Back to Journals » Drug Design, Development and Therapy » Volume 16
Antidepressant-Like Effect and Mechanism of Ginsenoside Rd on Rodent Models of Depression
Authors Li Y, Wang ML , Zhang B, Fan XX , Tang Q , Yu X, Li LN , Fan AR, Chang HS , Zhang LZ
Received 29 November 2021
Accepted for publication 3 March 2022
Published 28 March 2022 Volume 2022:16 Pages 843—861
DOI https://doi.org/10.2147/DDDT.S351421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yu Li,1,* Mei-Ling Wang,1,* Bo Zhang,1 Xiao-Xu Fan,1 Qin Tang,1 Xue Yu,2 Li-Na Li,2 Ang-Ran Fan,2 Hong-Sheng Chang,1 Lan-Zhen Zhang1
1School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, 102488, People’s Republic of China; 2School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, 102488, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lan-Zhen Zhang; Hong-Sheng Chang, School of Chinese Materia Medica, Beijing University of Chinese Medicine, Northeast Corner of the Intersection of Sunshine South Street and Baiyang East Road, Fangshan District, Beijing, 102488, People’s Republic of China, Tel +86 10 5391 2122, Email [email protected]; [email protected]
Background: There is growing evidence to suggest that ginsenoside Rd (GRd) has a therapeutic effect on depression, but the specific mechanisms behind its activity require further study.
Objective: This study is designed to investigate the antidepressant-like effect and underlying mechanisms of GRd.
Methods: In this study, the behavioral despair mouse model of depression and chronic unpredictable mild stress (CUMS) rat model of depression were established to explore the effects of GRd on depression-like behavior and its underlying mechanisms. Behavioral tests were used to evaluate the replication of animal models and depression-like behaviors. The hypoxia-inducible factor-1α (HIF-1α) blocker 2-methoxyestradiol (2-ME) was injected to determine the role of HIF-1α in the antidepressant-like effect of GRd. In addition, molecular biology techniques were used to determine the mRNA and protein expression of HIF-1ɑ signaling pathway and synaptic plasticity-related regulators, that is synapsin 1 (SYN 1) and postsynaptic density protein 95 (PSD 95). In silico binding interaction studies of GRd with focused target proteins were performed using molecular docking to predict the affinity and optimal binding mode between ligands and receptors.
Results: Our data show that GRd significantly reversed depression-like behavior and promoted mRNA and protein expression of HIF-1ɑ signaling pathway and synaptic plasticity-related regulators. However, the antidepressant-like effect of GRd disappeared upon inhibition of HIF-1α expression following administration of 2-ME. Furthermore, molecular docking results showed that GRd possessed significant binding affinity for HIF-1α, VEGF, and VEGFR-2.
Conclusion: Our results show that GRd exhibits significant antidepressant-like effect and that HIF-1α signaling pathway is a promising target for the treatment of depression.
Keywords: ginsenoside Rd, antidepressant effect, HIF-1α-VEGF signaling pathway, VEGFR-2, synaptic plasticity-related regulators, molecular docking
Introduction
Depression is a mental affective disorder characterized by significant and long-time decreased interest, low mood, slow thinking, activity decline, and loss of appetite, which seriously affects the physical and mental health of patients.1 According to a report from the World Health Organization, depression is predicted to become the main cause of the global burden of disease by 2030.2 At present, the treatment strategy for depression still involves selective serotonin (5-HT) reuptake inhibitors (SSRIs) which have been traditionally used in the clinic. Unfortunately, SSRIs do not meet the criteria for an excellent therapy, including rapid onset of action, high curative rate, and fewer side effects. SSRIs are ineffective in up to 35% of patients and they cause many side effects, such as gastrointestinal adverse reactions (particularly nausea and vomiting) and neurological side effects (especially headaches and tremors).3–5 Moreover, depression has become a serious worldwide public health problem and its complicated pathological mechanisms need to be further explored. Consequently, addressing this unmet need requires further study to develop safe and effective antidepressant drugs and explore innovative mechanisms of action.
Panax ginseng Meyer, a precious traditional Chinese medicine, is indigenous to the Far East countries (most notably China and Korea).6 According to the Pharmacopoeia of the People’s Republic of China (2020), it has the effect of reinforcing vital energy, calming the mind, and boosting cognitive functions. Further, antidepressant was one of the clinical indications of ginseng according to traditional Chinese medicine, with ginseng extract also being reported to improve depression in postmenopausal women.7,8 Modern research has proven that ginsenosides are the active ingredients of ginseng, traditionally used to treat insomnia, heart palpitations, mood disorders, and depression.9–11 Kaixin San has been used clinically for the prevention and treatment of depression, and many studies have shown that ginsenosides are most likely its main medicinal ingredients.12,13 In rodent depression models, ginseng total saponins and monomers, including but not limited to, Rb1, Rg1, and Rg3 have shown great antidepressant activity.14–17 These studies support the hypothesis that ginsenosides may be promising antidepressant drugs.
Naturally occurring ginsenosides have poor oral availability. Researchers generally believe that ginsenosides act as prodrugs in pharmacology when taken orally, and that their metabolites may be responsible for their observed efficacy due to their improved pharmacological activity.18 Ginsenoside Rd (GRd), which is produced by the metabolism of the hydrophilic protopanaxadiol ginsenoside via intestinal microbiota, is one of the most active and abundant components involved in the efficacy of ginseng. GRd has a variety of pharmacological activities, especially in terms of neuroprotection and shows improved efficacy compared to other ginsenosides.19 The potential mechanisms of GRd neuroprotection may be related to anti-inflammation, antioxidant, anti-apoptosis, and the regulation of Ca2+ and nerve factors by modulation of NF-κB, PI3K, and MAPK signaling pathway.20 Recent studies have demonstrated that GRd could improve cognitive impairment caused by chronic stress and play a great antidepressant effect in rodent models of depression.21,22 However, the mechanism by which GRd improves depression is not clear, thereby necessitating further investigation.
Hypoxia-inducible factor-1 (HIF-1), a heterodimer composed of HIF-1α and HIF-1β, plays an important role in the process of cells perceiving and adapting to changes in oxygen partial pressure in the internal environment. As the regulatory subunit of HIF-1, HIF-1α regulates HIF-1 activity through its sensitivity to changes in oxygen concentration.23 There is increasing evidence to suggest that HIF-1α may be a new target to treat depression. Studies have shown that intermittent hypoxia could promote hippocampal neurogenesis in adult rats and exert antidepressant-like effects on a variety of animal models for screening antidepressant activity, which is closely related to the activation of HIF-1α.24 The activation of HIF-1α may mediate synaptic plasticity to exert an antidepressant-like effect, which can be completely blocked when HIF-1α synthesis is inhibited by lentiviral infection with HIF-1α small hairpin RNAs.25 HIF-1α targeting genes vascular endothelial growth factor (VEGF) and erythropoietin (EPO) also have similar effects. VEGF is essential for inducing the rapid antidepressant-like effects, neurotrophic actions, and synaptic effects of ketamine. Peripheral administration of EPO can also produce a strong antidepressant effect. These studies suggest that HIF-1α is a promising therapeutic target for depression.26,27
Many studies have shown that GRd acts on the HIF-1α-VEGF pathway to play multiple positive roles. These include pro-angiogenesis after ischemic stroke and neurogenesis after transient focal cerebral ischemia.28,29 This suggests that GRd may also achieve a therapeutic effect in depression through this signaling pathway. To verify this hypothesis, the behavioral despair mouse model of depression and the chronic unpredictable mild stress (CUMS) rat model of depression were established to explore the antidepressant-like effect and specific mechanisms of GRd. Moreover, GRd was selected as the ligand for focused docking into HIF-1α, VEGF, and VEGFR-2 for molecular docking studies to evaluate protein-ligand binding and to further validate GRd as a potential ligand in other targets for therapeutic intervention in disease.
Materials and Methods
Animals and Drugs
Experiments were performed on adult male ICR mice (SPF grade, 18–22 g) and male Sprague Dawley rats (SPF grade, 180–220 g). All animals were obtained from SiPeiFu (Beijing) Biotechnology Co., Ltd., animal license number: SCXK (Beijing) 2019-0010. Before the actual experiments, animals were allowed one week of adaptive feeding. In addition, all animals were kept in the following standard conditions, including a 12/12 h light/dark cycle (light on 8:00 a.m. and light off 8:00 p.m.), at ambient temperature (21–23°C), humidity range of 30–50%, and adequate standard food and water. All steps involving animals were authorized by the Experimental Animal Ethics Committee of the Academic Committee of Beijing University of Chinese Medicine (project identification code: BUCM-2021082802-3002). The experimental procedures of our study were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
GRd and paroxetine were obtained from Yuanye (purity >98%, Shanghai, China) and 2-Methoxyestradiol (2-ME) was obtained from APE×BIO (Houston, USA).
Animal Study Design
Behavioral Despair Mouse Model of Depression
Forty mice were randomly divided into four groups: control (Con), paroxetine (Par, 10 mg/kg, i.p.), low-dose GRd (L-GRd, 10 mg/kg, i.g.), and high-dose GRd (H-GRd, 20 mg/kg, i.g.). All drugs were administered once a day for 14 days, while mice in the Con group received an equal volume of saline. Behavioral tests were performed on day 14. All mice were intraperitoneally anesthetized with 1% pentobarbital. The hippocampus of each mouse was removed and stored on ice for analysis.
HIF-1α Inhibition
Forty mice were randomly divided into four groups: control (Con), 2-Methoxyestradiol (2-ME), high-dose GRd (H-GRd) and H-GRd+2-ME. Mice in the H-GRd group were intragastrically treated with GRd (20 mg/kg) once daily for 14 days. The GRd dose used was optimal dose identified in Behavioral Despair Mouse Model of Depression. Mice in the GRd+2-ME group were intraperitoneally injected with 2-ME (5 mg/kg) after intragastric administration of GRd. The Con group received equal volumes of saline and mice in the 2-ME group were intraperitoneally injected with 2-ME (5 mg/kg) after receiving saline. Behavioral tests were performed on day 14.
CUMS Rat Model of Depression
Sixty rats were randomly divided into six groups: control (Con), model (CUMS, CUMS+saline), CUMS+Par (CUMS+4.8 mg/kg paroxetine, i.p.), CUMS+L-GRd (CUMS+10 mg/kg GRd, i.g.), CUMS+H-GRd (CUMS+20 mg/kg GRd, i.g.), and H-GRd (20 mg/kg GRd, i.g.). All rats, except those in the Con and H-GRd groups, were exposed to unpredictable mild stressors for 28 days. Subsequently, each group of rats received corresponding drug for 21 days. The behavioral tests including open field tests (OFTs) and sucrose preference tests (SPTs) were performed on day 21 of dosing. All rats were anesthetized by intraperitoneal injection of 1% pentobarbital and the hippocampus of each rat was removed and stored on ice for analysis.
CUMS Protocol
With the exception of the Con and H-GRd groups, rats were exposed to one or two of the following stressors accompanied by single cage breeding for 28 days: deprivation of food and water, reversal of day and night, cage tilt, 4°C ice water swimming, noise, stroboscopic, wet bedding in cages, body restraint, horizontal shaking, and tail pinching. The same stressor was not repeated within a 7 day period to prevent the rats from predicting upcoming stressors.
Forced Swimming Tests
Mice were taken to the testing room to adapt for 1 h before forced swimming tests (FSTs). A single mouse was placed in a cylinder-shaped glass container (height: 25 cm, diameter: 10 cm) filled with tap water (water height: 10 cm, temperature: 24±1°C). When mice were swimming, their tails and limbs could not reach the bottom of container while the tip of their nose was above water to breathe. Containers were separated by an opaque plate to prevent two mice from observing each other. After 2 min of adaptive swimming, the cumulative immobility time of mouse within 4 min was automatically collected and analyzed by behavior analysis system (Etho-Vision XT9, Noldus, Netherlands). The standard criterion for determining the immobility of mice was as follows: mouse stopped struggling and floated on the water, or made only minor movements necessary to keep its head above the water.
OFTs
Animals were taken to the testing room to adapt for 1 h before OFTs. The tests used an opaque square autonomous activity box (mice: 45 cm × 45 cm × 30 cm; rats: 100 cm × 100 cm × 40 cm). The bottom of the box was divided into twenty-five identical squares with nine squares in the middle set as the central area. A single mouse or rat was placed in the center of the autonomous activity box and the behavior analysis system described in Forced Swimming Tests was used to record the movement distance, velocity, frequency of entering the center, and the duration of the stay in the center within 5 min. The environment was kept quiet with stable light during the tests. Before each experiment, the area was cleaned with alcohol to remove all feces and to remove any odor left by previous mice.
SPTs
The SPT is a classic method to assess anhedonia in animals. Briefly, rats were individually placed in cages equipped with two bottles of 1% (w/v) sucrose solution for adaptation training for 24 h. Subsequently, one of the bottles of sucrose solution was replaced with tap water for 24 h. During the third 24 h period, rats were deprived of food and water. Immediately after the third phase, rats had free access to two bottles of solution in the cage for 2 h, one bottle filled with 100 mL of 1% sucrose solution and the other bottle containing 100 mL of tap water. The positions of the two bottles were alternated every hour to prevent positional preference. The consumption of both solutions were recorded and the sucrose preference was calculated as the sucrose preference rate (%): sucrose consumption/(sucrose consumption + tap water consumption) × 100.
Real-Time Fluorescence Quantitative Polymerase Chain Reaction Analysis
To detect mRNA expression of relevant genes, total RNA was first extracted from hippocampal tissue using the Hipure Total RNA Mini Kit (MAGEN) according to the manufacturers instructions. An ultraviolet spectrophotometer (UV-2000, Unico) was used to measure RNA concentration of each sample and RNA samples were maintained on ice to prevent degradation. Reverse transcription was performed using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific) and the Power SYBR Green PCR Master Mix (Invitrogen) according to the manufacturer’s instructions. The following conditions were used on a T100 Thermal Cycler PCR machine (Bio-Rad, USA): 42°C for 1 h, 70°C for 5 min, storage at 4°C. Amplification and quantitative detection were performed in a Real-Time PCR machine (Bio-Rad, USA) using the following protocol: initial denaturation at 95°C for 10 min, denaturation at 95°C for 10s, annealing at 60°C and extension for 30s with a total of 50 amplification cycles. We used the 2−ΔΔCt method to calculate the relative quantitative analysis of the results. All primer sequences are shown in Table 1.
 |
Table 1 Primer Sequences Used for RT-PCR |
Western Blotting
Hippocampal tissue was homogenized in RIPA lysis buffer with protease- and phosphatase inhibitors using an electric homogenizer then lysed on ice for 20 min before centrifugation at 13,000 rpm for 20 min at 4°C. The supernatant was collected and the pellet was discarded to obtain the whole cell lysates. The total protein concentration was determined according to the instructions of a BCA protein quantification kit. A total of 40 µg of protein was separated by electrophoresis on a 5–8% sodium dodecyl sulfate-polyacrylamide gel and then transferred to a 0.45 µm PVDF membrane by wet transfer at 100 V constant voltage for 1.5 h. Subsequently, the membrane was blocked with 5% (w/v) non-fat powdered milk at room temperature for 2 h. After 3×10 min washes in TBST buffer, the membrane was incubated with primary antibody at 4°C overnight and then further washed 3 times before incubation with secondary antibodies at room temperature for 1 h. Blots were developed using an ECL chemiluminescence detection kit. The labeled proteins were visualized using an Azure multifunctional molecular imaging system (C600, Azure, USA).
The primary antibodies and their dilution ratios were as follows: anti-HIF-1α (1:1000, Abcam), anti-VEGF (1:3000, Proteintech), anti-VEGFR-2 (1:800, Proteintech), anti-t-PI3K (1:10,000, Proteintech), anti-p-PI3K (1:500, Abcam), anti-t-Akt (1:5000, Proteintech), anti-p-AKT (1:5000, Proteintech), anti-t-mTOR (1:25,000, Proteintech), anti-p-mTOR (1:1000, Cell Signaling Technology), anti-SYN 1 (1:6000, Proteintech), anti-PSD 95 (1:3000, Proteintech), and anti-α-Tubulin (1:5000, Proteintech). The secondary antibodies and their dilution ratios were as follows: HRP-conjugated affinipure goat anti-rabbit IgG (1:5000, Proteintech) or HRP-conjugated affinipure goat anti-mouse IgG (1:5000, Proteintech).
Molecular Docking
Three-dimensional (3D) structures of focused target proteins including HIF-1α, VEGF, and VEGFR-2 implicated in depression were obtained from the online RCSB Protein Data Bank (PDB; https://www.rcsb.org/). PyMOL 2.4 (Delano Scientific LLC, Italy) was used to remove water molecules and small molecule ligands from protein structure. AutoDock Tools was then used to add hydrogen and atom types, to calculate charge, and finally for conversion into the PDBQ format. A two-dimensional (2D) structure of the small molecule ligand (ie GRd) was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Chem3D 19.0 software was then used to convert this into a 3D structure and to optimize the mechanical structure to minimize energy. AutoDock Tools was used to detect the root of the ligand and select the rotatable bond. Finally, this was saved in a PDBQT format. Molecular docking experiments were performed in Autodock Vina (PyRx 0.8).
Statistical Analysis
Data are expressed as mean ± standard error mean (SEM), and data with normal distribution and homogeneity of variance were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. The Kruskal–Wallis test was used for data with non-normal distribution. Statistical analysis was performed using SPSS 26 (SPSS, Chicago, Illinois, USA) and GraphPad Prism 8.0 (GraphPad Software Inc, San Diego, CA, USA). A p value < 0.05 was considered to be statistically significant.
Results
Effects of GRd on Behavioral Despair Mouse Model of Depression
Effects of GRd on Immobility Time in FSTs
The FST is still one of the most commonly used methods for screening antidepressants in all animal models. Compared to the Con group, the forced swimming immobility time of H-GRd, D-GRd, and Par groups were significantly shorter (*p < 0.05; **p < 0.01) (Figure 1).
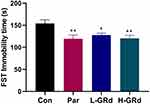 |
Figure 1 The effects of GRd on immobility time in the FST. Data are expressed as mean ± SEM (n = 10/group). *p < 0.05 vs control group; **p < 0.01 vs control group. |
Effects of GRd on OFTs
OFTs were designed to eliminate the interference of immobile time in the FST. There were no significant differences in movement distance, velocity, frequency of entering the center, and the duration of stay in the center between all groups (Figure 2).
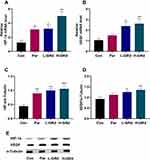
Effects of GRd on HIF-1α-VEGF Signaling Pathway
Changes in the HIF-1α-VEGF signaling pathway were examined in the behavioral despair mouse model of depression to explore potential mechanisms underlying the antidepressant-like effect of GRd. We found that mRNA and protein expression of HIF-1α and VEGF in low and high dose groups of GRd were significantly higher than those in the control group (Figure 3; *p < 0.05; **p < 0.01; ***p < 0.001).
Effects of HIF-1α Inhibitor on the Behavioral Despair Mouse Model of Depression
Effects of 2-ME on Immobility Time in the FST
We used 2-ME, a widely used and effective inhibitor of HIF-1α, to block HIF-1α expression to explore its role in the antidepressant-like effect of GRd. Our data show that the forced swimming immobility time of the GRd group was significantly shorter than that of the control group (Figure 4; **p < 0.01). This was not the case for the GRd+2-ME group mice. These data suggests that (1) the antidepressant-like effect of GRd can be eliminated by 2-ME, and (2) HIF-1α is essential for this effect. In addition, the forced swimming immobility time of mice treated with 2-ME alone was no different from that of the control group, which ruled out the potential of 2-ME-mediated interference.
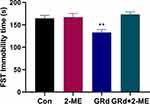 |
Figure 4 The effects of 2-ME on immobility time in the FST. Data are expressed as mean ± SEM (n = 10/group). **p < 0.01 vs control group. |
Effects of 2-ME on OFTs
There were no significant differences in movement distance, velocity, frequency of entering the center, and the duration of stay in the center between all groups (Figure 5).
Effects of GRd on CUMS Depressive Model in Rats
Effects of GRd on SPT
The SPT is a classic test for anhedonia in animals. The sucrose preference of rats in the CUMS group was significantly lower than that of control group (Figure 6; ###p < 0.001). In comparison, the sucrose preference of rats following treatment with low and high doses of GRd was significantly higher than the CUMS group (Figure 6; *p < 0.05, **p < 0.01). There were no differences in sucrose preference between the GRd and paroxetine groups. These results indicate that GRd can reverse CUMS-induced anhedonia in rats.
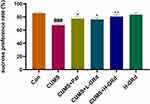 |
Figure 6 The effects of GRd on sucrose preference tests. Data are expressed as mean ± SEM (n = 10/group). ###p < 0.001 vs control group. *p < 0.05 vs CUMS group; **p < 0.01 vs CUMS group. |
Effects of GRd on OFTs
The OFT is also a classic behavioral test. The movement distance, velocity, frequency of entering the center, and the duration of stay in the center of CUMS group were all lower than the control group (Figure 7; ##p < 0.01; ###p < 0.001). However, the observation indexes of OFTs in GRd treatment group were better than in the CUMS group (Figure 7; *p < 0.05; **p < 0.01; ***p < 0.001).
SPT and OFT data showed that the CUMS model was successfully replicated and that GRd reverses CUMS-induced depression-like behavior to exhibit a significant antidepressant-like effect.
Effects of GRd on the HIF-1α-VEGF Signaling Pathway
RT-PCR and Western blotting were used to measure mRNA and protein expression of HIF-1α and VEGF, respectively. The data show that mRNA and protein expression of HIF-1α and VEGF in the CUMS group were significantly downregulated compared to the control group (Figure 8; #p < 0.05; ###p < 0.001). Both low- and high-dose GRd significantly increased mRNA expression of HIF-1α and VEGF, and HIF-1α protein expression in CUMS rats (Figure 8; **p < 0.01; ***p < 0.001). In addition, high-dose GRd significantly increased VEGF protein expression in the CUMS rats (Figure 8; **p < 0.01).
Effects of GRd on mRNA and Protein Expression of VEGFR-2
VEGFR-2, a high-affinity receptor of VEGF, is implicated in depression. We measured mRNA and protein expression of VEGFR-2 to understand whether GRd can act on VEGFR-2 to exert its antidepressant-like effects. The data showed that hippocampal mRNA and protein expression of VEGFR-2 in the CUMS group were severely downregulated compared to the control group (Figure 9; ##p < 0.01). Following treatment with low-dose GRd, VEGFR-2 protein expression increased significantly (Figure 9; ***p < 0.001). Further, treatment with high-dose GRd, significantly increased both mRNA and protein levels of VEGFR-2 (Figure 9; *p < 0.05; ***p < 0.001). These data confirm the involvement of VEGFR-2 in the antidepressant-like effect of GRd.
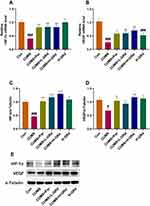
Effects of GRd on PI3K/AKT/mTOR Pathway
We used RT-PCR and Western blotting to explore VEGF- and VEGFR-2-mediated changes in mRNA and protein expression in the PI3K/AKT/mTOR signaling pathway, respectively. Our data showed that the mRNA expression of PI3K, AKT, and mTOR, and the protein expression of p-PI3K, p-AKT, and p-mTOR in the hippocampus of CUMS model rats were significantly lower compared to the control group (Figure 10; #p < 0.05; ##p < 0.01; ###p < 0.001). Moreover, low-dose GRd significantly increased mRNA expression of AKT and p-PI3K, p-AKT, and p-mTOR protein expression in the hippocampus of CUMS rats (Figure 10; *p < 0.05; **p < 0.01; ***p < 0.001). High-dose GRd significantly increased mRNA expression of PI3K, AKT and mTOR, while also upregulating p-PI3K, p-AKT, and p-mTOR protein expression (Figure 10; *p < 0.05; **p < 0.01; ***p < 0.001). Herein, we confirm the involvement of the PI3K/AKT/mTOR signaling pathway in GRd-induced antidepressant-like effects.
Effects of GRd on Synaptic Plasticity-Related Regulators
We also sought to understand whether GRd exerts an antidepressant-like effect by regulating synaptic plasticity-related regulators (ie SYN 1, PSD 95). We found that compared to the control group, SYN 1 and PSD 95 protein expression were significantly reduced (Figure 11; ##p < 0.01; ###p < 0.01). This could be reversed by low- and high-dose GRd (Figure 11; *p < 0.05; **p < 0.01; ***p < 0.001). These data suggest that GRd may exert its antidepressant-like effects by regulating the expression of synapse-related regulatory factors.
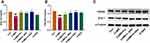
Molecular Docking of GRd with HIF-1α, VEGF, and VEGFR-2
A negative binding energy between a ligand and receptor is generally suggestive of spontaneous binding. The smaller the binding energy, the greater the docking activity and the greater the possibility of action. A binding energy below −5 kcal/mol is indicative of strong protein-ligand binding. Our molecular docking data suggest that the binding energy between GRd and HIF-1α, VEGF, VEGFR-2 were all less than −7 kcal/mol (−7.2, −8.3, −7.8 kcal/mol, respectively). This is indicative of a high likelihood of spontaneous binding. PyMOL was used to visualize these modelled binding data. The number of hydrogen bonds formed between GRd and HIF-1α, VEGF, and VEGFR-2 are 7, 7, and 10, respectively. The amino acid residues involved in these interactions are shown in Table 2. Further, molecular docking patterns of GRd with target proteins are shown in Figure 12.
 |
Table 2 Binding Affinity Between GRd and Protein Targets |
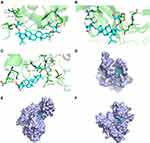 |
Figure 12 Molecular docking patterns of GRd with target proteins. Molecular docking patterns of GRd with target proteins such as HIF-1α (A and D); VEGF (B and E); VEGFR-2 (C and F). |
Discussion
Many ginsenosides are not easily absorbed from the intestine into the blood. Therefore, these components require metabolism by the microbiota in the gastrointestinal tract to be broken down into more easily absorbed compounds. Studies have shown that the antidepressant effect of red ginseng depends on the metabolite GRd being absorbed into the blood. Moreover, GRd has the highest absorption rate in the blood following oral administration of red ginseng in human and mice.22 Our previous studies have shown that Ginsenoside Rb1 (GRb1) induces antidepressant activity. GRd, a metabolite of GRb1, which is produced by removal of a β-D-glucose, was also identified in the blood, brain, feces, and urine of normal and depressed mice after administration of GRb1. In addition, our network pharmacological analysis results showed that GRd is implicated in antidepressant activity.30
The behavioral despair mouse model of depression is a proven depression model which places animals in irresistible adversity to breed despair. The immobility of animals after forced swimming stress is sensitive to antidepressants, making it an ideal behavioral experiment for preliminarily testing of the pharmacodynamics of antidepressants.31,32 Compared to the control group, both high and low doses of GRd can significantly shorten the forced swimming immobility time. This suggests that GRd improves the behavioral despair of mice and exerts a significant antidepressant-like effect.
The CUMS depression model simulates unpredictable stress events and induces various long-term physical, behavioral, neurochemical, and neuroendocrine changes, similar to those observed in depressed patients.33,34 Exposure to CUMS can reduce the movement distance, velocity, frequency of entering the center, the duration of stay in the center in OFTs, and the sucrose preference rate in SPTs, which shows that CUMS depression model is successfully reproduced. Both high and low doses of GRd reversed the depression-like behavior of CUMS rats and showed a significant antidepressant effect.
HIF-1, a base-helix-loop-helix PAS heterodimer composed of active subunit HIF-1α and constitutive subunit HIF-1β, mediates the adaptive response of cells to hypoxia. The transcriptional activity and protein stability of HIF-1α are extremely sensitive to changes in oxygen concentration. Moreover, HIF-1α is crucial for maintaining the integrity and function of HIF-1.35 Under conditions of hypoxia, HIF-1α binds to hypoxia response elements (HREs) on a variety of target genes to induce transcription of target genes. Many studies have shown that HIF-1α, not only in response to hypoxic stress but also in response to severe psychoemotional stress, is a promising target for the treatment of depression. HIF-1α prevents oxygen homeostasis disorders, promotes neuroplasticity, and exerts neuroprotective effects by activating a series of target genes involved in regulation of angiogenesis, vasomotor control, energy and ion metabolism, erythropoiesis, and apoptosis. Triggering these protective mechanisms may have a beneficial impact on the depression, thereby preventing the development of a depressive state caused by severe psychological and emotional stress.36–38
Recently, many studies have shown that VEGF, a secreted angiogenic mitogen regulated by HIF-1α, plays a beneficial role in the treatment of depression.39,40 Long-term stress decreases cell proliferation near the vascular system and expression of VEGF. Decreased expression of VEGF can be seen in the prefrontal cortex and hippocampus of patients with chronic stress-induced major depressive disorder. Further, VEGF protein expression in the cerebrospinal fluid of individuals who have attempted suicide is significantly lower compared to healthy people.41–43 In comparison, intracerebroventricular infusion of VEGF provides an exceedingly effective antidepressant-like effect.44 This antidepressant effect is associated with the ability of VEGF to improve cognitive function, promote neurogenesis, and facilitate hippocampal synaptic plasticity.45–48 Comprehensively, activation of the HIF-1α-VEGF signaling pathway may be implicated in the treatment of depression, which is highly consistent with our experimental results. Compared to the CUMS group, high and low doses of GRd can significantly increase mRNA and protein expression of hippocampal HIF-1α and VEGF in both the behavioral despair mouse model of depression and the rat CUMS model.
Intriguingly, 2-ME, a natural metabolite of estradiol and an effective HIF-1α inhibitor, inhibits the transcriptional activity and protein expression of HIF-1α by inducing microtubule depolymerization and the production of reactive oxygen species.49,50 In our HIF-1α inhibition experiment, the forced swimming immobility time of mice treated with 2-ME following oral administration of GRd was not shorter than the control group. This indicates that the antidepressant effect of GRd can be blocked by 2-ME, further confirming that HIF-1α is essential for the antidepressant effect of GRd.
The antidepressant effect of GRd benefits from the increased expression of hippocampal the synaptic plasticity-related regulators, SYN 1 and PSD 95. There is increasing evidence to suggest that synaptic plasticity disorder may be a pathological mechanism involved depression. Moreover, an improvement in synaptic plasticity plays a critical role in the treatment and prognosis of depression.51–53 Synaptic plasticity is generally coordinated with the synapse-associated proteins, mainly including presynaptic SYN 1 and postsynaptic PSD 95. SYN 1, a neuron-specific phosphoprotein associated with synapses, is distributed in almost all nerve terminals and located on the surface of the presynaptic vesicle membrane. SYN 1 regulates the transportation and circulation of synaptic vesicles to promote neurodevelopment, neuronal information transmission, and synaptic plasticity.54–56 PSD 95, a protein located on the cytoplasmic side of the postsynaptic membrane and related to synaptic signal transmission, plays a significant role in regulating neuron survival and synaptic function. Further, PSD 95 is widely involved in the onset and treatment of cognitive and emotional related mental diseases.57,58 Many studies have shown that protein expression of SYN 1 and PSD 95 are decreased in the prefrontal cortex of patients with major depression. A decrease of presynaptic SYN 1 and postsynaptic PSD 95 in the hippocampus and prefrontal cortex due to chronic stress may lead to depressive behavior and a severe decline in cognitive function in rodents.59,60 Conversely, increased SYN 1 and PSD 95 expression can improve depression.61,62 Consistent with our findings, GRd greatly reversed the significant decrease in SYN 1 and PSD 95 protein levels in the hippocampus of CUMS rats. This shows that the antidepressant-like effect of GRd is linked to the expression of hippocampal synaptic plasticity-related regulators.
There is a close correlation between the activation of the HIF-1α-VEGF signaling pathway and increased expression of synaptic plasticity-related regulatory factors. Both are beneficial for improving depression.63–65 Our results show that the activation of the HIF-1α-VEGF signaling pathway may promote the expression of synaptic plasticity-related regulatory factors through regulation of the PI3K-AKT-mTOR signaling transduction pathway mediated by vascular endothelial growth factor receptor 2 (VEGFR-2, also known as Flk-1). The downstream signal transduction molecular mechanism of VEGF is complex. VEGFR-2, a high-affinity receptor tyrosine kinase of VEGF, which is highly expressed in the rodent hippocampus, can activate the PI3K signaling pathway to stimulate cell proliferation and survival.66,67 VEGF-Flk-1 signaling brings into play a considerable role for synaptic plasticity in hippocampal dependent processes, such as learning and memory, and regulating synaptic transmission. These effects are important in the behavioral responses of animal models receiving multiple antidepressants and can, therefore, be used as valuable predictors of antidepressant activity.68–71
Mammalian target of rapamycin (mTOR), one of the downstream signaling molecules of PI3K, is activated and regulated by the PI3K-AKT pathway, which is a pivotal target for glutamatergic and cholinergic drugs which exert rapid antidepressant effects.72–74 Studies have shown that the mTOR signal is closely related to synaptic plasticity. When the signal is stimulated, it will rapidly increase the expression of synaptic proteins (eg PSD 95 and SYN 1) and the number of spinal synapses,75–77 which is consistent with our research results. After CUMS rats were subjected to chronic stress, we found that mRNA and protein expression of VEGFR-2, PI3K, AKT, and mTOR were significantly reduced. These can all be reversed by GRd.
In recent years, molecular docking has become an important technology in the field of computer-aided drug design. This method is a theoretical simulation method for studying the interactions between molecules (eg ligands and receptors) and predicting their binding mode(s) and affinity.78 Our molecular docking studies revealed additional insights into the effects of GRd on HIF-1α signaling pathway related target proteins for therapeutic intervention in depression. In the present study, a molecular docking study of GRd (ligand) was performed against focused target proteins including HIF-1α, VEGF, and VEGFR-2. Our data reveal that GRd docks well into HIF-1α, VEGF, and VEGFR-2 with docking scores of −7.2, −7.8, and −8.3 kcal/mol, respectively. This suggests that all three proteins may have a high binding affinity for GRd. We also analyzed the hydrogen bonds between GRd and the target protein. The number of hydrogen bonds formed by GRd with HIF-1α, VEGF, and VEGFR-2 were 7, 7 and 10, respectively. The details of the binding amino acid residues are shown in Table 2. In addition, the molecular docking patterns of GRd with the target protein are shown in Figure 12. The modelled antidepressant mechanism of GRd is shown in Figure 13.
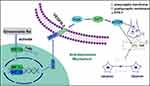 |
Figure 13 The antidepressant mechanism of GRd. |
Conclusion
Our data indicate that GRd exhibits a significant antidepressant-like effect. The observed beneficial effects may be attributed to the activation of the HIF-1α-VEGF signaling pathway, VEGFR-2-mediated PI3K-AKT-mTOR signaling pathway, and increased expression of the synaptic plasticity-related proteins, SYN 1 and PSD 95.
Abbreviations
2-ME, 2-methoxyestradiol; CUMS, chronic unpredictable mild stress; EPO, erythropoietin; FST, forced swimming tests; GRd, ginsenoside Rd; HIF-1α, hypoxia-inducible factor-1α; HREs, hypoxia response elements; mTOR, mammalian target of rapamycin; OFT, open field tests; PSD 95, postsynaptic density protein 95; SPT, sucrose preference tests; SSRIs, selective serotonin reuptake inhibitors; SYN 1, synapsin 1; VEGF, vascular endothelial growth factor; VEGFR-2, vascular endothelial growth factor receptor 2.
Institutional Review Board Statement
The study was approved by The Experimental Animal Ethics Committee of the Academic Committee of Beijing University of Chinese Medicine (project identification code: BUCM-2021082802-3002).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2017YFC1702104.
Disclosure
The authors report no conflicts of interest in this work.
References
1. LeMoult J, Gotlib IH. Depression: a cognitive perspective. Clin Psychol Rev. 2019;69:51–66. doi:10.1016/j.cpr.2018.06.008
2. World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization;2017.
3. Edwards JG, Anderson I. Systematic review and guide to selection of selective serotonin reuptake inhibitors. Drugs. 1999;57(4):507–533. doi:10.2165/00003495-199957040-00005
4. Brambilla P, Cipriani A, Hotopf M, Barbui C. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69–77. doi:10.1055/s-2005-837806
5. Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101–112. doi:10.4068/cmj.2018.54.2.101
6. Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16:S3–S5. doi:10.3346/jkms.2001.16.S.S3
7. Wang GL, He ZM, Zhu HY, et al. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng C.A. Meyer. J Ethnopharmacol. 2017;204:118–124. doi:10.1016/j.jep.2017.04.009
8. Kennedy DO, Scholey AB. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75(3):687–700. doi:10.1016/S0091-3057(03)00126-6
9. Kim Y, Cho SH. The effect of ginsenosides on depression in preclinical studies: a systematic review and meta-analysis. J Ginseng Res. 2021;45(3):420–432. doi:10.1016/j.jgr.2020.08.006
10. Ratan ZA, Haidere MF, Hong YH, et al. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 2021;45(2):199–210. doi:10.1016/j.jgr.2020.02.004
11. Yu HL, Fan CQ, Yang LJ, et al. Ginsenoside Rg1 prevents chronic stress-induced depression-like behaviors and neuronal structural plasticity in rats. Cell Physiol Biochem. 2018;48(6):2470–2482. doi:10.1159/000492684
12. Zhu Y, Duan X, Cheng X, et al. Kai-Xin-San, a standardized traditional Chinese medicine formula, up-regulates the expressions of synaptic proteins on hippocampus of chronic mild stress induced depressive rats and primary cultured rat hippocampal neuron. J Ethnopharmacol. 2016;193:423–432. doi:10.1016/j.jep.2016.09.037
13. Wang J, Zhou XJ, Hu Y, et al. Research progress on pharmacodynamic material basis and pharmacological action mechanism of Kai-Xin-San. Chin Tradit Herb Drugs. 2020;51(18):4780–4788.
14. Dang H, Chen Y, Liu X, et al. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1417–1424. doi:10.1016/j.pnpbp.2009.07.020
15. Zhang JH, Yang HZ, Su H, et al. Berberine and Ginsenoside Rb1 ameliorate depression-like behavior in diabetic rats. Am J Chin Med. 2021;49(5):1195–1213. doi:10.1142/S0192415X21500579
16. Li Y, Wang L, Wang P, et al. Ginsenoside-Rg1 rescues stress-induced depression-like behaviors via suppression of oxidative stress and neural inflammation in rats. Oxid Med Cell Longev. 2020;2020:2325391. doi:10.1155/2020/2325391
17. Zhou T, Sun L, Yang S, et al. 20(S)-Ginsenoside Rg3 protects kidney from diabetic kidney disease via renal inflammation depression in diabetic rats. J Diabetes Res. 2020;2020:7152176. doi:10.1155/2020/7152176
18. Xu J, Chen HB, Li SL. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med Res Rev. 2017;37(5):1140–1185. doi:10.1002/med.21431
19. Liu JF, Yan XD, Qi LS, et al. Ginsenoside Rd attenuates Aβ25-35-induced oxidative stress and apoptosis in primary cultured hippocampal neurons. Chem Biol Interact. 2015;239:12–18. doi:10.1016/j.cbi.2015.06.030
20. Chen YY, Liu QP, An P, et al. Ginsenoside Rd: a promising natural neuroprotective agent. Phytomedicine. 2021;95:153883. doi:10.1016/j.phymed.2021.153883
21. Wang HX, Jiang N, Lv JW, Huang H, Liu XM. Ginsenoside Rd reverses cognitive deficits by modulating BDNF-dependent CREB pathway in chronic restraint stress mice. Life Sci. 2020;258:118107. doi:10.1016/j.lfs.2020.118107
22. Han SK, Joo MK, Kim JK, Jeung W, Kang H, Kim DH. Bifidobacteria-fermented red ginseng and its constituents Ginsenoside Rd and protopanaxatriol alleviate anxiety/depression in mice by the amelioration of gut dysbiosis. Nutrients. 2020;12(4):901. doi:10.3390/nu12040901
23. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88(4):1474–1480. doi:10.1152/jappl.2000.88.4.1474
24. Zhu XH, Yan HC, Zhang J, et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30(38):12653–12663. doi:10.1523/JNEUROSCI.6414-09.2010
25. Huang YF, Yang CH, Huang CC, Hsu KS. Vascular endothelial growth factor-dependent spinogenesis underlies antidepressant-like effects of enriched environment. J Biol Chem. 2012;287(49):40938–40955. doi:10.1074/jbc.M112.392076
26. Kenwood MM, Roseboom PH, Oler JA, Kalin NH. New insights into the mechanisms of Ketamine’s antidepressant effects: understanding the role of VEGF in mediating plasticity processes. Am J Psychiatry. 2019;176(5):333–335. doi:10.1176/appi.ajp.2019.19030282
27. Hemani S, Lane O, Agarwal S, Yu SP, Woodbury A. Systematic review of erythropoietin (EPO) for neuroprotection in human studies. Neurochem Res. 2021;46(4):732–739. doi:10.1007/s11064-021-03242-z
28. Zeng LT, Shi M, Hui J, Hao MH, Weng DD, Han JL. Ginsenoside Rd promotes angiogenesis of rat after cerebral ischemic stroke through PI3K/AKT/mTOR/Hif-1α pathway. Chin J Neurol. 2015;31(06):686–692.
29. Liu XY, Zhou XY, Hou JC, et al. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol Sin. 2015;36(4):421–428. doi:10.1038/aps.2014.156
30. Liang W, Liu Y, Zhou K, et al. Ginsenoside Rb1 prevents lipopolysaccharide-induced depressive-like behavior by inhibiting inflammation and neural dysfunction and F2 elicits a novel antidepressant-like effect: a metabolite-based network pharmacology study. J Ethnopharmacol. 2021;282:114655. doi:10.1016/j.jep.2021.114655
31. Zhang B, Wang PP, Hu KL, et al. Antidepressant-like effect and mechanism of action of Honokiol on the mouse lipopolysaccharide (LPS) depression model. Molecules. 2019;24:11.
32. Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177(3):245–255. doi:10.1007/s00213-004-2048-7
33. Liu XJ, Li ZY, Li ZF, et al. Urinary metabonomic study using a CUMS rat model of depression. Magn Reson Chem. 2012;50(3):187–192. doi:10.1002/mrc.2865
34. Peng Z, Zhang C, Yan L, et al. EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippcampal apoptosis signaling in a chronic stress-induced rat model of depression. Int J Mol Sci. 2020;21(5). doi:10.3390/ijms21051769.
35. Jiang Q, Geng X, Warren J, et al. Hypoxia inducible factor-1α (HIF-1α) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience. 2020;448:126–139. doi:10.1016/j.neuroscience.2020.09.036
36. Baranova KA, Mironova VI, Rybnikova EA, Samoilov MO. Characteristics of the transcription factor HIF-1α expression in the rat brain during the development of a depressive state and the antidepressive effects of hypoxic preconditioning. Neurocheml J. 2010;4(1):35–40. doi:10.1134/S1819712410010071
37. Li G, Zhao M, Cheng X, et al. FG-4592 improves depressive-like behaviors through HIF-1-mediated neurogenesis and synapse plasticity in rats. Neurotherapeutics. 2020;17(2):664–675. doi:10.1007/s13311-019-00807-3
38. Kang I, Kondo D, Kim J, et al. Elevating the level of hypoxia inducible factor may be a new potential target for the treatment of depression. Med Hypotheses. 2021;146:110398. doi:10.1016/j.mehy.2020.110398
39. Valiuliene G, Valiulis V, Dapsys K, et al. Brain stimulation effects on serum BDNF, VEGF, and TNF alpha in treatment-resistant psychiatric disorders. Eur J Neurosci. 2021;53(11):3791–3802. doi:10.1111/ejn.15232
40. Amidfar M, Reus GZ, de Moura AB, Quevedo J, Kim YK. The role of neurotrophic factors in pathophysiology of major depressive disorder. Adv Exp Med Biol. 2021;1305:257–272.
41. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi:10.1016/j.biopsych.2006.02.013
42. Isung J, Aeinehband S, Mobarrez F, et al. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry. 2012;2:e196. doi:10.1038/tp.2012.123
43. Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21(5):1304–1314. doi:10.1111/j.1460-9568.2005.03951.x
44. Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104(11):4647–4652. doi:10.1073/pnas.0610282104
45. Castillo MFR, Cohen A, Edberg D, et al. Vascular endothelial growth factor in bipolar depression: a potential biomarker for diagnosis and treatment outcome prediction. Psychiatry Res. 2020;284:112781. doi:10.1016/j.psychres.2020.112781
46. Sideromenos S, Lindtner C, Zambon A, Horvath O, Berger A, Pollak DD. VEGF treatment ameliorates depression-like behavior in adult offspring after maternal immune activation. Cells. 2020;9(4):1048. doi:10.3390/cells9041048
47. Viikki M, Anttila S, Kampman O, et al. Vascular endothelial growth factor (VEGF) polymorphism is associated with treatment resistant depression. Neurosci Lett. 2010;477(3):105–108. doi:10.1016/j.neulet.2010.04.039
48. Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides. 2012;46(1):1–10. doi:10.1016/j.npep.2011.05.005
49. Khan M, Dhammu TS, Baarine M, et al. GSNO promotes functional recovery in experimental TBI by stabilizing HIF-1α. Behav Brain Res. 2018;340:63–70. doi:10.1016/j.bbr.2016.10.037
50. Khan M, Dhammu TS, Matsuda F, et al. Promoting endothelial function by S-nitrosoglutathione through the HIF-1alpha/VEGF pathway stimulates neurorepair and functional recovery following experimental stroke in rats. Drug Des Devel Ther. 2015;9:2233–2247. doi:10.2147/DDDT.S77115
51. Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11(8):1169–1180. doi:10.1017/S1461145708009309
52. Liu Y, Lan N, Ren J, et al. Orientin improves depression-like behavior and BDNF in chronic stressed mice. Mol Nutr Food Res. 2015;59(6):1130–1142. doi:10.1002/mnfr.201400753
53. Shen C, Cao K, Cui S, et al. SiNiSan ameliorates depression-like behavior in rats by enhancing synaptic plasticity via the CaSR-PKC-ERK signaling pathway. Biomed Pharmacother. 2020;124:109787. doi:10.1016/j.biopha.2019.109787
54. Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989;108(1):111–126. doi:10.1083/jcb.108.1.111
55. Xu QQ, Huang SS, Song MK, et al. Synaptic mutant huntingtin inhibits synapsin-1 phosphorylation and causes neurological symptoms. J Cell Biol. 2013;202(7):1123–1138. doi:10.1083/jcb.201303146
56. Tang J, Lu L, Wang Q, et al. Crocin reverses depression-like behavior in Parkinson disease mice via VTA-mPFC pathway. Mol Neurobiol. 2020;57(7):3158–3170. doi:10.1007/s12035-020-01941-2
57. Dore K, Malinow R. Elevated PSD-95 blocks ion-flux independent LTD: a potential new role for PSD-95 in synaptic plasticity. Neuroscience. 2021;456:43–49. doi:10.1016/j.neuroscience.2020.02.020
58. Ma X, Zhu Z, Guo S, Duan J. The effect of deoxyschizandrin on chronic unpredictable mild stress-induced depression. Biotechnol Appl Biochem. 2021;68(1):52–59. doi:10.1002/bab.1893
59. Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibanez JM, Crespo C, Nacher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci Lett. 2012;530(1):97–102. doi:10.1016/j.neulet.2012.09.032
60. Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):70–75. doi:10.1016/j.pnpbp.2008.10.005
61. Shao S, Yao D, Li S, et al. N-cadherin regulates GluA1-mediated depressive-like behavior in adolescent female rat offspring following prenatal stress. Neuroendocrinology. 2021. doi:10.1159/000518383
62. Methiwala HN, Vaidya B, Addanki VK, Bishnoi M, Sharma SS, Kondepudi KK. Gut microbiota in mental health and depression: role of pre/pro/synbiotics in their modulation. Food Funct. 2021;12(10):4284–4314. doi:10.1039/D0FO02855J
63. Sun P, Wei S, Wei X, et al. Anger emotional stress influences VEGF/VEGFR2 and its induced PI3K/AKT/mTOR signaling pathway. Neural Plast. 2016;2016:4129015. doi:10.1155/2016/4129015
64. Zhang N, Xing M, Wang Y, Tao H, Cheng Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience. 2015;311:284–291. doi:10.1016/j.neuroscience.2015.10.038
65. Tillo M, Ruhrberg C, Mackenzie F. Emerging roles for semaphorins and VEGFs in synaptogenesis and synaptic plasticity. Cell Adh Migr. 2012;6(6):541–546. doi:10.4161/cam.22408
66. Cao Y, Ye Q, Zhuang M, et al. Ginsenoside Rg3 inhibits angiogenesis in a rat model of endometriosis through the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway. PLoS One. 2017;12(11):e0186520. doi:10.1371/journal.pone.0186520
67. Roslavtceva VV, Salmina AB, Prokopenko SV, Pozhilenkova EA, Kobanenko IV, Rezvitskaya GG. The role of vascular endothelial growth factor in the regulation of development and functioning of the brain: new target molecules for pharmacotherapy. Biomed Khim. 2016;62(2):124–133. doi:10.18097/PBMC20166202124
68. Wu KW, Mo JL, Kou ZW, et al. Neurovascular interaction promotes the morphological and functional maturation of cortical neurons. Front Cell Neurosci. 2017;11:290. doi:10.3389/fncel.2017.00290
69. Schmidt HD, Banasr M, Duman RS. Future antidepressant targets: neurotrophic factors and related signaling cascades. Drug Discov Today Ther Strateg. 2008;5(3):151–156. doi:10.1016/j.ddstr.2008.10.003
70. Sun L, Sun Q, Qi J. Adult hippocampal neurogenesis: an important target associated with antidepressant effects of exercise. Rev Neurosci. 2017;28(7):693–703. doi:10.1515/revneuro-2016-0076
71. Nowacka-Chmielewska MM, Paul-Samojedny M, Bielecka-Wajdman AM, Barski JJ, Obuchowicz E. Alterations in VEGF expression induced by antidepressant drugs in female rats under chronic social stress. Exp Ther Med. 2017;13(2):723–730. doi:10.3892/etm.2017.4022
72. Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi:10.3389/fnmol.2011.00051
73. Pazini FL, Cunha MP, Rosa JM, et al. Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone via PI3K/Akt/mTOR pathway. Mol Neurobiol. 2016;53(10):6818–6834. doi:10.1007/s12035-015-9580-9
74. Neis VB, Moretti M, Rosa PB, et al. The involvement of PI3K/Akt/mTOR/GSK3beta signaling pathways in the antidepressant-like effect of AZD6765. Pharmacol Biochem Behav. 2020;198:173020. doi:10.1016/j.pbb.2020.173020
75. Xia B, Huang X, Sun G, Tao W. Iridoids from Gardeniae fructus ameliorates depression by enhancing synaptic plasticity via AMPA receptor-mTOR signaling. J Ethnopharmacol. 2021;268:113665. doi:10.1016/j.jep.2020.113665
76. Um SW, Kim MJ, Leem JW, Bai SJ, Lee BH. Pain-relieving effects of mTOR inhibitor in the anterior cingulate cortex of neuropathic rats. Mol Neurobiol. 2019;56(4):2482–2494. doi:10.1007/s12035-018-1245-z
77. Ryskalin L, Limanaqi F, Frati A, Busceti CL, Fornai F. mTOR-related brain dysfunctions in neuropsychiatric disorders. Int J Mol Sci. 2018;19:8. doi:10.3390/ijms19082226
78. Kumar S, Saini V, Maurya IK, et al. Design, synthesis, DFT, docking studies and ADME prediction of some new coumarinyl linked pyrazolylthiazoles: potential standalone or adjuvant antimicrobial agents. PLoS One. 2018;13(4):e0196016. doi:10.1371/journal.pone.0196016
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.