Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Antidepressant medication use and nasopharyngeal cancer risk: a nationwide population-based study
Authors Lin C, Chan HL, Hsieh YH, Liang H , Chiu WC , Lee Y, McIntyre RS, Chen VC
Received 29 December 2017
Accepted for publication 13 March 2018
Published 30 April 2018 Volume 2018:14 Pages 1101—1106
DOI https://doi.org/10.2147/NDT.S161049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Chiao-Fan Lin,1,2,* Hsiang-Lin Chan,1,2 Yi-Hsuan Hsieh,1,2 Hsin-Yi Liang,1,2 Wei-Che Chiu,3,4,* Yena Lee,5 Roger S McIntyre,5,6 Vincent Chin-Hung Chen2,7,8
1Department of Child Psychiatry, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan; 2Department of Psychiatry, Chang Gung University, Taoyuan, Taiwan; 3Department of Psychiatry, Cathay General Hospital, Taipei, Taiwan; 4School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan; 5Mood Disorders Psychopharmacology Unit, Brain and Cognition Discovery Foundation, Toronto, Ontario, Canada; 6Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada; 7Medical Research Department, Health Information and Epidemiology Laboratory, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan; 8Department of Psychiatry, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan
*These authors contributed equally to this work
Background: The association between antidepressant exposure and nasopharyngeal cancer (NPC) has not been previously explored. The purpose of this study was to investigate the association between antidepressant prescription, including novel antidepressants, and the risk of NPC in a population-based study.
Materials and methods: Data for the analysis were derived from National Health Insurance Research Database. We identified 16,957 cases with a diagnosis of NPC and 83,231 matched controls by using a nested case–control design. A conditional logistic regression model was used, with adjustments for potentially confounding variables (eg, comorbid physical diseases, comorbid psychiatric diseases, and other medications).
Results: We report no association between NPC incidence and antidepressant prescription. For all classes of antidepressants, antidepressant exposure, regardless of cumulative dose, had no significant effect on NPC incidence (adjusted odds ratio of cumulative selective serotonin reuptake inhibitor exposure ≥336 defined daily dose was 1.18 [95% CI: 0.90–1.53]; tricyclic antidepressant exposure ≥336 defined daily dose was 1.18 [95% CI: 0.80–1.74]).
Conclusion: There was no association between antidepressant prescription and incident NPC.
Keywords: nasopharyngeal cancer, antidepressants, Taiwan national insurance
Introduction
Antidepressants are the first line of treatment for major depressive disorder1 and a number of other psychiatric disorders, including anxiety disorders and obsessive compulsive disorder.2 They can also be prescribed for the treatment of insomnia, chronic pain, and inflammatory disorders, such as irritable bowel syndrome.3 The increase in prescription of antidepressants, especially selective serotonin reuptake inhibitors (SSRIs), was reported from 1999 to 2012.4
Procarcinogenic effects of some antidepressants have been reported in non-Asian populations. Amerio et al reviewed the US Food and Drug Administration preclinical in vivo evidence comparing the carcinogenic risk of various psychotropic drugs. Among antidepressants, 63.6% (7/11) of examined agents were associated with carcinogenicity.5 However, epidemiologic evidence of a relationship between antidepressant use and cancer risk remains inconclusive. Results from a meta-analysis suggested that there is a modest increase in the risk of breast and ovarian cancers with the use of antidepressants, with a pooled odds ratio (ORs) of 1.11 (95% CI: 1.03–1.20). Another nested case–control study indicated that SSRIs, tricyclic antidepressants (TCAs), and serotonin–norepinephrine reuptake inhibitors (SNRIs, eg, duloxetine and venlafaxine) did not significantly increase gastric cancer risk in the overall group, including those with a history of peptic ulcers.6 Similarly, Chan et al reported no association between the classes of antidepressants and invasive cervical cancer.7 In contrast, a separate population-based case–control study found that antidepressant use may actually reduce the risk of colorectal cancer.8 Other study also showed that use of TCAs and SSRIs was associated with lower risk of hepatocellular carcinoma.9 The main cancer types investigated in previous studies include breast, ovarian, and colorectal. To our knowledge, no epidemiological study has assessed the association between antidepressant use and nasopharyngeal cancer (NPC).
NPC is a prevalent cancer in several specific populations, including natives of south China, Southeast Asia, and North Africa. In these regions, the age-standardized incidence rate of NPC is >20 per 100,000 person-years.10 However, it is rare in most parts of the world, where the age-standardized incidence rate is <1 per 100,000 person-years.11 Evidence strongly suggests that Epstein–Barr virus (EBV) infection and genetic susceptibility are important etiologic factors for NPC.12 Several epidemiological studies showed an association of NPC with early childhood EBV infection and with chronic ear, nose, and sinus conditions.13 Nonviral environmental contributors to NPC development include smoking, preserved food, and exposure to formaldehyde.14 In most cases, NPC is regarded to be squamous in origin.15 Kinjo et al proposed that TCAs inhibit the growth of squamous cell carcinoma cell line.16 However, little is known about the relationship between antidepressant medication use and incident NPC.
Accordingly, the purpose of the present study was to assess the association between the prescription of antidepressants and the diagnosis of NPC. Toward addressing our primary purpose, we used a nationwide population-based registry database from Taiwan, taking into consideration the effects of several potentially confounding factors.
Materials and methods
Source population
The National Health Insurance (NHI) program was launched by the Taiwan government on March 1, 1995. In the 15 years that followed, it covered over 23 million residents, ~99% of Taiwan’s population. The National Health Insurance Research Database (NHIRD), derived from the original claims data of the NHI program, includes patients’ sociodemographic information, diagnostic codes, and records of medical procedures, ambulatory care, hospital inpatient care, and prescription claims. Cases and controls were selected from the NHIRD between January 1, 1997 and December 31, 2008. NHIRD does not contain identifying patient information. This study was approved by the Institutional Review Board of Chiayi Chang Gung Memorial Hospital.
In Taiwan, patients with definitive cancer diagnoses register with the Catastrophic Illness Registry and apply for a Catastrophic Illness Certificate. Issuance of the Certificate requires a diagnosis made by a physician and a formal panel review by the Bureau of National Health Insurance. A diagnosis of cancer must be confirmed by tissue pathology.
The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) was used to define cases: NPC was coded as 147.xx, and a diagnosis of cancer was confirmed with the Catastrophic Illness Registry Dataset. Cases were defined as people with newly diagnosed NPC between January 1, 1999, and December 31, 2008. Individuals with NPC who had any previous cancer diagnoses in the dataset between January 1, 1997, and December 31, 2008, were excluded. Index date was the date of the first NPC claim. Using an incidence density sampling method,17 we randomly selected five matched controls for each case. To be included in the control population, individuals were required to have been without a cancer diagnosis before the index date. Controls were individually matched to the case by birth year.
Exposure assessment
The pharmacological coding system we used is based on the Anatomical Therapeutic Chemical classification system. Antidepressants were identified as N06A. In the present study, antidepressants were classified as SSRIs (ie, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline), SNRIs (eg, duloxetine and venlafaxine), TCAs (eg, amitriptyline, clomipramine, dothiepin, doxepin, imipramine, maprotiline, and melitracen), reversible inhibitor of monoamine oxidase A (eg, moclobemide), noradrenergic and specific serotonergic antidepressant (eg, mirtazapine), serotonin antagonist and reuptake inhibitors (eg, trazodone), and norepinephrine–dopamine reuptake inhibitors (ie, bupropion). Information on exposure to antidepressants for all participants was confirmed with the prescription claims in the NHIRD. Antidepressants prescribed within the 365 days preceding the index date were selected as the unit of analysis. All antidepressant exposures after the index date were excluded from the analysis.
Each patient’s exposure to an antidepressant was determined using the cumulative dose of antidepressants, which is quantified by a defined daily dose (DDD), defined by World Health Organization.18 The cumulative doses were divided into four exposure dose levels as follows: ≥28 DDD, ≥84 DDD, ≥168 DDD, and ≥336 DDD.
The use of potentially confounding drug (metformin) prescribed before the index date was also confirmed in cases and controls. Confounding medical conditions (eg, depressive disorder, type 2 diabetes mellitus [T2DM], hypertension, hypercholesterolemia, EBV infection, alcohol-related disorders, COPD, and tobacco use disorder) were also identified.
Statistical analyses
Descriptive statistics of cancer cases and controls were reported for sociodemographic data (eg, age, income, and urbanization), comorbid disorders, and medication use. To assess the association between antidepressant use and NPC risk, we used SAS version 9.2 (SAS Institute, Cary, NC, USA) to carry out conditional logistic regression models. The different classes of antidepressants and four cumulative dose levels were assessed separately. Corrected ORs were calculated after adjusting for demographic data and confounding factors. The statistical significance of associations was assessed by a P-value <0.05 or a 95% CI.
Results
The study population consisted of 16,957 cases with a diagnosis of NPC and 83,231 controls identified between January 1, 1997 and December 31, 2008. Descriptive demographic data are shown in Table 1. Males had higher risk of NPC (male: 73.09%; female: 26.91% in cases group). Table 2 shows descriptive information related to comorbid mental and physical disorders and medication use. The incidence rates of hypertension and COPD were significantly higher in the cancer group than in the control group.
  | Table 1 Demographic data of cases and controls |
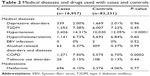  | Table 2 Medical diseases and drugs used with cases and controls |
Results of our primary analyses, after adjusting for age, urbanization, income, depressive disorders, T2DM, alcohol-related disorders, tobacco use disorder, and metformin use, can be found in Table 3. The adjusted OR for cumulative SSRI exposure ≥28 DDD was 0.94 (95% CI: 0.81–1.09) and the adjusted OR for cumulative TCA exposure ≥28 DDD was 1.02 (95% CI: 0.89–1.17). For all classes of antidepressants, antidepressant exposure, regardless of cumulative dose, was not significantly associated with the risk of developing NPC (adjusted OR for cumulative SSRI exposure ≥336 DDD was 1.18 [95% CI: 0.90–1.53]; adjusted OR for cumulative TCA exposure ≥336 DDD was 1.18 [95% CI: 0.80–1.74]).
Discussion
To our knowledge, this is the first population-based nested case–control study to evaluate the association between antidepressant use and the risk of NPC. Results of our primary analyses indicate that there is no association between antidepressant use and the risk of NPC. The lack of a significant association remained after adjusting for different cumulative doses and various classes of antidepressants.
NPC is distinct from other types of cancer in terms of its geographical distribution. A preponderance of etiological studies of NPC have hitherto focused on exposure to EBV, as well as environmental factors and genetic components.13,14 Relatively few studies have evaluated the potential iatrogenic contribution to NPC incidence. Di Maso et al conducted a hospital-based case–control study in Italy, enrolling 198 Caucasian patients with NPC. They reported an inverse association between aspirin use and NPC risk. However, generalization of their results to a larger population is limited by their small sample size (n=3 cases [1.5%] and 27 controls [4.5%] reported regular aspirin use [OR=0.24; 95% CI: 0.07–0.87]).19
It was proposed that antidepressants use is associated with increased risk of cancer, with breast, ovarian, and colon cancers being the most studied malignancies. Moorman et al reported long-term use of SSRIs could increase the risk of breast cancer. However, further studies demonstrated that breast cancer risk was not a concern for female patients who require long-term antidepressant use.20,21 Harlow and Cramer first reported that antidepressants could be associated with an increased risk of ovarian cancer.22 However, later studies indicated that there was no association between risk of ovarian cancer and use of antidepressant drugs.23,24 In colon cancer, Haukka et al reported a bias in the association of antidepressants with increased risk of colon cancer.25 Azmitia reported that serotonin acted as a carcinogen in the development of colorectal cancer due to its role in stimulating cell division.26 However, some other studies have found either no effect on cancer growth or even an antineoplastic effect.23,27,28 Taken together, the results from experimental studies can be summarized as providing inconsistent findings.
Several in vivo studies have investigated the relationship between antidepressant use and oral cancer cells. One study reported that SSRIs (ie, paroxetine and sertraline) induced a dose-dependent inhibition of cell viability and proliferation in two cell lines.29 Similarly, another study reported that in human oral cancer cells, paroxetine induced cytosolic-free Ca2+ concentrations rise by causing phospholipase C-independent Ca2+ release from the endoplasmic reticulum and Ca2+ influx via store-operated Ca2+ channels in a manner regulated by protein kinase C and phospholipase A2. Paroxetine (up to 50 μM) induced human oral cancer cell death in a Ca2+-independent manner.30
The study herein is strengthened by our population-based nested case–control design, utilizing a large, representative database. The information regarding sociodemographic data, diagnostic codes, and medical prescriptions was extracted from NHIRD, reducing the opportunity for selection bias and recall bias. Moreover, confounding factors such as income, urbanization, other medication (ie, metformin) prescribed before the index date, and comorbid medical conditions (ie, depressive disorder, T2DM, hypertension, hypercholesterolemia, EBV infection, alcohol-related disorders, COPD, and tobacco use disorder) were adjusted for in our analyses. Moreover, medical diagnoses were established by a physician using standardized codes of the ICD-9-CM. Finally, we explored the temporal relationship between all classes of antidepressants and NPC, and the cumulative dose effect of antidepressants on the risk of NPC.
Limitations
Notwithstanding the foregoing methodological strengths, interpretation of our study findings needs to take into consideration several methodological limitations. First, several confounding factors such as lifestyle, diet, and smoking were not included in our database. Diet, such as consuming nitrosamine-containing preserved foods, is an important nonviral environmental risk factor for NPC.14 Hsu et al also reported a relationship between smoking and NPC: the longer and heavier the cigarette smoking habit, the higher is the NPC risk.31 Second, using prescription records might overestimate cumulative doses of antidepressant use because drug adherence could not be determined. Further studies analyzing the relationship between antidepressant use, lifestyle factors, and risk of NPC are needed.
Conclusion
Our results indicate that there is no association between prescription of antidepressant drugs and the risk of NPC. Antidepressants are widely prescribed for the treatment of psychiatric and medical disorders, with increasing utilization in some jurisdictions. Moreover, prescription renewal, a proxy of longer-term antidepressant exposure, has been increasing, as has the coprescription of multiple antidepressants. The foregoing pharmacoepidemiological observations provide further impetus for scrutiny of possible tolerability and/or safety concerns associated with greater antidepressant exposure. We did not find any association between variable antidepressant exposure and incident NPC.
Acknowledgments
The authors would also like to thank the Health Information and Epidemiology Laboratory (CLRPG6G0041) for their comments and assistance in data analysis. This study was based on the National Health Insurance Research Database provided by the Central Bureau of National Health Insurance, the Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Disclosure
The study was supported by a grant from the Chiayi Chang Gung Memorial Hospital in Taiwan (CMRPG6E0272, CMRPG6E0273) Dr Chen has been an investigator in clinical trials from Eli Lilly and Janssen and has received travel reimbursements for attending academic conferences from Eli Lilly, Janssen. He also has received speaking honoraria from Pfizer, Eli Lilly, Janssen, Astellas, GlaxoSmithKline, and AstraZeneca. The other authors report no conflicts of interest in this work.
References
Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. 2010;71 (Suppl E1):e04. | ||
Kellner M. Drug treatment of obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):187–197. | ||
Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460. | ||
Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314(17):1818–1831. | ||
Amerio A, Galvez JF, Odone A, Dalley SA, Ghaemi SN. Carcinogenicity of psychotropic drugs: a systematic review of US Food and Drug Administration-required preclinical in vivo studies. Aust N Z J Psychiatry. 2015;49(8):686–696. | ||
Hsieh YH, Chiu WC, Lin CF, et al. Antidepressants and gastric cancer: a nationwide population-based nested case-control study. PLoS One. 2015;10(11):e0143668. | ||
Chan HL, Hsieh YH, Lin CF, et al. Invasive cervical cancer and antidepressants: a nationwide population-based study. Medicine (Baltimore). 2015;94(42):e1866. | ||
Chubak J, Boudreau DM, Rulyak SJ, Mandelson MT. Colorectal cancer risk in relation to antidepressant medication use. Int J Cancer. 2011;128(1):227–232. | ||
Chen VC, Lin CF, Hsieh YH, et al. Hepatocellular carcinoma and antidepressants: a nationwide population-based study. Oncotarget. 2017;8(18):30464–30470. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Young LS, Dawson CW. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):581–590. | ||
Poh SS, Chua ML, Wee JT. Carcinogenesis of nasopharyngeal carcinoma: an alternate hypothetical mechanism. Chin J Cancer. 2016;35(1):9. | ||
Jia WH, Qin HD. Non-viral environmental risk factors for nasopharyngeal carcinoma: a systematic review. Semin Cancer Biol. 2012;22(2):117–126. | ||
Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. | ||
Kinjo T, Kowalczyk P, Kowalczyk M, et al. Desipramine inhibits the growth of a mouse skin squamous cell carcinoma cell line and affects glucocorticoid receptor-mediated transcription. Mol Carcinog. 2009;48(12):1123–1130. | ||
Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. III. Design options. Am J Epidemiol. 1992;135(9):1042–1050. | ||
WHO. Collaborating Centre for Drug Statistics Methodology, ATC Classification Index with DDDs. 2013(2013). Available from: http://www.whocc.no/atc_ddd_publications/atc_ddd_index/. Accessed July 10, 2014. | ||
Di Maso M, Bosetti C, La Vecchia C, et al. Regular aspirin use and nasopharyngeal cancer risk: a case-control study in Italy. Cancer Epidemiol. 2015;39(4):545–547. | ||
Ashbury JE, Levesque LE, Beck PA, Aronson KJ. A population-based case-control study of Selective Serotonin Reuptake Inhibitors (SSRIs) and breast cancer: the impact of duration of use, cumulative dose and latency. BMC Med. 2010;8:90. | ||
Wernli KJ, Hampton JM, Trentham-Dietz A, Newcomb PA. Antidepressant medication use and breast cancer risk. Pharmacoepidemiol Drug Saf. 2009;18(4):284–290. | ||
Harlow BL, Cramer DW. Self-reported use of antidepressants or benzodiazepine tranquilizers and risk of epithelial ovarian cancer: evidence from two combined case-control studies (Massachusetts, United States). Cancer Causes Control. 1995;6(2):130–134. | ||
Wu CS, Lu ML, Liao YT, Lee CT, Chen VC. Ovarian cancer and antidepressants. Psychooncology. 2015;24(5):579–584. | ||
Coogan PF, Rosenberg L, Palmer JR, et al. Risk of ovarian cancer according to use of antidepressants, phenothiazines, and benzodiazepines (United States). Cancer Causes Control. 2000;11(9):839–845. | ||
Haukka J, Sankila R, Klaukka T, et al. Incidence of cancer and antidepressant medication: record linkage study. Int J Cancer. 2010;126(1):285–296. | ||
Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56(5):413–424. | ||
Cosgrove L, Shi L, Creasey DE, Anaya-McKivergan M, Myers JA, Huybrechts KF. Antidepressants and breast and ovarian cancer risk: a review of the literature and researchers’ financial associations with industry. PLoS One. 2011;6(4):e18210. | ||
Frick LR, Rapanelli M. Antidepressants: influence on cancer and immunity? Life Sci. 2013;92(10):525–532. | ||
Gil-Ad I, Zolokov A, Lomnitski L, et al. Evaluation of the potential anti-cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer-xenografted mice. Int J Oncol. 2008;33(2):277–286. | ||
Fang YC, Chou CT, Pan CC, et al. Paroxetine-induced Ca2+ movement and death in OC2 human oral cancer cells. Chin J Physiol. 2011;54(5):310–317. | ||
Hsu WL, Chen JY, Chien YC, et al. Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20-year follow-up study on 9,622 males without family history in Taiwan. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1218–1226. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

