Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 12
Antihypertensive Effect Of Amlodipine In Co-Administration With Omeprazole In Patients With Hypertension And Acid-Related Disorders: Cytochrome P450-Associated Aspects
Authors Dorofeeva MN, Shikh EV, Sizova ZM, Tarasenko AV , Denisenko NP , Smirnov VV, Ryzhikova KA , Sozaeva ZA, Grishina EA, Sychev DA
Received 30 May 2019
Accepted for publication 16 October 2019
Published 5 November 2019 Volume 2019:12 Pages 329—339
DOI https://doi.org/10.2147/PGPM.S217725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Margarita N Dorofeeva,1 Evgenia V Shikh,2 Zhanna M Sizova,1 Alisa V Tarasenko,3 Natalia P Denisenko,4,5 Valeriy V Smirnov,6,7 Kristina A Ryzhikova,4 Zhannet A Sozaeva,4 Elena A Grishina,4 Dmitriy A Sychev5
1Department of Social Expertise, Urgent and Outpatient Therapy, First Moscow State Medical University (Sechenov University), Ministry of Healthcare, Moscow, Russia; 2Department of Clinical Pharmacology and Propedeutics of Internal Diseases, First Moscow State Medical University (Sechenov University), Ministry of Healthcare, Moscow, Russia; 3Medicine of the Future, First Moscow State Medical University (Sechenov University), Ministry of Healthcare, Moscow, Russia; 4Research Institute, Russian Medical Academy of Continuous Professional Education, Ministry of Healthcare, Moscow, Russia; 5Department of Clinical Pharmacology and Therapy, Russian Medical Academy of Continuous Professional Education, Ministry of Healthcare, Moscow, Russia; 6Department of Pharmaceutical and Toxicological Chemistry, First Moscow State Medical University (Sechenov university), Ministry of Healthcare, Moscow, Russia; 7Laboratory of Clinical Pharmacology, National Research Centre - Institute of Immunology, Federal Medical Biological Agency, Moscow, Russia
Correspondence: Natalia P Denisenko
Research Institute, Russian Medical Academy of Continuous Professional Education, Ministry of Healthcare, Barrikadnaja Street, 2/1, Moscow 125993, Russia
Tel +7 495 945 81 39
Email [email protected]
Background: CYP2C19 and CYP3A are the main enzymes involved in omeprazole metabolism, while CYP3A is the principal enzyme family for amlodipine biotransformation. Concomitant use of these drugs in patients with hypertension and acid-related disorders (ARD) might lead to drug–drug interaction.
Purpose: The aim of the study was to find if adding omeprazole for treating ARD to amlodipine long-term therapy of hypertension influenced blood pressure of CYP2C19 polymorphism carriers.
Patients and methods: Fifty-one patients diagnosed with hypertension and ARD were enrolled in the study. Evaluation of antihypertensive therapy was performed by office (OBPM) and ambulatory (ABPM) blood pressure monitoring. Peripheral venous blood was collected for DNA extraction and real-time polymerase chain reaction was performed for CYP2C19*2G681A (rs4244285), CYP2C19*3G636A (rs4986893) and CYP2C19*17C−806T (rs12248560) polymorphisms analysis.
Results: Of 51 patients there were 21 extensive metabolizers (EMs), 18 ultrarapid metabolizers (UMs) and 12 intermediate metabolizers (IMs). The results of OBPM showed that antihypertensive effect was significantly more pronounced in IMs compared to EMs or UMs and the average group value in the following parameters: average office systolic blood pressure (BP), dynamics of the average office systolic BP. According to dynamics of diastolic BP, the antihypertensive effect was also significantly higher in IMs than in UMs and the average group value. The results of ABPM revealed that there was a significantly more pronounced antihypertensive effect in IMs compared to all other analyzed groups according to the dynamics of both daytime systolic and 24 hr diastolic BP. The average daytime diastolic BP and its dynamics, the average 24 hr systolic BP and its dynamics were higher in IMs compared to EMs and UMs.
Conclusion: Adding omeprazole to long-term amlodipine therapy in patients with hypertension and ARD may lead to a significantly more pronounced antihypertensive effect in patients genotyped CYP2C19 IMs.
Keywords: CYP2C19, CYP3A, pharmacogenetics, proton pump inhibitor
Introduction
Amlodipine is a dihydropyridine calcium-channel blocker. It blocks the inward movement of calcium by binding to L-type calcium channels in the heart and arteriolar vasculature. This causes vascular smooth muscle to relax, dilating mainly arterioles.1
Amlodipine is largely metabolized in the liver via the Cytochrome P450 3A4/5.2,3 CYP3A4 is the main contributor to amlodipine dehydrogenation. Plasma concentration of this drug depends on metabolic rate which in turn depends on the activity of cytochrome isoenzymes metabolizing the drug. Therefore, changes in CYP3A4 and CYP3A5 activity may lead to changes in the effectiveness and tolerability of amlodipine.4
Omeprazole is a prodrug which requires activation in the acidic environment of the parietal cells canaliculus, where it is converted to its active form inhibiting the enzyme H+/K+-ATPase (proton pump) irreversibly. The proton pump controls the last step in acid secretion, and by targeting this step, omeprazole and the other PPIs are able to potently inhibit gastric acid secretion.5
Omeprazole is metabolized and inactivated in the liver by the cytochrome P450 system. CYP2C19 is the principal isoenzyme involved, although CYP3A4 may also contribute.6 Omeprazole is metabolized to hydroxysulfone and desmethyl metabolites, which have no effect on gastric acid secretion. Omeprazole is both a substrate and an inhibitor of the above isoenzymes; therefore, its interaction with other substrates of them is possible.7,8 In CYP2C19 poor and intermediate metabolizers the CYP3A bypass for omeprazole activates.9 Omeprazole was found to reversibly inhibit both CYP2C19 and CYP3A4 in vitro.10,11 It was also found that in white omeprazole reduced the urinary excretion of dapsone’s metabolite mediated by CYP3A and possibly CYP2E1.12 The other study aimed to evaluate the contribution of omeprazole metabolites to in vivo CYP2C19 and CYP3A4 inhibition. They found that omeprazole metabolite 5′-O-desmethylomeprazole may be responsible for the majority of hepatic CYP3A4 inhibition.8
Since both amlodipine and omeprazole metabolisms are mediated by CYP3A, especially in CYP2C19 poor (PMs) and intermediate (IMs) metabolizers for omeprazole, we could predict the presence of drug–drug interactions in co-administration of these drugs. As PMs are rare among Caucasians, the group of IMs may represent data of patients with CYP2C19 reduced activity and low biotransformation of omeprazole.13
The aim of the study was to find if adding omeprazole for treating ARD to amlodipine long-term therapy of hypertension influenced blood pressure of CYP2C19 polymorphism carriers.
Materials And Methods
Study Design And Patients
A total of 51 patients (17 men, 34 women; mean age 66.6±9.8 years, age range 37–88 years) diagnosed with hypertension and acid-related disorders (ARD) were enrolled in the study in the period from January to December 2017. The inclusion criteria were: long-term 10 mg of amlodipine intake for treating hypertension together with the indications for 20 mg omeprazole assignment for treating ARD for a least 2 weeks. The exclusion criteria were: age under 18 years, severe hypertension (a systolic blood pressure of ≥180 mm Hg or a diastolic blood pressure of ≥110 mm Hg), a history of acute cerebrovascular accident or transient ischemic attack; severe heart rhythm and conduction disorders; chronic heart failure; chronic liver, kidney or adrenal gland diseases; a need for drug therapy for comorbidities; acute peptic ulcer (since standard therapy includes antibacterial drugs metabolized by cytochrome 3A4, which may affect the results of the study). The patients enrolled received only amlodipine and omeprazole with no other drugs during the period of study. The patients were already treated with 10 mg of amlodipine and were newly assigned for 20 mg of omeprazole. Blood pressure data were collected in patients already taking amlodipine therapy (baseline) and after 2 weeks of combined therapy of 10 mg of amlodipine and 20 mg omeprazole (endpoint).
All patients underwent physical examination, standard laboratory and instrumental examination including 12-lead ECG and 24-h ambulatory blood pressure monitoring (ABPM).
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of First Moscow State Medical University (Sechenov University). Written informed consent was obtained from all participants before entering the study.
Methods For Evaluating The Antihypertensive Efficacy Of Amlodipine
Evaluation of antihypertensive therapy was performed by two methods: office (OBPM) and ambulatory blood pressure monitoring (ABPM). OBPM was performed at each visit, ABPM twice: before and 2 weeks after the beginning of combination therapy of amlodipine and omeprazole.
OBPM was performed at each site in a sitting position 15 mins after the rest by the auscultatory method using a double measurement of BP with an interval of 3 mins with the average value calculation.
ABPM was carried out using the software-hardware complex “BPLab” within 24 hrs. The complex provides measurement of BP by oscillometric and auscultatory methods. Measurement of BP was recorded from 8:00 to 23:00 hrs (daytime) and from 23:00 to 8:00 hrs (nighttime). The intervals between measurements were 15 mins during the day and 30 mins during the night. All patients kept a diary of self-observation and recorded their physical activity.
The ABPM data were analyzed in case of 85% or more successful measurements per day. The average systolic and diastolic BP were estimated per 24 hrs, daytime and nighttime.
The following indicators were considered normal:
- average 24 hrs BP <130/80 mmHg, average daytime BP <135/85 mmHg, average nighttime BP <120/70 mmHg;
- variability in daytime average systolic and diastolic BP <15.5 mmHg and <13.3 mmHg, respectively; nighttime average systolic and diastolic BP <14.8 mmHg and <11.3 mm Hg, respectively;
- the degree of nocturnal BP dipping – 10–22%;
- the rate of morning increase in BP – less than 10 mmHg/hour.
The risk of amlodipine adverse drug reactions (ADR) was assessed by the results of tolerability in accordance with the following criteria:
- excellent – absence of ADR;
- satisfactory – ADR which required a dose adjustment of the drug;
- unsatisfactory – ADR which led to the discontinuation of the drug.
The electrical activity of the heart was recorded using a certified Heart Mirror electrocardiograph (Innomed, Hungary) in 12-lead ECG at a speed of 25 mm/s and a gain setting of 1 mV/cm. Heart rate was estimated by calculating RR interval in the second standard lead.
Genotyping
Peripheral venous blood (9 mL) of patients was collected in K3-EDTA (ethylenediaminetetraacetic acid) vacuum tubes for DNA extraction from whole blood samples. The DNA extraction process was carried out using the standard phenol method with protein kinase K using DNA-EXTRAN-1 equipment (Syntol, Russia). Genotyping was performed by PCR-RFLP (polymerase chain reaction and restriction fragment length polymorphism) method. The carriage of CYP2C19 polymorphic gene markers was determined by real-time polymerase chain reaction (Real-Time PCR) using the SNP-Screen reagent kits (Syntol, Russia) on the Real-Time CFX96 Touch amplifier (Bio-Rad Laboratories, Inc., USA).
Primer sequence synthesized by Syntol (Russia) was selected using the Primer Select program 4.05, DNASTAR Inc:
- rs4244285 (G681A, *2): direct 5′-AGAAGAATTGTTGTAAAAAGTAAG3′,
- reverse 5′-ATAAAGTCCCGAGGGTTGTTGATG-3′.
- rs4986893 (G636A, *3): direct 5′-GATCAGCAATTTCTTAACTTGATG-3′,
- reverse 5′-GACTGTAAGTGGTTTCTCAGGA-3′
- rs12248560 (C806T, *17): direct 5ʹ-AAATTTGTGTCTTCTGTTCTCAAA-3ʹ
- reverse 5ʹ-TAGCTGGCAGAACTGGGATT-3ʹ
For genotyping, equipment was used:
- real-time PCR instrument
- microcentrifuge
- 1.5 mL tubes
- 10 and 100 μL micropipettes
- 0.2 μL PCR tubes
For PCR amplification reagents (2.5 x Reaction mixture, 2.5 x Diluent and Taq-polymerase) were mixed with the calculation based on the number of samples tested plus 4 (one negative and three positive control samples).
- filter pipette tips
Sample preparation:
- Component thawing, mixing and centrifuging.
- Preparing a mixture of components by adding them in the order indicated in the table, mixing and centrifuging.
- Labeling PCR tubes.
- Adding 20 μl of the mixture to the PCR tubes.
- Adding 5 µl of control and test samples to each PCR tube according to the labeling.
- Short centrifugation.
- Placing tubes into the device in accordance with the study protocol.
Amplification program for all polymorphisms:
- 0 С - 3:00 min
- 0 C - 0:15 min
- 0 C - 0:40 min
- Plate reading.
- Repeating for 40 cycles.
- Finishing.
«SNP-screen» kit (Synthol, Russia) was used to determine single nucleotide polymorphisms (SNPs) rs4244285 (G681A, *2), rs4986893 (G636A, *3), rs12248560 (C806T, *17) of the CYP2C19 gene. In every «SNP-screen» kit, two allele-specific hybridizations were used, which allowed the determination of two alleles of the studied polymorphism separately on two fluorescence channels.
According to the results of genotyping, the following genotypes of SNPs rs4244285 (G681A, *2), rs4986893 (G636A, *3), rs12248560 (C806T, *17) of the CYP2C19 gene were obtained, which showed that three genotypes may be distinguished:
- Reference homozygote with G/G (*1/*1) or С/С genotype;
- Heterozygote with G/A (*1/*2 или *1/*3) or Т/С (*2/*17 или *3/*17) genotype;
- Variant homozygote with A/A (*2/*2 или *3/*3) or Т/Т genotype.
Assay
To determine the level of endogenous compounds a portion of the morning urine was used since the concentration of cortisol reaches its maximum value in the morning. A solvent extraction method was used for isolating steroids.
To analyze the effect of pharmacotherapy on the activity of CYP3A4, the urine of the patients was taken twice: before entering the study and after two weeks of treatment.
2 mL of urine was extracted with 4 mL of ethyl acetate/isopranolol (85:15). After orbital mixing for 10 min and centrifuging at 3000 × g for 5 min, the upper organic layer was separated. The layers were combined and evaporated in a vacuum evaporator.
Phenotyping
Cortisol and 6β-hydroxycortisol concentrations in urine were measured using Agilent G1978B Multimode Source for 6410 Triple Quade LC/MS (Agilent Technologies, Inc., USA) high-performance liquid chromatography with mass spectrometry. The isolation of the drug and its metabolite was performed on Waters Symmetry C18 Column (150 mm×4.6 mm; 5.0 μm). The column temperature was maintained at 35°C. UV-detector wavelength was set at 246 nm. The mobile phase contained 55% water formic acid solution (1 L of water:1 mL of formic acid) and 45% acetonitrile. The flow rate was 0.5 mL/min. Volumes of 10 μL were injected. The mass spectrometer was operated using the following conditions: positive polarity, MM-ES+APCI ionization.
The results were evaluated by the 6-β-hydroxycortisol/cortisol ratio (6 beta-OHC/C). The higher the urine metabolic ratio 6β-hydroxycortisol/cortisol, the higher the activity of CYP3A.
Statistical Analysis
The statistical analysis was performed using SPSS Statistics 22. The p-values <0.05 were considered statistically significant. To evaluate the deviation of genotype frequencies in the studied population from Hardy–Weinberg equilibrium, Fisher’s exact test was used. To evaluate continuous variables, the number of cases, the mean (M), the standard deviation (m), the minimum, the maximum, the median (Me) and quartiles were indicated. To compare two quantitative variables, the Student’s t-test and the Mann–Whitney test (U-test) were used. To compare three or more groups of quantitative data Kruskal-Wallis H-test has been used. To identify the relationship between the quantitative indicators in the groups, the non-parametric Spearman’s method was used.
Results
CYP2C19 Genotyping Results
Of the 51 patients, there were 21 (41.2%) extensive metabolizers (EMs; CYP2C19 *1/*1), 18 (35.3%) ultrarapid metabolizers (UMs; CYP2C19 *1/*17, CYP2C19 *17/*17) and 12 (23.5%) intermediate metabolizers (IMs; CYP2C19 *1/*2, CYP2C19 *2/*17, CYP2C19 *1/*3) (Table 1).
 |
Table 1 CYP2C19 Genetic Polymorphisms Frequency In Patients With Hypertension And ARD |
Patients were defined as EMs, UMs and IMs according to the guidelines of the Dutch Pharmacogenetics Working Group the Royal Dutch Pharmacists Association.14
The observed genotype frequencies were in Hardy–Weinberg equilibrium, p>0.05 (CYP2C19*2: χ2 = 0.74, р = 0.69; CYP2C19*17: χ2 = 0.02, р = 0.99).
The allele frequencies were as follows: CYP2C19*2 – 10.8%, CYP2C19*17 – 23.5%, CYP2C19*3 - 0.98% (Figure 1).
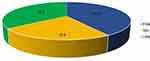 |
Figure 1 Predicted CYP2C19 phenotype of patients with hypertension and ARD. Abbreviations: EMs, extensive metabolizers; UMs, ultrarapid metabolizers; IMs, intermediate metabolizers. |
CYP3A Activity
Evaluation Of CYP3A4 Activity In Patients With Hypertension And ARD Receiving The Omeprazole And Amlodipine Combination
The effect of combined omeprazole and amlodipine pharmacotherapy in patients with grade 1–2 hypertension and ARD on CYP3A4 activity was evaluated using a non-invasive method by estimation of the 6β-hydroxycortisol/cortisol ratio (6 beta-OHC/C) in the urine.
Before the treatment, the mean 6β-hydroxycortisol/cortisol ratio in the average group of patients with hypertension and ARD was 1.09 [0.79; 1.30], which was comparable to the 6β-hydroxycortisol/cortisol ratio in EMs (1.08 [1.02; 1.23]), IMs (1.11 [0.96; 1.35]) and UMs (1.09 [0.86; 1.31]) (Table 2).
 |
Table 2 CYP3A4 Activity In Patients With Hypertension And ARD |
After 2 weeks of the treatment, the mean 6β-hydroxycortisol/cortisol ratio in the average group of patients was 0.92 [0.69; 1.09]. A statistically significant decrease in the 6β-hydroxycortisol/cortisol ratio was observed in IMs from 1.11 [0.96; 1.35] to 0.81 [0.69; 0.93] (p=0.03), while in EMs and UMs a slight decrease in the 6β-hydroxycortisol/cortisol ratio was observed (0.97 [0.70; 1.28] and 0.98 [0.60; 1.14], respectively), which was not statistically significant.
During the observation period, the metabolic ratio dynamics among all the examined patients was 0.17 [0.13; 0.24]; in EMs – 0.11 [0.09; 0.15]; in UMs – 0.11 [0.06; 0.15]. The maximum 6β-hydroxycortisol/cortisol ratio dynamics was found in IMs (0.30 [0.26; 0.35]).
Analysis of the 6β-hydroxycortisol/cortisol ratio dynamics in ∆% showed that maximum decrease was found in IMs (27.10 [24.26; 29.34]), which exceeded the decrease among all the examined patients (15.60 [12.49; 18.17], p=0.04), as well as in EMs (10.20 [6.00; 14.65], p=0.04) and UMs (10.09 [8.62; 13.85], p=0.03).
6-β-Hydroxycortisol/Cortisol Ratio (6 Beta-OHC/C) In Patients With CYP2C19 Genotypes During Omeprazole And Amlodipine Co-Administration
Analysis of the omeprazole and amlodipine co-administration effect on CYP3A4 activity in patients with grade I-II hypertension and different CYP2C19 metabolic status showed that CYP3A4 activity decreased in all the analyzed groups.
A higher decrease in the 6β-hydroxycortisol/cortisol ratio was found in IMs compared to EMs and UMs and the mean value among all the examined patients (Figure 2).
Antihypertensive Effect Of Amlodipine In Patients With CYP2C19 Polymorphisms Taking Omeprazole
OBPM results in patients with different metabolic status taking the combination of amlodipine and omeprazole showed that the dynamics (delta of baseline and endpoint of BP) of the average SBP was 14.90 [13.54; 15.73] mmHg for all the examined patients (Table 3).
 |
Table 3 The Dynamics Of OBPM Results In Patients Taking A Combination Of Amlodipine And Omeprazole |
IMs showed maximum dynamics (19.90 [18.15; 20.80] mmHg), which was significantly higher than dynamics in the group of UMs (12.50 [9.99; 14.29] mmHg, p = 0.03), EMs (13.20 [12.39; 14.81] mmHg, p = 0.04) and among all the examined patients (14.90 [13.54; 15.73] mmHg, p = 0.04).
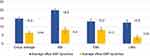
The dynamics of the average DBP was 6.44 [5.57; 7.24] mmHg for all the examined patients. The dynamics of the average DBP in IMs was 8.30 [6.50; 9.33] mmHg, which was almost comparable to the one in EMs (8.11 [7.41; 9.55] mmHg, p = 0.06), and was significantly higher than the average DBP in UMs (3.93 [2.27; 4.69] mmHg, p = 0.04) and among all the patients (6.44 [5.57; 7.24] mmHg, p = 0.04) (Figure 3).
The analysis of office heart rate measurement results did not demonstrate significant changes during the therapy.
The analysis of the OBPM results revealed that IMs showed a significantly more pronounced antihypertensive effect compared to the one in EMs, UMs and the average value for all the patients on the following parameters: the average SBP (p = 0.04), the dynamics of the average SBP (p = 0.04; p = 0.03, p = 0.04, respectively). IMs also showed a significantly more pronounced antihypertensive effect on DBP compared to the one in UMs and the average value for all the patients (p = 0.03, p = 0.04, respectively).
ABPM results in patients taking a combination of amlodipine and omeprazole with different metabolic status showed that dynamics of the average 24 hr SBP in IMs was 14.70 [12.29; 15.84] mmHg, which was comparable to the dynamics in EMs (11.40 [10.97; 13.58] mmHg, p = 0.05) and exceeded the dynamics in UMs (9.20 [6.60; 9.46] mmHg, p = 0.05) and in the average group of patients (11.80 [9.61; 14.64] mmHg, p = 0.05) (Table 4).
 |
Table 4 The Dynamics Of ABPM Results In Patients Taking A Combination Of Amlodipine And Omeprazole |
The results of ABPM showed that dynamics of the average 24 hr DBP in IMs was 11.72 [11.39; 12.88] mmHg, which exceeded the dynamics in EMs (5.80 [3.01; 8.29] mmHg, р=0.03), UMs (5.90 [4.99; 7.42] mmHg, р=0.03) and in the average group of patients (8.30 [6.43; 10.28] mmHg, р=0.04).
The dynamics of average daytime SBP in IMs was higher compared to other parameters (15.30 [14.26; 18.32] mmHg) and significantly exceeded one in EMs (8.20 [7.80; 9.82] mmHg, p = 0.04), in UMs (8.60 [7.64; 9.62] mmHg, p = 0.04) and in the average group of patients examined (10.70 [9.69; 11.92] mm Hg, p = 0.04).
The dynamics of the average nighttime SBP in IMs (9.9 [8.26; 11.34] mmHg) also significantly exceeded the dynamics in EMs (6.84 [5.19; 7.32] mmHg, p = 0.04), in UMs (3.1 [2.30; 4.48] mmHg, p = 0.04) and in the average group of patients (6.61 [4.96; 7.98] mmHg, p = 0.04).
The dynamics of the average daytime DBP in IMs (13.80 [12.39; 15.08] mmHg) significantly exceeded the dynamics in EMs (8.94 [8.27; 10.22] mmHg, p = 0.04) and UMs (8.40 [7.41; 9.54] mmHg, p = 0.04).
The dynamics of the average nighttime DBP in IMs (11.20 [9.74; 13.38] mmHg) significantly exceeded one in EMs (8.90 [7.60; 10.83] mmHg), in UMs (6.80 [5.99; 7.42] mmHg, р=0.04) and in the average group of patients examined (9.20 [7.65; 11.21] mmHg) (Figure 4).
Heart rate dynamics analysis showed a slightly more pronounced increase in IMs (3.30 [2.83; 3.76] bpm) compared to the group of UMs (1.20 [1.14; 1.53] bpm).
Antihypertensive effect was significantly more pronounced in IMs compared to UMs by the average nocturnal DBP (p = 0.04) and the dynamics of nocturnal DBP (p = 0.04).
Discussion
CYP2C19 and CYP3A are the main enzymes involved in omeprazole metabolism, while CYP3A is the principal enzyme family for amlodipine biotransformation. Concomitant use of these drugs in patients with hypertension and ARD might lead to variability of response to one of the drugs, particularly, in patients with CYP2C19 reduced activity.
Studies showed that such drug–drug interaction might occur in drugs, substrates of CYP2C19 and CYP3A enzymes. Concomitant use of clopidogrel, which converts to its active metabolite via CYP2C19 and also CYP3A, and amlodipine, might lead to poor response to clopidogrel.15 Dihydropyridine calcium-channel blockers were showed to inhibit the CYP3A4 enzyme, which led to reduced antiplatelet activity of clopidogrel.16 These findings were translated into conclusions to avoid combination use of clopidogrel and amlodipine.17
We included 51 patients (17 men, 34 women; mean age 66.6±9.8 years, age range 37–88 years) with long-term 10 mg of amlodipine intake for treating hypertension together with the indications for 20 mg omeprazole assignment for treating ARD. The distribution of CYP2C19 phenotypes based on genotyping among patients was as follows: EMs (CYP2C19*1/*1) - 41.1%; IMs (CYP2C19*1/*2, CYP2C19*1/*3, CYP2C19*2/*17, CYP2C19*3/*17) - 23.5%; UMs (CYP2C19 *1/*17, CYP2C19*17/*17) - 35.3%. The frequency of CYP2C19*2, CYP2C19*3, CYP2C19*17 alleles in our study reflects the data described for Europeans.18
The results of OBPM showed that antihypertensive effect was significantly more pronounced in patients with hypertension and ARD in case of a phenotype of IMs compared to the ones with a phenotype of EMs or UMs and the average group value in the following parameters: the average office systolic BP (p = 0.04), the dynamics of the average office systolic BP (p = 0.04; p = 0.03, p = 0.04, respectively). According to the dynamics of diastolic BP, the antihypertensive effect was also significantly higher in IMs than in UMs and the average group value (p = 0.03 and p = 0.04, respectively).
Furthermore, the results of ABPM revealed that there was a significantly more pronounced antihypertensive effect in IMs compared to all other analyzed groups according to the dynamics of both daytime systolic and 24 hr diastolic BP. The average daytime diastolic BP and its dynamics, the average 24 hr systolic BP and its dynamics were higher in IMs compared to EMs and UMs.
So we found that in IMs antihypertensive effect of long-term amlodipine intake was higher than in EMs or UMs when adding omeprazole in patients with hypertension and ARD. This could be explained using the following hypothesis: in CYP2C19 IMs omeprazole biotransformation converts to CYP3A to a higher extent, omeprazole inhibits CYP3A, which leads to amlodipine cumulation and stronger antihypertensive effect compared to CYP2C19 EMs and UMs. This hypothesis needs to be verified in large clinical studies.
Analysis of the 6β-hydroxycortisol/cortisol ratio dynamics in patients with grade 1–2 of hypertension showed that there was a decrease in CYP3A4 activity during the combined omeprazole and amlodipine pharmacotherapy in all the analyzed groups. The most pronounced decrease was found in the group of CYP2С19 IMs, which exceeded the group average decrease (p=0.04), the decrease in EMs (p=0.04) and UMs (p=0.03).
This finding also supports our hypothesis about CYP3A omeprazole bypass activation in IMs and possible drug–drug interaction and increase of antihypertensive effect of amlodipine.
Thus, in patients with hypertension and ARD, who need adding omeprazole to long-term amlodipine therapy, genetic testing for CYP2C19 polymorphisms may be useful before drug administration. In order to increase safety of combined pharmacotherapy in patients receiving omeprazole, preference should be given to antihypertensive drugs which are not metabolized by CYP3A4 and do not influence its activity. In outpatient practice for patients with hypertension and ARD taking omeprazole, amlodipine pharmacotherapy should be started with a dose of 5 mg in the absence of the results of pharmacogenetic testing.
Conclusion
Adding omeprazole to long-term amlodipine therapy in patients with hypertension and ARD may lead to a significantly more pronounced antihypertensive effect in patients genotyped CYP2C19 IMs.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McKeever RG, Hamilton RJ. Calcium channel blockers. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2019.
2. Bhatnagar V, Garcia EP, O’Connor DT, et al. CYP3A4 and CYP3A5 polymorphisms and blood pressure response to amlodipine among African-American men and women with early hypertensive renal disease. Am J Nephrol. 2010;31(2):95–103. doi:10.1159/000258688
3. Kim K-A, Park P-W, Lee O-J, et al. Effect of CYP3A5*3 genotype on the pharmacokinetics and pharmacodynamics of amlodipine in healthy Korean subjects. Clin Pharmacol Ther. 2006;80(6):646–656. doi:10.1016/j.clpt.2006.09.009
4. Zhu Y, Wang F, Li Q, et al. Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab Dispos. 2014;42(2):245–249. doi:10.1124/dmd.113.055400
5. Golan DE, Armstrong AW, Armstrong EJ. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Philadelphia: Wolters Kluwer; 2017.
6. Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27–37. doi:10.5009/gnl15502
7. Dean L. Omeprazole Therapy and CYP2C19 Genotype. In: Pratt V, McLeod H, Rubinstein W, Dean L, Kattman B, Malheiro A, editors. Medical Genetics Summaries. Bethesda (MD): National Center for Biotechnology Information (US); 2012.
8. Shirasaka Y, Sager JE, Lutz JD, Davis C, Isoherranen N. Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions. Drug Metab Dispos. 2013;41(7):1414–1424. doi:10.1124/dmd.113.051722
9. Li X-Q, Weidolf L, Simonsson R, Andersson TB. Enantiomer/enantiomer interactions between the S- and R- isomers of omeprazole in human cytochrome P450 enzymes: major role of CYP2C19 and CYP3A4. J Pharmacol Exp Ther. 2005;315(2):777–787. doi:10.1124/jpet.105.090928
10. Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–827. doi:10.1124/dmd.32.8.821
11. Zvyaga T, Chang SY, Chen C, et al. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metab Dispos. 2012;40(40):1698–1711. doi:10.1124/dmd.112.045575
12. Caraco Y, Wilkinson GR, Wood AJ. Differences between white subjects and Chinese subjects in the in vivo inhibition of cytochrome P450s 2C19, 2D6, and 3A by omeprazole. Clin Pharmacol Ther. 1996;60(4):396–404. doi:10.1016/S0009-9236(96)90196-4
13. Gardiner SJ, Begg EJ. Pharmacogenetic testing for drug metabolizing enzymes: is it happening in practice? Pharmacogenet Genomics. 2005;15(5):365–369. doi:10.1097/01213011-200505000-00013
14. Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte–an update of guidelines. Clin Pharmacol Ther. 2011;89(5):662–673. doi:10.1038/clpt.2011.34
15. Harmsze AM, Robijns K, van Werkum JW, et al. The use of amlodipine, but not of P-glycoprotein inhibiting calcium channel blockers is associated with clopidogrel poor-response. Thromb Haemost. 2010;103(5):920–925. doi:10.1160/TH09-08-0516
16. Seo K-D, Kim YD, Yoon Y-W, Kim J-Y, Lee K-Y. Antiplatelet effect of clopidogrel can be reduced by calcium-channel blockers. Yonsei Med J. 2014;55(3):683–688. doi:10.3349/ymj.2014.55.3.683
17. Wang Z-Y, Chen M, Zhu L-L, et al. Pharmacokinetic drug interactions with clopidogrel: updated review and risk management in combination therapy. Ther Clin Risk Manag. 2015;11:449–467. doi:10.2147/TCRM.S80437
18. Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317–323. doi:10.1038/clpt.2013.105
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.