Back to Journals » Journal of Experimental Pharmacology » Volume 13
Antidiarrheal Effect of DLBS1Y62, a Bioactive Fraction of Uncaria gambir Roxb. Dried Sap Extract, in Wistar Rats
Authors Wibowo DA, Nailufar F, Tjandrawinata RR
Received 24 December 2020
Accepted for publication 6 May 2021
Published 15 July 2021 Volume 2021:13 Pages 669—675
DOI https://doi.org/10.2147/JEP.S299001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bal Lokeshwar
Dicky A Wibowo,1 Florensia Nailufar,1 Raymond R Tjandrawinata2
1Animal Pharmacology Section, Dexa Laboratories of Biomolecular Sciences (DLBS), PT Dexa Medica, Cikarang, 17550, Indonesia; 2Dexa Laboratories of Biomolecular Sciences (DLBS), PT Dexa Medica, Cikarang, 17550, Indonesia
Correspondence: Raymond R Tjandrawinata
Dexa Laboratories of Biomolecular Sciences (DLBS), PT Dexa Medica, Industrial Estate Jababeka II, Industri Selatan V Block PP No. 7, Cikarang, 17550, Indonesia
Tel +62-21-89841901
Fax +62-21-89841905
Email [email protected]
Background: Diarrhea is a common health problem worldwide, especially in developing countries. It is the second leading cause of mortality for children. Uncaria gambir Roxb. extract has been used to treat diarrhea and dysentery, and as an astringent medicine, in Asian countries. Here, we investigated the antidiarrheal effect of DLBS1Y62, which is the bioactive fraction of dried sap extract from U. gambir, using castor oil-induced diarrhea and castor oil-induced enteropooling in rats.
Methods: DLBS1Y62 was obtained by crushing and milling the dried sap extract of U. gambir leaves. Male Wistar rats, 2– 3 months old, weighing 200– 250 g (n=30), were used for this study. Negative controls received 0.05 mL purified water. Positive controls were treated with 2 mg/kg BW loperamide orally as a suspension. Groups I, II, and III received 6.25, 12.5, and 25 mg/kg BW DLBS1Y62, respectively. Group IV received a combination of 6.25 mg/kg BW DLBS1Y62 and 20 mg/kg BW attapulgite. Diarrheal onset and frequency were observed; then, the weight and volume of intestinal contents were measured.
Results: DLBS1Y62 at all dose levels and in combination with attapulgite could inhibit the formation of further fecal forms of diarrhea, without delaying the onset of diarrhea. The rats that received DLBS1Y62 25 mg/kg BW had the lowest frequency of diarrhea and average intestinal contents compared with the treatment and negative control groups. DLBS1Y62 at a dose of 25 mg/kg BW also gave similar results to 2 mg/kg BW loperamide as a positive control in reducing diarrheal frequency and intestinal content.
Conclusion: The results of this study suggest that DLBS1Y62, particularly at a dose of 25 mg/kg BW, containing tannin as a compound, may become an alternative treatment for diarrhea.
Keywords: Uncaria gambir Roxb., DLBS1Y62, diarrhea, rats
Introduction
Diarrhea is a common illness worldwide and it is the second leading cause of mortality among children under 5 years old.1 WHO and Unicef have stated that there are about two billion cases of diarrheal disease worldwide every year and 1.9 million children die from diarrhea each year, mostly in developing countries.2 In Indonesia, diarrhea is the third leading cause of child death.3 Excessive loss of fluid in diarrhea causes dehydration, which leads to death in a short time, especially in children.4
Diarrhea is the frequent passage of liquid feces (three times in a 24-hour period) and it is characterized by increased gastrointestinal motility and secretion.5 Excessive loss of fluid in diarrhea is associated with an imbalance between the absorptive and secretory mechanisms of water and electrolytes in the intestinal tract, accompanied by hypermotility.6 This imbalance is possibly caused by an overstimulation of bacterial enterotoxins and inflammation, leading to the releases of prostaglandins in the intestinal mucosa.7 Besides bacterial infection, diarrhea can be caused by drugs, psychological factors, allergies,8 and food poisoning.9 More than 700 drugs, including laxatives, have been implicated in causing diarrhea.10 Castor oil is a stimulant laxative agent that could be used for inducing a diarrhea model.11
First line management of diarrhea for children under 5 years old comprises continued feeding, increased fluids, and supplemental zinc for 10–14 days to prevent dehydration. In addition, the WHO guideline recommends that children experiencing non-severe dehydration should receive oral rehydration therapy with oral rehydration solution in a health facility. For bloody diarrhea or severe dehydration in cases of suspected cholera, the guideline suggests antimicrobials.12 A study showed that inappropriate practices in the management of childhood diarrhea were prevalent; these included the curtailment of fluids and breastfeeding, food restriction, and the use of inappropriate medication, which can result in a higher risk of mortality or prolongation of the diarrhea condition.13
Some studies have researched the traditional use of antidiarrheal medicinal plants by investigating the biological activity of extracts of such plants, which contain phytochemicals (such as alkaloids, tannins, flavonoids, and terpenes) that are thought to be responsible for the antidiarrheal activity.14 Uncaria gambir Roxb. (Gambier) is a member of the Rubiaceae family which contains diverse and complex secondary metabolites, and particularly alkaloids and tannins.15 Uncaria gambir extract has been used for the treatment of diarrhea and dysentery, and as an astringent medicine, in Asian countries.16 In this study, we evaluate the antidiarrheal effect of the dried sap extract of U. gambir Roxb., named DLBS1Y62, on castor oil-induced diarrhea and castor oil-induced enteropooling in Wistar rats.
Materials and Methods
Materials
The leaves of U. gambir Roxb. were collected from Padang, West Sumatera, Indonesia. The leaves were boiled with water and pressed to collect the sap. The sap was molded into certain shapes (cylinders or blocks) and dried for about 1 week. The dried sap extract was crushed and milled in Dexa Laboratories of Biomolecular Sciences to obtain DLBS1Y62 (batch no. RL1607010). Other materials were castor oil (lot no. BCBR1629V; Sigma Aldrich, MO, USA), xylazine (batch no. 07063241 PKC; Interchemie Werken, Castenray, Netherlands), ketamine 10% (batch no. 16D131; Kepro BV, Deventer, Netherlands), ethanol 20% (batch no. 14B187; AST Farma BV, Oudewater, Netherlands), loperamide (Imodium®; Indonesia), and attapulgite (New Diatabs®; Indonesia).
Animals
Male Wistar rats, 2–3 months old, weighing 200–250 g (n=30), were used for this study. The animals were housed individually in polypropylene cages under standard conditions (12 hours light/dark cycle, temperature at 22±2°C, and humidity 60±10%) and acclimatized for 7 days prior to the study. Dry food pellets (Laboratory Rodent Diet 5001; minimum 23% protein, minimum 4.5% fat, and maximum 6% fiber) and water were provided ad libitum.
Ethical Approval
All procedures in this experiment have been reviewed and approved by the Institutional Animal Care and Use Committee, Dexa Laboratories of Biomolecular Sciences, with protocol no. DIS-DLBS-PROC-020. The guidelines followed by the committee are the Institutional Animal Care and Use Committee Guidebook, 2nd Edition (ARENA OLAW, 2002); Guide for the Care and Use of Laboratory Animals, 8th Edition (National Research Council (US) Committee, 2011); and Guidelines for the Euthanasia of Animals (AVMA, 2013).
Methods
Castor Oil-Induced Diarrhea
The experiment was carried out according to the methods of Girard et al (2005)17 and Sharma et al (2012).18 Animals were divided into six groups, each consisting of five rats, and were fasted for 4 hours before treatment, with free access to water. Negative controls received 0.05 mL purified water. Positive controls were treated with 2 mg/kg BW loperamide orally as a suspension. Groups I, II, and III received 6.25, 12.5, and 25 mg/kg BW DLBS1Y62, respectively. Group IV received a combination of 6.25 mg/kg BW DLBS1Y62 and 20 mg/kg BW attapulgite. Treatments were administered orally as suspensions.
After 1 hour of treatments, diarrhea was induced by the administration of 1 mL castor oil orally by gavage to each rat. The rats were placed individually in the metabolic cage. The floor of the cage consisted of a grid and all feces expelled from the rat fell through this grid onto a plate. The occurrence and severity of diarrhea were recorded for 3 hours. Onset and frequency of diarrhea were observed, and the fecal form was scored cumulatively according to the method of Hedge et al (1994),19 as follows: normal feces or no feces (score 0), well-shaped wet feces (score 1), shapeless feces (score 2), and unshaped feces with a large amount of liquid (score 3).
Castor Oil-Induced Enteropooling
The experiment was carried out according to the methods of Robert et al (1976)20 and Rahman et al (2013),21 with slight modifications. Thirty rats from the previous experiment were divided into six groups. Grouping of animals followed the previous experimental treatments. Before being used in this experiment, all animals were rested for 2 weeks to eliminate residual drugs, since the average of biological half-life of loperamide (used in the positive control group) is 10.8 hours.22 The rats were fasted for 6 hours before treatments and allowed free access to water. After 1 hour of treatment, the rats in each group received 1 mL of castor oil orally by gavage. One hour later, the rats were euthanized by sodium pentobarbital intracardiac injection (150 mg/kg BW) under ketamine (40–80 mg/kg BW) and xylazine (8 mg/kg BW) anesthesia. The small intestine from the pylorus to the cecum was isolated, then the intestinal contents were weighed and their volume was measured.
Statistical Analysis
The average weight and volume of intestinal contents were expressed as the mean ± standard deviation. Subsequently, the results were analyzed statistically using ANOVA followed by Tukey’s method for post-hoc analysis to determine significant differences between treatment groups and controls. The value of p<0.05 was considered as significant.
Results
Effect of DLBS1Y62 on Castor Oil-Induced Diarrhea
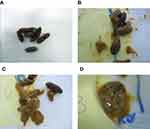
Observation of castor oil-induced diarrhea for 3 hours resulted in four fecal forms, as shown in Figure 1. According to Hedge et al (1994),19 rats were considered protected against diarrhea if they scored 0 or 1 and considered unprotected against diarrhea if they scored 2 or 3. Groups I–IV expressed two forms of feces, which were scored 1 and 2, which only showed a diarrhea condition with a shapeless feces form. A diarrhea condition was also shown by positive controls, with a score of 2. Meanwhile, the negative control group expressed three forms of feces, which were scored 1, 2, and 3, indicating a diarrhea condition with a shapeless feces form and unshaped feces with a large amount of liquid.
The first onset of diarrhea was observed in feces scored 2 or 3, as shown in Table 1. Group I, which received 6.25 mg/kg BW DLBS1Y62, showed the fastest first onset of diarrhea, at minute 61, of the groups with a fecal score of 2. Among the treatment groups that only received DLBS1Y62, Group II had longer first onset of diarrhea, with a fecal score of 2 at minute 65. Group IV showed the longest first onset of diarrhea of all treatment groups. Negative controls had a longer onset than the DLBS1Y62 treatment groups. Positive controls, which received 2 mg/kg BW loperamide, showed the longest onset of diarrhea.
 |
Table 1 First Onset Occurrence of Diarrhea (Minutes) |
Table 2 shows frequency of diarrhea during 3 hours of observation for individual rats in each group. The lowest frequency of diarrhea was shown by positive controls. Negative controls had a higher diarrheal frequency than the treatment groups. Group II had a similar diarrheal frequency to Group IV. Group III, which received 25 mg/kg BW DLBS1Y62, showed the lowest average frequency of diarrhea among the treatment groups.
 |
Table 2 Frequency of Diarrhea |
Effect of DLBS1Y62 on Castor Oil-Induced Enteropooling
The form of intestinal content in this study was a combination of liquid and semisolid forms. Intestinal content was assessed based on its weight and volume. Table 3 shows that the positive control and treatment groups had lower average weight of intestinal contents than the negative control group. The average weight of intestinal contents in negative controls was 41.39% higher than in positive controls. The highest difference in the average weight of intestinal contents was shown by positive controls (29.27%). Among the treatment groups, Group III, which received 25 mg/kg DLBS1Y62, showed the highest reduction in average weight of intestinal contents (27.41%) and its average weight was 2.63% higher than in positive controls.
 |
Table 3 Average Weight and Volume of Intestinal Contents |
Positive control and treatment groups had a lower average volume of intestinal contents than negative controls, as shown in Table 3. Group III showed the greatest reduction in average intestinal volume (39.97%) of all groups and the average volume in this group was 3.53% lower than for positive controls. Tables 2 and 3 show that oral administration of 25 mg/kg BW DLBS1Y62 had a similar effect to 2 mg/kg BW loperamide in inhibiting diarrheal frequency and reducing intestinal contents, but did not result in a longer time to first onset of diarrhea (Table 1).
Discussion
The effect of DLBS1Y62 on experimentally induced diarrhea was evaluated using castor oil-induced diarrhea and castor oil-induced enteropooling in Wistar rats. Castor oil is obtained from the seeds of Ricinus communis and is one of the oldest drugs used as a laxative.23,24,25 The results showed that administration of DLBS1Y62 and the combination of DLBS1Y62 with attapulgite could inhibit the formation of further forms of diarrheal feces(unshaped feces with a large amount of liquid). In contrast, rats that received purified water as a negative control formed three shapes of feces: well-shaped wet feces, shapeless feces, and unshaped feces with a large amount of liquid. According to Hedge et al (1994),19 rats were considered protected against diarrhea if they formed well-shaped wet feces and considered unprotected against diarrhea if they formed shapeless feces or unshaped feces with a large amount of liquid.
Castor oil is reported to cause diarrhea by increasing the volume of intestinal contents by preventing the reabsorption of water.16 Liberation of ricinoleic acid from castor oil results in irritation and inflammation of the intestinal mucosa, leading to the release of prostaglandin E2 (PGE2), which results in the stimulation of motility and secretion, and the prevention of reabsorption of NaCl and water.26,27 Rats that received DLBS1Y62 at all dose levels and in combination with attapulgite had a lower frequency of diarrhea and reduced intestinal content compared with negative controls. The decreased intestinal content in rats that received DLBS1Y62 could be caused by water reabsorption and a reduction in water secretion. Low diarrheal frequency in the DLBS1Y62 treatment groups was probably related to decreased intestinal motility, but DLBS1Y62 treatments did not delay the onset of diarrhea. Altered intestinal motility may alter fluid absorption by increasing or decreasing the exposure of the luminal content to the intestinal absorptive surface. Non-specific antidiarrheal agents decrease intestinal motility and decrease stool frequency, which may, in turn, limit the number of incontinent episodes.28,29 DLBS1Y62 at a dose of 25 mg/kg BW showed an inhibitory effect on diarrheal frequency and resulted in low intestinal contents, similar to the effect of loperamide at a dose 2 of mg/kg BW.
Antidiarrheal activity has been found in plants possessing tannins, alkaloids, saponins, flavonoids, steroids, and terpenoids.30 Most Gambier extracts contain catechine and catechu tannat acid (tannin), which are flavonoid derivatives.31 Tannins and flavonoids are suggested to be responsible for antidiarrheal activity by increasing colonic water and electrolyte reabsorption, and tannins could also decrease the irritability of the bowel, thereby reducing the peristaltic index.32 Tannins that are present in antidiarrheal plants could denature proteins in the intestinal mucosa by forming protein tennates, which may reduce secretion.33,34 Tannin produces a temporary protective layer of coagulated protein on the mucosal membrane of the gut, possibly desensitizing sensory nerve endings and reducing provocative peristaltic stimuli.35 Tannins also form a protective pellicle that prevents the absorption of toxic substances. Tannins are astringent, bitter plant polyphenols, which either bind and precipitate or shrink proteins.36,37 Moreover, herbs with astringent properties are recommended as a treatment for diarrhea.14
According to the results of the animal study, the possible antidiarrheal mechanism of action of DLBS1Y62 could be related to inhibition of water secretion, reduction of intraluminal fluid accumulation, or increasing water absorption. However, it did not delay the onset of diarrhea. This finding is in accordance with the goal of drug therapy in diarrhea, that is to reduce stool water by increasing fluid absorption, or reducing fluid secretion, or both.38
Conclusion
The present study showed that DLBS1Y62 at a dose of 25 mg/kg BW resulted in inhibition of diarrheal fecal formation and also reduced the fecal passage frequency of castor oil-induced diarrhea and enteropooling. However, the extract did not delay the onset of diarrhea. Although further studies are still required, this study indicates that DLBS1Y62 could be considered as a promising herbal treatment for diarrhea.
Acknowledgments
The authors would like to thank Irfan A. Darfiansyah, Theresia Ginting, and Evelyne Nadia Halim for their support in preparing the substance; Neny Agustianingsih and Dewi Andriani for daily animal care; and Imelda L. Winoto, Isabela Anjani, Shinta Alicia Sihombing, Kharisma Setianingrum Agpri, and Destrina Grace for reviewing this manuscript.
Disclosure
The authors were all employees of Dexa Laboratories of Biomolecular Sciences (DLBS), Dexa Medica during the study and manuscript writing. This study was financially funded by PT Dexa Medica. The authors report no other potential conflicts of interest in this work.
References
1. CDC. Diarrhea: common Illness, Global Killer; 2015. Available from: https://www.cdc.gov/healthywater/global/diarrhea-burden.
2. Farthing M, Salam MA, Lindberg G, et al. Acute diarrhea in adults and children: a global perspective. J Clin Gastroenterol. 2013;47(1):12–20. doi:10.1097/MCG.0b013e31826df662
3. Komarulzaman A, Smits J, de Jong E. Clean water, sanitation and diarrhoea in Indonesia: effects of household and community factors. Glob Public Health. 2017;12(9):1141–1155. doi:10.1080/17441692.2015.1127985
4. Budyanra DP. The Risk Factor that Affect Children Diarrhea in The Island of Java 2013 (Riskesdas 2013 Data Analysis). J Educational Health Commu Psychol. 2017;6(1):1. doi:10.12928/jehcp.v6i1.6615
5. Ezeja IM, Ezeigbo II, Madubuike KG, et al. Antidiarrheal activity of Pterocarpus erinaceus methanol leaf extract in experimentally–induced diarrhea. Asian Pac J Trop Med. 2012;5(2):147–150. doi:10.1016/S1995-7645(12)60014-5
6. Ezenwali MO, Njoku OU, Okoli CO. Studies on the anti-diarrheal properties of seed extract of Monodora tenuifolia. Int J App Res Nat Prod. 2010;2(4):20–26.
7. Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111(7):931–943. doi:10.1172/JCI200318326
8. Ma C, Wu S, Yang P, Li H, Tang S, Wang Q. Behavioural factors associated with diarrhea among adults over 18 years of age in Beijing, China. BMC Public Health. 2014;14(1):451. doi:10.1186/1471-2458-14-451
9. Schiller LR. Management of diarrhea in clinical practice: strategies for primary care physicians. Rev Gastroenterol Disord. 2007;7:S27.
10. Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Safety. 2000;22(1):53–72. doi:10.2165/00002018-200022010-00005
11. Portalatin M, Winstead N. Medical management of constipation. Clin Colon Rectal Surg. 2012;25(1):12. doi:10.1055/s-0032-1301754
12. World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. Geneva: World Health Organization; 2005:1–50.
13. Carter E, Bryce J, Perin J, Newby H. Harmful practices in the management of childhood diarrhea in low- and middle-income countries: a systematic review. BMC Public Health. 2015;15:788. doi:10.1186/s12889-015-2127-1
14. Palombo EA. Bryce JPerin JNewby HPhytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytotherapy Res. 2006;20(9):717–724. doi:10.1002/ptr.1907
15. Turner IM. Notes on the genus Uncaria (Rubiaceae) in Singapore. Gard Bull Sing. 2018;70(1):9–12. doi:10.26492/gbs70(1).2018-02
16. Taniguchi S, Kuroda K, Doi KI. Evaluation of gambir quality based on quantitative analysis of polyphenolic constituents. J Pharmaceutical Soc Japan. 2007;127(8):1291–1300. doi:10.1248/yakushi.127.1291
17. Girard P, Pansart Y, Gillardin JM. Inducible nitric oxide synthase involvement in the mechanism of action of Saccharomyces boulardii in castor oil-induced diarrhoea in rats. Nitric Oxide. 2005;13(3):163–169. doi:10.1016/j.niox.2005.06.001
18. Sharma P, Vidyasagar G, Bhandari A, et al. A pharmacological evaluation of antidiarrhoeal activity of leaves extract of Murraya koenigii in experimentally induced diarrhoea in rats. Asian Pacific J Tropical Dis. 2012;2(3):230–233. doi:10.1016/S2222-1808(12)60052-8
19. Hedge SS, Moy TM, Perry MR, Loeb M, Eglen RM. Evidence for the involvement of 5-hydroxytryptamine 4 receptors in 5-hydroxytryptophan-induced diarrhea in mice. J Pharmacol Exp Therapeutics. 1994;271(2):741–747.
20. Robert A, Nezamis JE, Lancaster C, Hanchar AJ, Klepper MS. Enteropooling assay: a test for diarrhea produced by prostaglandins. Prostaglandins. 1976;11(5):809–828. doi:10.1016/0090-6980(76)90189-1
21. Rahman MK, Barua S, Islam MF, et al. Studies on the anti-diarrheal properties of leaf extract of Desmodium puchellum. Asian Pac J Trop Biomed. 2013;3(8):639. doi:10.1016/S2221-1691(13)60129-X
22. Drugbank. Loperamide; 2015. Available from: https://www.drugbank.ca/drugs/DB00836.
23. Pinto A, Calignano A, Mascolo N, Autore G, Capasso F. Castor oil increases intestinal formation of platelet-activating factor and acid phosphatase release in the rat. Br J Pharmacol. 1989;96(4):872. doi:10.1111/j.1476-5381.1989.tb11897.x
24. Mascolo N, Izzo AA, Barbato F, Capasso F. Inhibitors of nitric oxide synthetase prevent castor‐oil‐induced diarrhoea in the rat. Br J Pharmacol. 1993;108(4):861–864. doi:10.1111/j.1476-5381.1993.tb13478.x
25. Tunaru S, Althoff TF, Nüsing RM, Diener M, Offermanns S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci U S A. 2012;109(23):9179–9184. doi:10.1073/pnas.1201627109
26. Das KA, Rohini RM, Hema A. Antidiarrheal activity of Rhizophora mucronata bark extracts. Int J Alt Med. 2007;7:1.
27. Tangpu V, Deori K, Yadav AK. Evaluation of safety and protective effects of Potentilla fulgens root extract in experimentally induced diarrhea in mice. J Intercultural Ethnopharmacology. 2014;3(3):103. doi:10.5455/jice.20140416104844
28. Sweetser S. Evaluating the patient with diarrhea: a case-based approach. Mayo Clin Proc. 2012;87(6):596–602. doi:10.1016/j.mayocp.2012.02.015
29. Scarlett Y. Medical management of fecal incontinence. Gastroenterology. 2004;126:S55–63. doi:10.1053/j.gastro.2003.10.007
30. Havagiray RC, Chandra R, Kaushik S. Studies on anti-diarrhoeal activity of Calotropis gigantea R.B.R. in experimental animals. J Pharm Pharm Sci. 2004;7:70–75.
31. Isnawati A, Raini M, Sampurno OD, Mutiatikum D, Widowati L, Gitawati R. Characterization of 3 types gambir extract (Uncaria gambir Roxb) from Sumatera Barat. Buletin Penelitian Kesehatan. 2012;40(4):201–208.
32. Tadesse WT, Hailu AE, Gurmu AE, Mechesso AF. Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneria scabra in mice. BMC Complement Altern Med. 2014;14(1):460. doi:10.1186/1472-6882-14-460
33. Adzu B, Amos S, Amizan MB, Gamaniel K. Evaluation of the antidiarrhoeal effects of Zizyphus spina-christi stem bark in rats. Acta Trop. 2003;87(2):245–250. doi:10.1016/S0001-706X(03)00114-1
34. Jia Q, Su W, Peng W, Li P, Wang Y. Anti-diarrhoea and analgesic activities of the methanol extract and its fractions of Jasminum amplexicaule Buch.-Ham. (Oleaceae). J Ethnopharmacol. 2008;119(2):299–304. doi:10.1016/j.jep.2008.07.014
35. Capasso F, Gaginella TS, Grandolini G, Izzo AA. Phytotherapy: A Quick Reference to Herbal Medicine. Springer Science & Business Media; 2003.
36. De Jesus NZ, Falcao HD, Gomes IF, et al. Tannins, peptic ulcers and related mechanisms. Int J Mol Sci. 2012;13(3):3203–3228. doi:10.3390/ijms13033203
37. Ashok PK, Upadhyaya K. Tannins are astringent. J Pharmacognosy Phytochemistry. 2012;1(3):45–50.
38. Van Den Eynden B, Spaepen W. New approaches to the treatment of patients with acute, nonspecific diarrhea: a comparison of the effects of loperamide and loperamide oxide. Curr Therapeutic Res. 1995;56(11):1132–1141. doi:10.1016/0011-393X(95)85123-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.