Back to Journals » Drug Design, Development and Therapy » Volume 15
Antifungal Efficacy and Safety of Cycloheximide as a Supplement in Optisol-GS
Authors Dal Pizzol M, Freitas EC, Locatelli C, Guareze F, Reginatto P, Machado G, Fuentefria A, Marinho D
Received 22 January 2021
Accepted for publication 5 March 2021
Published 18 May 2021 Volume 2021:15 Pages 2091—2098
DOI https://doi.org/10.2147/DDDT.S298059
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Melissa Dal Pizzol,1,2 Eduarda Correa Freitas,2 Claudete Locatelli,2 Felipe Guareze,2 Paula Reginatto,3 Gabriella Machado,3 Alexandre Fuentefria,3 Diane Marinho1,2
1Programa de Pós-Graduação em Medicina: Ciências Cirúrgicas, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; 2Ophthalmology Department, Hospital De Clínicas De Porto Alegre, Porto Alegre, Brazil; 3Programa de Pós-Graduação em Ciências Farmacêuticas, Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
Correspondence: Melissa Dal Pizzol
Ophthalmology Department, Hospital de Clínicas de Porto Alegre, Rua Ramiro Barcelos, 2350, Zona 17, Porto Alegre, CEP 90035-903, RS, Brazil
Tel +55 5133598306
Email [email protected]
Purpose: The incidence of fungal infection after corneal transplant has increased significantly in recent years, especially Candida spp. This study aimed to evaluate the efficacy and safety of the addition of cycloheximide in Optisol-GS media in decreasing the growth of Candida spp. strains.
Methods: This in vitro laboratory efficacy study measured fungal colony growth in 24 vials of Optisol-GS that were divided into 6 groups of 4 vials each, as follows: (1) MIC/2 cycloheximide, (2) MIC cycloheximide, (3) MICx5 cycloheximide, (4) MICx10 cycloheximide, from MIC values obtained for each strain, (5) unsupplemented optisol-GS as a positive control (added inoculum), and (6) unsupplemented optisol-GS as a negative control (no inoculum). In each group was added Candida albicans, C. glabrata and C. parapsilosis, except in the negative control. The evaluated variables were fungal colony growth from the Optisol-GS vials, corneal endothelial cell density and endothelial cell viability at different concentrations of cycloheximide.
Results: In the efficacy study, all strains showed a reduction in fungal cell growth from the second day at all evaluated concentrations of optisol-GS supplemented with cycloheximide, even at subinhibitory concentrations (MIC/2). For C. glabrata, the colony count was reduced to 99%. No evidence of corneal endothelial toxicity was found at any concentration, in the safety study, compared with the paired control.
Conclusion: The addition of cycloheximide to optisol-GS decreased the fungal growth, demonstrating fungicide action against C. glabrata and fungistatic action against C. albicans and C.parapsilosis. This drug did not demonstrate toxicity to the corneal endothelium at different concentrations.
Keywords: cornea, eye banking, infection, pharmacology
Introduction
Although fungal infection after corneal transplantation is a rare complication, the high visual impact and morbidity of these infections have led to the study of the possibility of supplementation of corneal preservation media with antifungal drugs.1,2 At present, none of the commercially hypothermic preservation media contain any antifungal action agent and the colorimetric indicators of microbial contamination do not consistently detect fungal contamination.3 Organ culture at 34°C is the most frequent method used for corneal storage in Europe, while in North America, hypothermic storage at 4°C is the preference of most eye banks. A survey involving 1.6 European eye banks, using a database of 16,862 corneas distributed for endothelial keratoplasty over 5 years from 2012, compared the incidences of fungal infection. As a result, the study demonstrated an infection rate of 0.5% in the cases in which the corneas were preserved in hypothermic media, compared to an incidence of 0.02% when the corneas were preserved in organ culture media (p < 0.0001).4 This difference is likely because the organ culture media has been added into the formula 0.25 μg/mL amphotericin B, which is different from the hypothermic media, which has no antifungal additive.4
Approximately all cases of fungal infection after corneal transplants were caused by Candida species, in particular Candida albicans, and C. glabrata. In addition, there was an increase in this microorganism after endothelial keratoplasty.1–7 In 2010, the Eye Bank Association of America (EBAA) created a subcommittee to investigate the benefit of fungal supplementation in Optisol-GS storage media in the United States of America (USA).3 This subcommittee concluded an increasing trend in the incidence of post keratoplasty fungal infection. However, supplementation with antifungals is not recommended because of the lack of evidence proving the efficacy, safety, and cost-benefit of supplementation. Some studies evaluated the efficacy, safety, and light stability of amphotericin B and voriconazole addition in hypothermic storage at many different concentrations and found endothelial toxicity at a high concentration of amphotericin B and lack of effect at a low concentration of voriconazole.5,6
Cycloheximide is an antibiotic with significant antifungal properties. It is produced by some streptomycin-producing strains of Streptomyces griseus and acts by inhibiting protein synthesis.7,8 Fungal resistance selection is an important trend and makes therapy difficult. There is already evidence of resistance to voriconazole and other azole derivatives in the treatment of fungal ulcers.9,10 Cycloheximide is efficient for mutagenic strains resistant to multiple drugs,11 and also has the advantage of having a low cost. In addition to presenting a broad spectrum of antimicrobial action, cycloheximide is widely used as a preservative agent in culture media to inhibit fungi and bacteria. Thus, this drug can be a good antifungal agent in the Optisol medium for corneal preservation.12–14 There are still no studies evaluating its efficacy and safety in the treatment of fungal ulcers or its supplementation in hypothermic preservation media. This study aimed to evaluate the efficacy and safety of the addition of cycloheximide in Optisol-GS media in decreasing the growth of the main Candida spp. strains.
Materials and Methods
Fungal Strains
In this study, three Candida spp. strains of clinical relevance were tested: C. albicans (CA MS2), C. glabrata (CG 05) and C. parapsilosis (ATCC 22019). The strains were analyzed phenotypically by Vitek Yeast Biochemical Card (BioMerieux Vitek – Hazelwood, Missouri, USA). All strains were obtained from the mycology collection of the Laboratory of Applied Mycology of the Federal University of Rio Grande do Sul (Porto Alegre, Brazil). The standard strain (ATCC 22019) was obtained from ATCC (American Type Culture Collection, Manassas, VA, USA) and used as a control.
Antifungal Susceptibility Testing
The minimum inhibitory concentration (MIC) of the fungal strains selected for this study was determined by the broth microdilution method, according to the M27-A3 protocol.15,16 The solution containing cycloheximide was prepared in RPMI 1640 media (Roswell Park Memorial Institute 1640; Sigma-Aldrich, St. Louis, Missouri, USA) pH 7.0 buffered with MOPS (morpholinopropanesulfonic acid; Dynamic, Diadema, São Paulo, Brazil), at twice the concentration to be tested, ranging from 250 to 0.97 μg/mL. The fungal inoculum was prepared in a 0.85% sterile saline solution (Dynamic; Diadema, São Paulo, Brazil), containing cell culture with 24 h of growth on Sabouraud Dextrose Agar (SDA – HiMedia, India) at 35°C. The fungal suspension was adjusted to 0.5 McFarland scale, approximately 1.0–5.0×106 CFU/mL, in a spectrophotometer (GT220, Global Trade Technology) at 530 nm. Then, two subsequent dilutions, at 1:50 and 1:20 were performed to obtain a cell concentration of approximately 1.0–5.0×103 CFU/mL.
The assay was performed on 96-well sterile polystyrene microplates. After the microdilution of the cycloheximide, 100 µL of each Candida inoculum suspension were added to the microplates, obtaining the same inoculum concentration (0.5–2.5×103 CFU/mL) in all wells of the microplate. This ensured the same microorganism dose in all cycloheximide concentrations evaluated. Thus, the microplates were incubated at 35°C for 48 h. The MIC values were determined as the lowest concentrations capable of inhibiting 100% of fungal visual growth. The same procedures were performed to test the corneal preservation media, Optisol-GS (Bausch and Lomb, USA), this was used to replace the RPMI 1640 media.
Efficacy Study
For each Candida spp. strain tested, twenty-four sterile vials of Optisol-GS were used. The vials were divided into 6 groups, as follows: (1) MIC/2 cycloheximide (2) MIC cycloheximide, (3) MICx5 cycloheximide, (4) MICx10 cycloheximide, from MIC values obtained for each strain, (5) unsupplemented Optisol-GS as a positive control (added inoculum) and (6) unsupplemented Optisol-GS as a negative control (no inoculum). All the groups were evaluated in quadruplicate (using 4 vials per group). In each group was added a Candida spp. strain, except in the negative control. In this study, C. albicans, C. glabrata, and C. parapsilosis were tested. The strains were grown on SDA for 24 h at 35°C. Colonies were harvested from the plates and suspended in sterile water to obtain turbidity equivalent to 0.5 McFarland standards (1–5×106 CFU/mL). Then, the fungal suspensions were diluted to approximately 2.5×103 CFU/mL for groups 1 to 5. All vials of Optisol-GS were refrigerated at 2 to 8°C according to manufacturer recommendations. After 2, 7 and 14 days of incubation, 1 mL of solution was removed, and 10, and 100 µL aliquots were diluted 1:10 with sterile water to minimize any cycloheximide carryover. Then, the samples were immediately taken from 1mL of solution and cultured in SDA. Then, all plates were incubated at 35°C for 36 h for subsequent counting of viable fungal colonies on the plates. The methodology followed Layer et al17 with modifications.
Two-way ANOVA followed by Dunnett’s test was performed to assess the effectiveness of cell reduction of Candida cells when different concentrations of cycloheximide was added to Optisol and compared to the control. P < 0.05 was considered statistically significant.
Safety Study
Five pairs of donor corneas not suitable for transplantation due to positive serology were obtained from Hospital de Clínicas de Porto Alegre Corneal Eye Bank. All donor corneas were donated voluntarily, with written informed consent, and the study methodology followed the guidelines of the Helsinki and Istanbul declaration. Ethical committee approval and institutional research board clearance were obtained from Hospital de Clínicas de Porto Alegre. Two pairs of corneas were used to test cycloheximide at MIC and 3 pairs to test cycloheximide concentration at MICx10. Randomly, by sortation, the Optisol-GS flask with the raffled cornea was supplemented with cycloheximide, and the other cornea from the pair was maintained without cycloheximide as a control. The endothelial cell density (ECD) of all corneas was evaluated via specular microscopy (Konan KSS-EB10) on day 0 and after 3 and 14 days of cycloheximide supplementation. Paired t-test was used to compare the mean change in ECD between controls and supplemented Optisol-GS from 0, 3, and 14 days. In cases when ECD could not be performed due to corneal oedema or inability to identify the cells borders, a value of 500 cells/mm2 was used to perform the statistical analyses, assuming to be secondary to severe endothelial damage.
All donor corneal endothelium was evaluated with two different vital dyes staining at day 14. The first was 0.4% trypan blue (Sigma-Aldrich Corp), a vital dye that stains severely damaged and dead endothelial cells, and the second was 0.5% alizarin red (GFS Chemical, Inc), which stains cell borders and denudes Descemet membrane. The staining protocol used was based on the protocol published by Park et al,18 that consisted of placing each donor cornea in a culture dish with the endothelial facing up. The endothelium was covered with 0.4% trypan blue for 60 seconds. Trypan blue was then gently poured off, and the cornea was rinsed with phosphate-buffered saline (PBS). The cornea was then immersed in 0.5% alizarin red for 90 seconds, followed by rinsing with PBS. An 8 mm trephine was used to cut the central cornea and then transferred to microscope slides with the endothelium facing up. The endothelium was examined under an Olympus BX51 light microscope (Olympus, Germany) at 200x magnification, attached to an Olympus digital camera. Five different photographs within the central and paracentral cornea were taken for each cornea. Two blinded examiners counted, in a given area in each photograph, viable and nonviable cells (stained with trypan blue). The mean percentage of nonviable cells (the number of trypan blue cells divided by the total number of cells) was calculated for each cornea and each examiner (Figure 1). The intraclass correlation coefficient was used to assess whether there was a correlation of means between the two examiners. Paired t-test was used to compare the average of the 2 examiners of percentage of nonviable cells between controls and supplemented Optisol-GS.
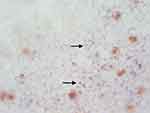 |
Figure 1 Corneal endothelium vital dyes stained with 0.4% trypan blue and 0.5% alizarin red (arrows indicate positive trypan blue). |
Results
All evaluated Candida species were sensitive to cycloheximide in the RPMI media and to Optisol-GS supplemented with this antimicrobial at zero time (t=0), demonstrating MIC values between 1.95 and 62.5 µg/mL for both media. After 14 days (t=14) of incubation in Optisol-GS supplemented with cycloheximide there was an increase in MIC values of this drug against all strains tested (MIC = 3.90–125 µg/mL). In addition, after 14 days of incubation, supplementation with cycloheximide in both RPMI and Optisol-GS media was more effective against C. glabrata and C. parapsilosis strains (MIC = 3.90 µg/mL) (Table 1).
 |
Table 1 Determination of MIC Values (µg/mL) for Cycloheximide in RPMI Media and in Optisol-GS at Time Zero (t=0) and After 14 Days of Incubation (t=14) |
Efficacy Study
The three Candida spp. strains showed a reduction in fungal cell growth from the second day of incubation in Optisol-GS supplemented with cycloheximide, at all evaluated concentrations, even at subinhibitory concentrations (MIC/2) (Figure 2). For the C. glabrata (Figure 2B), there was an expressive reduction in the colony counts from the 2nd day. This reduction became more pronounced until the 14th day, mainly at MICx5 and MICx10 concentrations, in which the colony count was reduced to 99%. For C. albicans (Figure 2A), there was a reduction in fungal growth after the 2nd day, which remained constant until the 14th day at all concentrations evaluated. C. parapsilosis (Figure 2C) showed an expressive reduction in growth from the 7th day, remaining constant until the 14th day, with a reduction viable cells ranging between 77 and 92%.
For all strains there was a significant reduction in the number of viable cells at all cycloheximide concentrations evaluated when compared to the untreated control (P <0.05), demonstrating the anti-Candida efficacy of this drug.
Safety Study
From day 0 to day 3 of supplementation and day 14 at both concentrations (MIC and MICx10), there was a reduction in ECD. However, there was no difference in ECD reduction between supplemented Optisol-GS with cycloheximide and paired controls, as shown in Table 2. The evaluation with vital dye staining demonstrated similar percentage of nonviable endothelial cells between their paired controls and antifungal supplemented Optisol-GS at both concentrations (Table 2).
 |
Table 2 Changes in Safety Study Variables |
Discussion
Post-keratoplasty fungal infections have increased significantly in recent years.3,19–21 These increase in cases are probably associated with endothelial keratoplasty (EK) and Candida spp have been the most common isolates, especially C. albicans and C. glabrata.2,20 The other reason for this increase can be the corneal tissue processing, especially for EK, which requires removal of the tissue from the storage medium and increasing the tissue temperature. In addition, some surveys associated a greater fungal infection risk after EK when the tissue was prepared in the eye bank compared with tissue prepared in the operating room2–4 or with surgeons prepared tissue.1
Hypothermic media are the most commonly used media in the USA and Latin America. In these media, the prevalence of cases of fungal infection was higher than bacterial infection, the mean incidence fungal rim culture is approximately 1.5%, with Candida spp being the most common contaminant. Fungal infection was more likely when grafts were prepared from corneas that had been stored in hypothermic media (0.5%) compared with organ culture (0.02%) (p < 0.0001) as shown by Lau et al.4 Other papers report lower rates of donor rim contamination and postoperative infection when organ culture media were used.17,22,23 These differences are possibly attributed to the absence of antifungals in the hypothermic media.
Ritterband et al published the first study about the addition of voriconazole (100 μg/mL) to Optisol-GS media. The rate of positive cultures in the Optisol-GS media without the supplementation of voriconazole was 12.3% (66/533), of which 7 (10%) were fungi C. glabrata and C. albicans were the most prevalent strains. In the rims with voriconazole addition, the positivity culture rate was 11.1% (59/533), but there was no fungal growth, this difference was statistically significant (p = 0.015). Endothelial toxicity was evaluated with vital dyes of trypan blue and red alizarin. The percentage of non-viable cells was 0.17% in the group with the addition of voriconazole and 0.22% without the addition, which was not significantly different (p < 0.05). In the stability test, the effectiveness of voriconazole supplementation in eliminating fungal growth was between 6 and 7 days.6 Interestingly, our study found a time-kill plot earlier than voriconazole, starting within the second day, especially, in C. glabrata and C. parapsilosis. For C. albicans, these findings occurred slightly later, around the seventh day.
Other authors have evaluated the supplementation of amphotericin B and voriconazole at different concentrations in Optisol-GS.17 The efficacy of both supplementations was evaluated in 20 vials of Optisol-GS and divided into groups, as follows: (1) MIC voriconazole (1 μg/mL), (2) MIC x 10 voriconazole (10 μg/mL), (3) MIC x 25 voriconazole (25 μg/mL), (4) MIC x 50 voriconazole (50 μg/mL), (5) MIC x 0.25 amphotericin B (0.0625 μg/mL), (6) MIC x 0.5 amphotericin B (0.125 μg/mL), (7) MIC amphotericin B (4 μg/mL), (8) MIC x10 amphotericin B (40 μg/mL), (9) Optisol-GS media inoculated (positive control) and (10) only Optisol-GS media (negative control). The study tested two Candida spp. strains (C. albicans and C. glabrata). Due to the known toxicity of amphotericin B, concentrations below the MIC were chosen. Voriconazole, on the other hand, was chosen over the MIC due to studies that reported the absence of toxicity, including the paper of Ritterband et al6 which added MICx100 to Optisol-GS. In the group that added C. albicans to voriconazole there was a similar growth of colony-forming units in all concentrations of voriconazole and in the positive control group and in the group that added C. glabrata there was a reduction of only 70% of the colony-forming units, with no relation to the concentration. In the amphotericin B group, there was no fungal growth of C. albicans at any concentration tested. For C. glabrata, fungal growth occurred only at the lowest concentrations (MICx0.25 and MICx0.5), but colony-forming units were reduced by 99% and 96%, respectively. Safety was achieved through the analysis of the endothelium by specular microscopy and through the vital dyes of trypan blue and red alizarin. Compared to the control, there was only a significant decrease in endothelial cell count against amphotericin B MICx10 (p = 0.04) and a trend at MIC (p = 0.07). On the other hand, in the other concentrations and in the voriconazole group there was no significant difference. The percentage of nonviable cells was also significant only at MICx10 in amphotericin B (78% amphotericin B x 10% control p = 0.02). This study corroborates with a study that evaluated the effectiveness of amphotericin B at 0.25 μg/mL, which is widely used in European eye banks to supplement the organ culture medium.24,25 On the other hand, this study is contradictory to Ritterband et al,6 which demonstrated a decrease in the incidence of positive cultures of corneal rims with supplementation with voriconazole. However, these authors used concentrations two times higher (100 μg/mL) that could justify this difference.
In our study, supplementation of Optisol-GS media was performed with cycloheximide, an antimicrobial with a known antifungal action, which is inexpensive and works quickly. In the efficacy study, a reduction in fungal cell growth from the 2nd day of incubation in Optisol-GS media, in all evaluated concentrations, even in sub-inhibitory concentrations (MIC/2), was observed. For C. glabrata, there was an expressive reduction of 99% in the colony count, on the 14th day at MICx5 and MICx10 concentrations. This indicates that the cycloheximide has a fungicidal action (defined as a reduction >99% in CFU/mL) dependent on the drug concentration and time. For C. albicans and C. parapsilosis, the cycloheximide presents only a fungistatic action over the time elapsed, since a reduction of less than 99% in CFU/mL was observed. In low concentrations, such as MIC, the cycloheximide has a fungistatic action against all Candida species that can explain the increase in the MIC values of this drug after 14 days of exposure to yeasts (Table 1).
Our results demonstrate the excellent antifungal activity of cycloheximide against the main strains involved in fungal infections after corneal transplantation, even at subinhibitory concentrations (MIC/2). Moreover, our findings suggest that the Optisol-GS supplemented with cycloheximide may have a better performance than Optisol-GS supplemented with voriconazole or amphotericin B, particularly for C. glabrata. Furthermore, its antifungal effect seems to be present even at lower concentrations, overcoming the voriconazole results,6,17 and the absence of toxicity even at higher concentrations, surpassing the amphotericin B findings.5,17,25
The small sample size of our toxicity evaluation was one major limitation of our study and new studies must be carried out to strengthen our findings. However, our results suggest a possible benefit in adding cycloheximide to Optisol-GS media, considering the high morbidity of a fungal infection after corneal transplantation. Another limitation of the study is the non-testing of the different dosages of cycloheximide using yeast inoculated into the corneoscleral tissue rather than yeast alone in storage media to better simulate the realistic scenario. The Candida species seems to create a biofilm in mammalian cells, becoming more resistant to azoles drugs and more virulent, explaining higher positive yeast cultures in the Optisol-GS with the corneoscleral tissue than the Optisol-GS alone.25
In future perspectives, we suggest studies that assess the synergism of two drugs with antifungal action to decrease the concentration and toxicity of one antifungal alone as already suggested by Kowalski et al.26
Conclusion
Cycloheximide in Optisol-GS decreased significantly the yeast fungal concentration from the second day of preservation. This antifungal agent also demonstrated fungicide action against C. glabrata and fungistatic action against C. albicans and C. parapsilosis. The addition of cycloheximide at different concentrations in Optisol-GS did not demonstrate toxicity to the corneal endothelium even at high concentrations. Due to its fast action and low cost, cycloheximide can be considered a possible additive to the hypothermic corneal preservation medium.
Acknowledgments
The authors thank the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS – EDITAL 04/2016 – PRONUPEQ 2016) for financial support and research fellowships. This study was financed in part by the Associação Fundo de Incentivo à Pesquisa e Eventos (AFIP) – Finance Code 001, and was approved by science ethics committee of Hospital de Clínicas de Porto Alegre. We would like to inform that the manuscript is submitted on behalf of all authors and that it is an original research not previously published and that it is not being considered for publication elsewhere.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declared they have no conflicts of interest for this work.
References
1. Mian SI, Aldave AJ, Tu EY, et al. Incidence and outcomes of positive donor rim cultures and infections in the cornea preservation time study. Cornea. 2018;37(9):1102–1109. doi:10.1097/ICO.0000000000001654
2. Brothers KM, Shanks RMQ, Hurlbert S, Kowalski RP, Tu EY. Association between fungal contamination and eye bank–prepared endothelial keratoplasty tissue - temperature-dependent risk factors and antifungal supplementation of optisol–gentamicin and streptomycin. JAMA Ophthalmol. 2017;135(11):1184–1190. doi:10.1001/jamaophthalmol.2017.3797
3. Aldave AJ, Dematteo J, Glasser DB, et al. Report of the eye bank association of America medical advisory board subcommittee on fungal infection after corneal transplantation. Cornea. 2013;32(2):149–154. doi:10.1097/ICO.0b013e31825e83bf
4. Lau N, Hajjar Sesé A, Augustin VA, et al. Fungal infection after endothelial keratoplasty: association with hypothermic corneal storage. Br J Ophthalmol. 2018;103:1–4. doi:10.1136/bjophthalmol-2018-313295
5. Duncan K, Parker J, Hoover C, Jeng BH. The effect of light exposure on the efficacy and safety of amphotericin B in corneal storage media. JAMA Ophthalmol. 2016;134(4):432–436. doi:10.1001/jamaophthalmol.2016.0008
6. Ritterband DC, Shah MK, Meskin SW, et al. Efficacy and safety of voriconazole as an additive in Optisol GS: a preservation medium for corneal donor tissue. Cornea. 2007;26(3):343–347. doi:10.1097/ICO.0b013e31802d82e8
7. Kominek LA. Cycloheximide production by Streptomyces griseus: control mechanisms of cycloheximide biosynthesis. Antimicrob Agents Chemother. 1975;7(6):856–860. doi:10.1128/AAC.7.6.856
8. Dehoux P, Davies J, Cannon M. Natural cycloheximide resistance in yeast: the role of ribosomal protein L41. Eur J Biochem. 1993;213(2):841–848. doi:10.1111/j.1432-1033.1993.tb17827.x
9. Venkatesh Prajna N, Lalitha P, Rajaraman R, et al. Changing azole resistance: a secondary analysis of the MUTT I randomized clinical trial. JAMA Ophthalmol. 2016;134(6):693–696. doi:10.1001/jamaophthalmol.2016.0530
10. Florcruz N, Evans JR. Medical interventions for fungal keratitis (Review) summary of findings for the main comparison. Cochrane Database Syst Rev. 2015;(4):1469–1493. doi:10.1002/14651858.CD004241
11. De Souza CC, Pellizzon CH, Hiraishi M, Goldman MHS, Goldman GH. Isolation and characterisation of cycloheximide-sensitive mutants of Aspergillus nidulans. Curr Genet. 1998;33(1):60–69. doi:10.1007/s002940050309
12. Ali. AA. In vitro antifungal effect of potassium sorbate and sodium benzoate on the growth of fungi causing sinusitis. Afr J Microbiol Res. 2017;11(6):232–236. doi:10.5897/ajmr2016.8414
13. Petruzzi L, Corbo MR, Campaniello D, Speranza B, Sinigaglia M, Bevilacqua A. Antifungal and antibacterial effect of propolis: a comparative hit for food-borne pseudomonas, enterobacteriaceae and fungi. Foods. 2020;9(5):559. doi:10.3390/foods9050559
14. Jin J, Nguyen TTH, Humayun S, et al. Characteristics of sourdough bread fermented with Pediococcus pentosaceus and Saccharomyces cerevisiae and its bio-preservative effect against Aspergillus flavus. Food Chem. 2021;345(November 2020):128787. doi:10.1016/j.foodchem.2020.128787
15. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Third Informational Supplement - M27-S3. Clinical and Laboratory Standards Institute- NCCLS; 2008.
16. Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-; CLSI Document M27-A3. Clinical and Laboratory Standards Institute; 2008.
17. Layer N, Cevallos V, Maxwell AJ, Hoover C, Keenan JD, Jeng BH. Efficacy and safety of antifungal additives in Optisol-GS corneal storage medium. JAMA Ophthalmol. 2014;132(7):832–837. doi:10.1001/jamaophthalmol.2014.397
18. Park S, Fong AG, Cho H, et al. Protocol for vital dye staining of corneal endothelial cells. Cornea. 2012;31(12):1476–1479. doi:10.1097/ICO.0b013e31824d0dda
19. Mathes KJ, Tran KD, Mayko ZM, Stoeger CG, Straiko MD, Terry MA. Reports of post-keratoplasty infections for eye bank-prepared and non–eye bank-prepared corneas. Cornea. 2019;38(3):263–267. doi:10.1097/ico.0000000000001839
20. Edelstein SL, DeMatteo J, Stoeger CG, MacSai MS, Wang CH. Report of the Eye Bank Association of America Medical Review Subcommittee on adverse reactions reported from 2007 to 2014. Cornea. 2016;35(7):917–926A. doi:10.1097/ICO.0000000000000869
21. Borkar DS, Wibbelsman TD, Buch PM, et al. Endophthalmitis rates and clinical outcomes following penetrating and endothelial keratoplasty. Am J Ophthalmol. 2019;205:82–90. doi:10.1016/j.ajo.2019.05.004
22. Fontana L, Errani PG, Zerbinati A, Musacchi Y, Di PB, Tassinari G. Frequency of positive donor rim cultures after penetrating keratoplasty using hypothermic and organ-cultured donor corneas. Cornea. 2007;26(5):552–556. doi:10.1097/ICO.0b013e3180415d7e
23. Elisabeth P, Hilde B, Ilse C. Eye bank issues: II. Preservation techniques: warm versus cold storage. Int Ophthalmol. 2008;28(3):155–163. doi:10.1007/s10792-007-9086-1
24. Ling MLH, Wells M, Petsoglou C, et al. Factors affecting corneal organ culture contamination: a 6-year study at the New South Wales tissue bank. Cornea. 2019;38(7):829–835. doi:10.1097/ICO.0000000000001936
25. Tran D, Dhaliwal D, Kamyar R, Jhanji V, Kowalski RP. Effect of optisol supplementation with 0.255 m g/mL. Cornea. 2019;38(7):901–904. doi:10.1097/ICO.0000000000001969
26. Kowalski RP, Raj CVS, Stuart JC, Dunn DS. Antifungal synergism: a proposed dosage for corneal storage medium. Arch Ophthalmol. 1985;103(2):250–256. doi:10.1001/archopht.1985.01050020102030
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

