/s13756-017-0236-6
18. Quinn PJ, Carter ME, Markey BK, Carter GR. Clinical Veterinary Microbiology. London, UK: Mosby International Limited; 1999.
19. Aylate A, Agize M, Ekero D, Kiros A, Ayledo G, Gendiche K. In-vitro and in-vivo antibacterial activities of Croton macrostachyus methanol extract against E. coli and S. aureus. Adv Anim Vet Sci. 2017;5(3):107–114.
20. Olasehinde GI, Okolie ZV, Oniha MI, Adekeye BT, Ajayi AA. In vitro antibacterial and antifungal activities of Chrysophyllum albidum and Diospyros monbuttensis leave. J Pharmacogn Phytother. 2015;7:23.
21. Sule IO, Agbabiaka TO. Antibacterial effect of some plant extracts on selected Enterobacteriaceae. Ethnobot leafl. 2008;1:137.
22. Suurbaar J, Mosobil R, Donkor AM. Antibacterial and antifungal activities and phytochemical profile of leaf extract from different extractants of Ricinus communis against selected pathogens. BMC Res Notes. 2017;10(1):660. doi:10.1186/s13104-017-3001-2
23. Jagessar RC, Mohamed A, Gomes G. An evaluation of the antibacterial and antifungal activity of leaf extracts of Momordica Charantia against Candida albicans, Staphylococcus aureus and Escherichia coli. Nat Sci. 2008;6(1):1–14.
24. Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agents: current methods and future trends. J Med Plant Res. 2010;4:104–111.
25. Bussmann RW, Malca-García G, Glenn A, et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J Ethnopharmacol. 2010;132(1):101–108. doi:10.1016/j.jep.2010.07.048
26. Nigussie D, Davey G, Legesse BA, Fekadu A, Makonnen E. Antibacterial activity of methanol extracts of the leaves of three medicinal plants against selected bacteria isolated from wounds of lymphoedema patients. BMC Complement Altern Med. 2021;21(1):1–10. doi:10.1186/s12906-020-03183-0
27. Lumpu N, Manienga K, Bumoyi M. Antibacterial and antifungal screening of extracts from six medicinal plants collected in Kinshasa-Democratic Republic of Congo against clinical isolate pathogens. J Pharmacognosy Phytother. 2014;6(3):24–32. doi:10.5897/JPP2013.0263
28. Gupta A, Naraniwal M, Kothari V. Modern extraction methods for the preparation of bioactive plant extracts. Int J Appl Nat Sci. 2012;1:8–26.
29. Fawole OA, Finnie JF, Van Staden J. Antimicrobial activity and mutagenic effects of twelve traditional medicinal plants used to treat ailments related to the gastrointestinal tract in South Africa. S Afr J Bot. 2009;75(2):356–362. doi:10.1016/j.sajb.2008.11.002
30. Singariya P, Kumar P, Mourya KK. Phytochemical screening and antimicrobial activities of dhaman grass and Indian Ginseng. J Pharm Res. 2012;5:135–139.
31. Florence AR, Joselin J, Brintha TS, Sukumaran S, Jeeva S. Preliminary phytochemical studies of select members of the family Annonaceae for bioactive constituents. Bio Disc Res J. 2014;5:85–96.
32. Chauhan A, Rijhwani S. A comprehensive review on phytochemistry of Ageratum conyzoides Linn. (Goatweeds). International Journal of Engineering Technology. Manag Appl Sci. 2015;3:348–358.
33. Vlietinck AJ, Van Hoof L, Totte J, et al. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J Ethnopharmacol. 1995;46(1):31–47. doi:10.1016/0378-8741(95)01226-4
34. Rabe T, Van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J Ethnopharmacol. 1997;56(1):81–87. doi:10.1016/S0378-8741(96)01515-2
35. Rizwana H, Al Hazzani AA, Shehata AI, Moubayed NMS. Antibacterial potential of Withania somnifera L. against human pathogenic bacteria. Afr J Microbiol Res. 2012;6:4810–4815.
36. Saidulu CH, Venkateshwar C, Gangadhar R. Preliminary phytochemical studies of medicinal plant drug: withania somnifera L. Biolife. 2014;2:306–312.
37. Jamal Q, Munir S, Sherwani SK, et al. Antibacterial activity of two medicinal plants: Withania somnifera and Curcuma longa. Eur Acad Res. 2013;1:1335–1345.
38. Matu NE, Kirira GP, Kigondu MVE, Moindi E, Amugune B. Antimicrobial activity of organic total extracts of three Kenyan medicinal plants. Afr J Pharm Pharmacol. 2012;1(1):14–18.
39. Bukuru J. Isolation and structural elucidation of natural products from Pentas bussei K. Krause, Pentas lanceolata (Forsk.) Deflers and Pentas parvifolia Hiern (Rubiaceae) [Doctoral dissertation, Ghent University]; 2003.
40. Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA. Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern Med. 2005;5(1):1–7. doi:10.1186/1472-6882-5-6
41. Agarwal P, Agarwal N, Gupta R, Gupta M, Sharma B. Antibacterial activity of plant extracts against methicillin-resistant Staphylococcus aureus and Vancomycin-resistant Enterococcus faecalis. J Microb Biochem Technol. 2016;8(5):404–407. doi:10.4172/1948-5948.1000316
42. Mitra PK. Antibacterial activity of an isolated compound (AC-1) from the leaves of Ageratum conyzoides Linn. J Med Plants Stud. 2013;1:23.
43. Hutchings A. Zulu Medicinal Plants: An Inventory.University of Natal press; 1996.
44. Bokaeian M, Saeidi S. Evolution of antimicrobial activity of leaf extract of withania somnifera against antibiotic resistant Staphylococcus aureus. Zahedan J Res Med Sci. 2015;17(7):e1016. doi:10.17795/zjrms1016
45. Budiman A, Aulifa DL. A Study Comparing Antibacterial Activity of Ageratum Conyzoides L. extract and Piper Betle L. extract in gel dosage forms against Staphylococcus aureus. Pharmacogn J. 2020;12(3):473–477. doi:10.5530/pj.2020.12.73
46. Harjanti DW, Ciptaningtyas R, Wahyono F. Phytochemical properties and antibacterial activity of Ageratum conyzoides, Piper betle, Muntingia calabura and Curcuma domestica against mastitis bacteria isolates. IOP Conf Ser Earth Environ Sci. 2019;247:012049. doi:10.1088/1755-1315/247/1/012049
47. Trinh PC, Thao LTT, Ha HTV, Nguyen TA. DPPH-scavenging and antimicrobial activities of Asteraceae medicinal plants on uropathogenic bacteria. Evid Based Complement Altern Med. 2020;2020:1–9. doi:10.1155/2020/7807026
48. Faye G, Birhanu T, Belete T. Survey and antimicrobial activity study of ethnomedicinal plants in selected districts of North Shewa Zone, Oromia, Ethiopia. Infect Drug Resist. 2021;14:5511.
49. Heba-tollah MS, Abd-Alla HI, Abdelwahab AB, Gabr MM, Kirsch G. Secondary metabolites and biological activity of Pentas species: a minireview. J Adv Res. 2018;10:21–30. doi:10.1016/j.jare.2017.12.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.