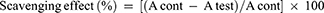Back to Journals » Journal of Experimental Pharmacology » Volume 14
Antidiabetic Activity of Hot Tea Infusion of Leaves of Moringa stenopetala in Streptozotocin-Induced Diabetic Rats
Authors Toma A
Received 13 May 2022
Accepted for publication 15 October 2022
Published 25 October 2022 Volume 2022:14 Pages 309—316
DOI https://doi.org/10.2147/JEP.S371354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Allan Zi-Jian Zhao
Alemayehu Toma
Department of Pharmacology, Hawassa University, Hawassa, Ethiopia
Correspondence: Alemayehu Toma, Tel +251913259141, Email [email protected]
Background: Moringa stenopetala is a traditionally used medicinal plant that has been used for the management of different disorders including diabetes in Ethiopia. This study was aimed to assess the antidiabetic activity of hot tea infusion of leaves of Moringa stenopetala in streptozotocin-induced diabetic rats.
Methods: Experimental animals were Wistar male rats aged 4– 6 weeks weighing 200– 250 gram. The animals were maintained in equal light/dark series of laboratory conditions, and the average ambient temperature was 23± 2 °C. The hot tea infusion of Moringa stenopetala leaves of different concentrations (as fine and coarse powder decoction), distilled water (10 mL/kg), and 150 mg/kg body weight of metformin were administered to diabetic rats as test, negative control, and positive standard drugs, respectively. Sucrose oral challenge test was also carried out to assess the effect of Moringa stenopetala hot tea infusion on postprandial glucose level. Blood glucose level was measured at 0, 30, 60, 90, and 180 minutes. Statistical analysis was conducted by SPSS package version 23, and the p-value less than 0.05 was declared as level of statistical significance.
Results: The phytoconstituents that tested positive in hot tea infusion of Moringa stenopetala leaves were alkaloids, tannins, flavonoids, saponins, and terpenoids. Different experimental groups treated with the hot tea infusion of Moringa stenopetala leaves showed significant reduction of blood glucose level after 30 minutes of hot tea infusion administration. In addition, the hot tea infusion at a different concentration for both fine and coarse powders reduced significantly raised blood glucose level. The present findings revealed that the hot tea infusion has blood glucose-lowering and antioxidant effects with wide safety margin.
Conclusion: The findings indicated that the hot tea infusion of the leaves of Moringa stenopetala shows a credible reduction in blood glucose level in rats.
Keywords: Moringa stenopetala leaves, blood glucose, antioxidant, antidiabetic activity, hot tea infusion
Background
Globally, the number of people with pre-diabetes and diabetes is increasing exponentially due to aging, urbanization, unreliable food consumption, growing prevalence of overweight, and decreased level of physical movement. Diabetes mellitus (DM) is a principal cause of morbidity and mortality worldwide, with 422 million adults being affected in 2014, and a majority of cases are from low- and middle-income countries. DM is the main cause of death in the world with an estimated number of one and half million deaths.1 The prevalence is predicted to be 643 million in the year 2030 and 783 million by 2045, with the greatest burden anticipated in low- and middle-income nations. At present, 3 in 4 adults of the worlds’ population with diabetes live in low- and middle-income countries.2 Diabetes is also associated with a congregation of life-threatening and potentially disabling micro- and macro-vascular complications.1 In the year 2021 alone, 541 million adults were at increased risk of developing type 2 diabetes.2 Hence, there is a much greater burden in the form of loss of productivity which may result in high economic and health impact to the nations.
Moringa stenopetala of the family Moringaceae is commonly planted in Omo Rift Valley regions in southern part of Ethiopia.3 The leaf part of this plant is cooked and eaten as a vegetable, and all parts are used to treat most infectious and non-infectious diseases.4–10 The leaves and roots of Moringa stenopetala have demonstrated antitrypanosomal effect according to the previous reports.11 Antispasmodic activities of the leaves on smooth muscle tissues and antimicrobial properties of the seeds4,9 have also been reported. The crude aqueous and ethanol extract/fraction of the leaves have demonstrated hypoglycemic and antihyperglycemic activities.12–14 Additionally, on chronic administration of the butanol fraction of Moringa stenopetala leaves antihyperglycemic and antihyperlipidemic effects were shown in alloxan-induced diabetic mice with wide margin of safety, indicating its potential for long-term management of diabetes and related complications including hyperlipidemia.12,13,15,16 The antiglycation effect in in vitro studies also demonstrated the role of Moringa stenopetala in inhibiting diabetes-associated complications.17 Hot tea infusion of Moringa stenopetala leaves has long been used by the local community in the southern part of Ethiopia and has a range of promising medicinal claims including on diabetes mellitus and hypertension, which is yet to be supported by scientific evidence.
However, the models used on the above efficacy and safety evaluations did not imitate the existing communities’ practice in evaluating antidiabetic properties. Therefore, this study was aimed to evaluate the effect of hot tea infusion on blood glucose level and antioxidant properties to confirm traditional hot tea consumption claims in the local community.
Materials and Methods
Chemicals
Metformin was purchased from Merc (Santé, France). Streptozotocin was procured from AdooQ BioScience, United States. Kits for determining blood glucose level were obtained from MyBioSource, United States. All other chemicals used in the present study for dilution and other purposes were purchased from reliable sources and were of standard quality.
Collections and Preparation of Moringa stenopetala Leaves
The Moringa stenopetala leaves were collected from Arbaminch town, South Nation’s Nationalities Peoples Region, 522 kilometers south of the capital city of Ethiopia. A taxonomist, Dr. Getechaw Addis, identified and authenticated the plant material, and then the plant material was deposited in the herbarium of Ethiopia Public Health Institute with AT-002 as a voucher number. Then the plant material was dried under shade and crushed to powder, fine and coarse powder, for tea preparation.
Preparation of Moringa stenopetala Hot Tea Infusion
The shed-dried leaves of Moringa stenopetala were powdered using a pestle and mortar until a free tea leaf-like consistency was reached. The ensuing tea leaves were drenched in hot distilled water (94.5 °C) for 10 minutes and filtered through cotton gauze. The filtrate was permitted to cool to room temperature before administering to overnight-fasted male Wistar rats.
Dose Calculation
Based on existing community practice, the Moringa stenopetala leaves hot tea infusion was prepared by soaking two teaspoons (tsp) (2 grams) of loose tea leaves in 100 milliliters of water (0.68 gram dried tea extract), which would be consumed by an adult with the average weight reflected to be 60 kilograms. Based on this documented information, the effective therapeutic human dose is calculated to be 11.33 milligrams per kilogram body weight, from which the animal dose was derived.
Animal dose = Human dose × Conversion factor
Animal dose = 11.33 mg/kg × 6.17
Dose for Group III and VI = 69.91 mg/kg (Moringa stenopetala leaves infusion 1 based on 2 tsp/100 mL)
Dose for Group IV and VII = 139.81 mg/kg (Moringa stenopetala leaves infusion 2 based on 4 tsp/100 mL)
Dose for Group V and VIII = 279.62 mg/kg (Moringa stenopetala leaves infusion 3 based on 6 tsp/100 mL)
To mimic the traditional approach precisely, both fine and coarse powders of the Moringa stenopetala leaves were used for the experimental purpose. The dose determination was based on minor modifications of a previous report.18 Coarse powders are particles that pass through a number twenty sieve (0.84 mm) and not more than forty percent pass through a number sixty sieve. Fine powders are particles that pass through a number sixty sieve (0.25 mm) and not more than forty percent pass through a number hundred sieve.19
Sample Size Determinations
The sample size was calculated by using version 3.1.9.7 G* power software with t-tests family through correlation of point biserial model, with a p-value of 0.05, a power of 0.90, and with a standardized effect size of 0.41.13 The needed total sample size for intervention is 40 rats, with 5 rats in each experimental group.
Experimental Animals
Experimental animals were Wistar male rats aged 4–6 weeks, weighing 200–250 gram. All animals used for this experiment were acquired from a reputable breeding facility for laboratory animals. The animals were permitted acclimatization to the laboratory environment for a one-week period. The animals were maintained in equal light/dark series of laboratory conditions, and the average ambient temperature was 23±2 °C. The rats were provided with a standard laboratory animal diet and food ad libitum. Approval for the ethical aspects of this study was obtained from Hawassa University College of Medicine and Health Sciences Institutional Review Board (IRB/046/2020). All rats used in this study were handled ethically throughout the study period conforming to internationally accepted care and use guidelines.
Induction of Diabetes
Five rats were selected randomly as normal controls. The remaining seven groups were induced by streptozotocin (STZ) 45 mg/kg through intraperitoneal injection (IP). Citrate buffer solution (0.01M, pH=4.5) was used to dissolve streptozotocin. All animals were allowed free access to water and a standard pellet diet after half an hour of administration. Six days thereafter the fasting blood glucose levels of rats were determined using the glucose oxidase method. Rats with a blood glucose level of more than 126 mg/dL were considered diabetic.13 Streptozotocin-induced diabetic rats were randomly selected and divided into seven groups negative control, positive control, and test groups.
Test for Sucrose Tolerance
The normal rats were divided into eight groups containing five animals each. Distilled water was administered to control groups. The test groups were fed orally with three different doses of hot tea infusion of Moringa stenopetala leaves (2, 4, and 6 spoonfuls) of both fine and coarse powder preparations. For the fifth group, acarbose (3 mg/kg) was administered as a positive control. Loading of sucrose (3 g/kg) was done for all groups after five minutes of administering the treatments.14 Collection of blood samples was done from a tail vein at 0, 30, 60, 90, and 180 min. The glucose oxidase method was used to determine the blood glucose levels.
Effect of Moringa stenopetala Leaves Hot Tea Infusion on Blood Glucose Levels
Streptozotocin-induced diabetic rats were administered with different levels of teaspoonfuls of hot tea infusion via gavage orally. The streptozotocin-induced diabetic and normal rats were given 10 mL/kg body weight of normal saline via gavage orally, and positive control rats were given 150 mg/kg of metformin via gavage orally.18 At times 0, 30, 60, 90, and 180 minutes the blood samples were collected from a tail vein and blood glucose levels were measured using a glucometer.
DPPH Radical Scavenging Activity Assay
The effect of decoction of hot tea infusion of Moringa stenopetala leaves on free radicals was measured in vitro by an assay using 2,2′-diphenyl-1-picrylhydrazyl (DPPH) according to the method described previously. Twenty-four milligrams of DPPH were prepared by dissolving with 100 mL methanol as stock solution and stored at 20 °C until required. The working solution was obtained by diluting the DPPH solution with methanol to attain an absorbance of about 0.98±0.02 at 517 nm using the spectrophotometer. A 3 mL aliquot of this solution was mixed with 100 μL of the hot tea infusion of Moringa stenopetala leaves at various concentrations (10–500 μg/mL). The mixture was shaken well for reactions and incubated in the dark for 15 min at room temperature. Then the absorbance was measured at 517 nm. The control was prepared using the same procedure without hot tea infusion of Moringa stenopetala leaves.20 The free radical scavenging activity was determined based on the percentage of DPPH radical scavenged using the following equation:
Where A cont. is the absorbance of control reaction and A test is the absorbance in the presence of extract.
Study for Acute Toxicity
Randomly selected rats of either sex were used for the acute toxicity study. The animals were well-kept, and fasted overnight providing only water ad libitum. They were divided into four groups, six animals in each group (three males and three females), and then the hot tea infusion of Moringa stenopetala was administered orally in an increasing dose level of 0.3, 2, and 5 g/kg via oral gavage according to the guidelines of the Organization for Economic Cooperation and Development.21 Animals were kept under close observation for 4 hours after administering the hot tea infusion of Moringa stenopetala leaves for neurological, behavioral, and autonomic profile, and then they were observed for any change in the general behavior and/or other physical activities, and mortality was recorded within 24 hours.
Screening of Phytochemical Constituents
The hot tea infusion of Moringa stenopetala leaves used for the antidiabetic activity evaluation was subjected to phytochemical screening following methods previously described by Trease and Evans.22 The tea infusion of Moringa stenopetala leaves was tested for the presence of alkaloids, saponins, flavonoids, terpenoids, and tannins.
Statistical Analysis
Descriptive observation was used to determine the existence of phytochemical constituents. All the values of blood sugar were expressed as mean±standard error of the mean (SEM). The values were analyzed by one-way analysis of variance (ANOVA). Tukey’s post hoc test was used to determine the level of significance. The statistical significance level was declared at p<0.05. SPSS software package version 23 was used to perform statistical analysis.
Results
Effect of Hot Tea Infusion of Moringa stenopetala Leaves on Postprandial Glycemia in Non-Diabetic Rats
An oral sucrose tolerance test was done by a single oral administration of hot tea infusion of Moringa stenopetala leaves to non-diabetic rats to confirm its effect on the postprandial blood glucose level. The results revealed that the maximum blood glucose level was reached at 30 min after administration of sucrose in all experimental groups. In addition, the hot tea infusion at a different concentration for both fine and coarse powders reduced significantly the raised blood glucose level. Similarly, the positive control also significantly reduced blood glucose after 30, 60, and 120 min of administration relative to non-treated control rats (Table 1).
 |
Table 1 Sucrose Challenge Test of Hot Tea Infusion Extracts of Moringa stenopetala Leaves in Rats |
Effect of Hot Tea Infusion of Moringa stenopetala Leaves on Blood Glucose Level in Streptozotocin-Induced Diabetic Rats
Acute blood glucose level-lowering effect of the hot tea infusion of Moringa stenopetala was seen in streptozotocin-induced diabetic rats. The blood glucose levels were measured at different time intervals (0, 30, 60, 90, and 180 minutes) in diabetic and normal rats given a hot tea infusion of Moringa stenopetala leaves after experimental induction of diabetes using streptozotocin. The results are presented as a summary in Table 2. Before experimental induction of diabetes, there was no significant difference in blood glucose levels between diabetic and non-diabetic control groups (p>0.05). After experimental induction, diabetic rats showed significant differences in blood glucose levels compared to normal rats (p<0.05). The groups treated with the hot tea infusion of Moringa stenopetala leaves showed a significant reduction in blood glucose levels after 30 minutes of hot tea infusion administration.
 |
Table 2 Effect of Hot Tea Infusion Extracts of Moringa stenopetala Leaves in Blood Glucose Level |
Effect of Hot Tea Infusion of Moringa stenopetala Leaves on 2,2-Diphenyl-1-Picryl Hydrazyl (DPPH) Free Radical
In order to determine the extent of the scavenging effect, hot tea infusion of the leaves of Moringa stenopetala was tested for antioxidant activity using 2,2-diphenyl-1-picryl hydrazyl (DPPH) free radical. The hot tea infusion of Moringa stenopetala showed antioxidant activity as described in Table 3.
 |
Table 3 Free Radical Scavenging Activity of Hot Tea Infusion of Moringa stenopetala Leaves |
Phytoconstituents in Hot Tea Infusion of Moringa stenopetala Leaves
The phytochemical identification tests carried out on the hot tea infusion of Moringa stenopetala leaves showed the presence of different secondary metabolites (Table 4). The phytoconstituents that tested positive in hot tea infusion of Moringa stenopetala leaves were alkaloids, tannins, flavonoids, saponins, and terpenoids.
 |
Table 4 Qualitative Determination for Phytochemical Constituents in Hot Tea Infusion of Moringa stenopetala Leaves |
Acute Oral Toxicity Test
The current acute toxicity studies indicated that the administration of graded concentrations of hot tea infusion of Moringa stenopetala (up to a dose of 5 g/kg) did not yield substantial changes in autonomic and behavioral patterns including alertness, motor activity, respiration, agitation, diarrhea, seizures, coma, and physical appearance of the animals. No death was identified up to the dose of 5 g/kg body weight, indicating that the medium toxic dose (LD50) is greater than 5 g/kg body weight in mice. All mice were physically alert.
Discussion
The local community has long used Moringa stenopetala leaves hot tea infusion for diabetes mellitus, with a wide range of hopeful therapeutic claims, which are yet to be supported by scientific confirmation. As the antidiabetic activity is no exception to this, the present study set out to demonstrate the effect of the folkloric consumption of the hot tea infusion prepared from the dried leaves for its antidiabetic property in contrast to the aqueous crude extract. In this investigation, the antidiabetic activity of the hot tea infusion of Moringa stenopetala leaf was compared to that of a potent antidiabetic standard conventional drug, metformin.
In this study, a statistically significant acute antihyperglycemic effect of hot tea infusion of Moringa stenopetala was shown after 120 minutes of treatment for 4 and 6 teaspoonfuls fine and coarse powders of Moringa stenopetala leaves. The streptozotocin-induced diabetic rats were treated with different concentrations of hot tea infusion of Moringa stenopetala leaves, which exhibited significant antidiabetic effect at 120 and 180 minutes after administration of the infusion, suggesting that the infusion has a role in management of hyperglycemia on acute administration. The overall percent reduction effected by the hot tea infusion of Moringa stenopetala leaves was found to be comparable with that of the standard drug. This is in line with a previous study on its acute and chronic antihyperglycemic effect which showed relatively comparable blood glucose reduction compared to the standard drug after 180 minutes.15,16
Currently, there is mounting interest in traditional medicinal plants in the treatment of diabetes due to the side effects associated with conventional antidiabetic drugs. Hence, herbal medicines play an imperative role in the comprehensive management of diabetes mellitus including prevention and treatment. Nowadays, herbal medicines have started to gain significance as a source of antidiabetic agents due to their less potential adverse effects, efficacy, and low cost. It was estimated that more than a thousand plant species are being used as alternative medicine to standard drugs for diabetes.23 Pharmacological actions of the plant products used as alternative medicines to treat diabetes are related to their phytochemical constituents. Medicinal plants are rich in phenolic compounds, flavonoids, terpenoids, coumarins, and other constituents which show a lowering effect in blood glucose levels.15,24
The presence of phytochemicals in plant products offers great potential for balancing metabolic disturbances. Several phytomolecules, including flavonoids, total phenolic compounds, alkaloids, glycosides, saponins, glycolipids, dietary fibers, polysaccharides, peptidoglycans, carbohydrates, amino acids, and others obtained from various plant sources, have been reported as potent hypoglycemic and antihyperglycemic agents.25 Alkaloids, flavonoids, and saponins, which are present in the hot tea infusion of the leaves of Moringa stenopetala, have been linked with the antidiabetic activity of Moringa stenopetala leaves.18,26 At this juncture, however, it is practically impossible to determine the specific phytochemical that bring about the detected antihyperglycemic activity of the Moringa stenopetala leaves of hot tea infusion. Nevertheless, it can be suggested that the range of polar phenolic compounds such as flavonoids and tannins in combination with alkaloids might be responsible for the apparent antidiabetic activity of the plant materials.
Additional observation gained from this study is the relative safety of the hot tea infusion of Moringa stenopetala leaves at the graded concentration of up to 5000 mg/kg. According to scientific reports, any compound or drug with the oral LD50 estimate greater than 1 g/kg could be considered less toxic and safe.25 Arising from this documented evidence, the hot tea infusion of Moringa stenopetala leaves at an oral dose of 5 g/kg could be seen as moderately safe on acute exposure. The present findings reveal that the hot tea infusion has blood glucose-lowering and antioxidant effects with wide safety of margin. The mechanisms of action of Moringa stenopetala leaves in previously reported investigations are alpha glucosidase inhibition, pancreatic beta cell regenerative effects, and inhibition of advanced glycation end products.12,13,20 However, further studies should be done to investigate the active chemical ingredient(s) responsible for the antihyperglycemic effects.
Conclusion
The findings of this investigation shows that the hot tea infusion of the leaves of Moringa stenopetala possesses antidiabetic activity. However, in-depth investigations are essential to elucidate the pharmacological mechanism(s) of action and the active constituents accountable for the observed antidiabetic action of the hot tea infusion.
Data Sharing Statement
All data used in this investigation are within the manuscript.
Ethics Approval and Consent to Participate
Ethical approval for this study was obtained from Institutional Review Board of Hawassa University College of Medicine and Health Sciences with approval number IRB/046/2020. All rats used in this study were treated ethically throughout the study period conforming to the International Guidelines of Laboratory Animal Care and Use.
Acknowledgment
I am grateful to the Ethiopian Public Health Institute for providing me bench space to carry out this study.
Disclosure
The author reports no conflicts of interest.
References
1. World Health Organization. Diabetes fact sheet; 2021. Available from: https://wwwwhoint/news-room/fact-sheets/detail/diabetes.
2. Federation ID. International diabetes federation, diabetes atlas; 2021. Available from: https://diabetesatlasorg/atlas/tenth-edition/.
3. Abuye C, Urga K, Knapp H, et al. A compositional study of Moringa stenopetala leaves. East Afr Med J. 2003;80(5):247–252. doi:10.4314/eamj.v80i5.8695
4. Mekonnen Y. Effects of ethanol extract of Moringa stenopetala leaves on Guinea-pig and mouse smooth muscle. Phytother Res. 1999;13(5):442–444. doi:10.1002/(SICI)1099-1573(199908/09)13:5<442::AID-PTR476>3.0.CO;2-7
5. Mengistu M, Abebe Y, Mekonnen Y, Tolessa T. In vivo and in vitro hypotensive effect of aqueous extract of Moringa stenopetala. Afr Health Sci. 2012;12(4):545–551.
6. Geleta B, Makonnen E, Debella A, Abebe A, Fekadu N. vitro vasodilatory activity and possible mechanisms of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in isolated thoracic aorta of Guinea pigs. Prevn Nutr Food Sci. 2016;8:35–42.
7. Geleta B, Makonnen E, Debella A, Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in rats. Front Pharmacol. 2016;7:97. doi:10.3389/fphar.2016.00097
8. Nibret E, Wink M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. PLoS One. 2010;17(12):911–920.
9. Seleshe S, Kang SN. In vitro antimicrobial activity of different solvent extracts from Moringa stenopetala leaves. Diabetes Metab Syndr Obes. 2019;24(1):70–74.
10. Tamrat Y, Nedi T, Assefa S, Teklehaymanot T, Shibeshi W. Anti-inflammatory and analgesic activities of solvent fractions of the leaves of Moringa stenopetala Bak. (Moringaceae) in mice models. BMC Complement Altern Med. 2017;17(1):473. doi:10.1186/s12906-017-1982-y
11. Mekonnen Y, Yardley V, Rock P, Croft S. In vitro antitrypanosomal activity of Moringa stenopetala leaves and roots. Phytother Res. 1999;13(6):538–539. doi:10.1002/(SICI)1099-1573(199909)13:6<538::AID-PTR486>3.0.CO;2-K
12. Toma A, Makonnen E, Mekonnen Y, Debella A, Addisakwattana S. Intestinal α-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complement Altern Med. 2014;14(1):180. doi:10.1186/1472-6882-14-180
13. Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15(1):242. doi:10.1186/s12906-015-0779-0
14. Woldekidan S, Mulu A, Ergetie W, et al. Evaluation of antihyperglycemic effect of extract of Moringa stenopetala (Baker f.). aqueous leaves on alloxan-induced diabetic rats. Diabetes Metab Syndr Obes. 2021;14:185–192.
15. Seleshe S, Ameer A, Kim B-J, Kang SN. Ethanolic extract of moringa stenopetala leaves enhances the quality characteristics and shelf-life of vacuum-packed pork patty during refrigeration storage. Prev Nutr Food Sci. 2021;26(3):357–365. doi:10.3746/pnf.2021.26.3.357
16. Toma A, Makonnen E, Debella A, Tesfaye B. Antihyperglycemic effect on chronic administration of butanol fraction of ethanol extract of Moringa stenopetala leaves in alloxan induced diabetic mice. Asian Pac J Trop Med. 2012;3(2):S1606–S10.
17. Toma A, Makonnen E, Mekonnen Y, Debella A, Addisakwattana S. Extract of leaves of Moringa stenopetala alleviates glycation in in vitro model. EC Nutr. 2017;11(6):218–222.
18. Fekadu N, Basha H, Meresa A, Degu S, Girma B, Geleta B. Diuretic activity of the aqueous crude extract and hot tea infusion of Moringa stenopetala (Baker f.) Cufod. leaves in rats. J Exp Pharmacol. 2017;9:73–80. doi:10.2147/JEP.S133778
19. Allen L, Ansel HC. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. Lippincott Williams and Wlkins: Philadephia; 2014.
20. Habtemariam S. Investigation into the antioxidant and antidiabetic potential of Moringa stenopetala: identification of the active principles. Nat Prod Commun. 2015;10(3):475–478. doi:10.1177/1934578X1501000324
21. Development OfECa. Test No. 249: fish Cell line acute toxicity - The RTgill-W1 cell line assay; 2021.
22. Evans WC. Trease and Evans’ Pharmacognosy.
23. Bindu JNRT. Role of medicinal plants in the management of diabetes mellitus: a review. J Toxicol. 2019;9(1):23.
24. Chetna MBS, Seema S, Siddiqui M, Abbas A. Role of phytochemicals in diabetes lipotoxicity: an overview. Int J Res Dev Pharm L Sci. 2015;4(4):1604–1610.
25. Clemen-Pascual LM, RA Macahig, Rojas NR. Comparative toxicity, phytochemistry, and use of 53 Philippine medicinal plants. Toxicol Rep. 2022;9:22–35. doi:10.1016/j.toxrep.2021.12.002
26. Bennett RN, Mellon FA, Foidl N, et al. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. BMC Complement Altern Med. 2003;51(12):3546–3553.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.