Back to Journals » OncoTargets and Therapy » Volume 8
Anticoagulant therapy for venous thromboembolism detected by Doppler ultrasound in patients with metastatic colorectal cancer receiving bevacizumab
Authors Suenaga M , Mizunuma N, Shinozaki E , Matsusaka S, Ozaka M, Ogura M , Chin K , Yamaguchi T
Received 13 October 2014
Accepted for publication 1 December 2014
Published 27 January 2015 Volume 2015:8 Pages 243—249
DOI https://doi.org/10.2147/OTT.S75722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Mitsukuni Suenaga, Nobuyuki Mizunuma, Eiji Shinozaki, Satoshi Matsusaka, Masato Ozaka, Mariko Ogura, Keisho Chin, Toshiharu Yamaguchi
Department of Gastroenterology, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan
Background: Doppler ultrasound imaging is useful for management of venous thromboembolism associated with a subclavicular implantable central venous access system in patients receiving bevacizumab (Bev). We investigated the efficacy and safety of our anticoagulant regimen based on Doppler findings.
Methods: Patients aged ≤75 years with metastatic colorectal cancer, no history of thromboembolism, and no prior use of Bev received chemotherapy plus Bev. Doppler ultrasound imaging of the deep venous system to detect thrombosis was performed after the first course of Bev and repeated after the third course in patients with asymptomatic thrombosis. Indications for anticoagulant therapy in patients with asymptomatic thrombosis were as follows: enlarging thrombus (E), thrombus >40 mm in diameter (S), thrombus involving the superior vena cava (C), and decreased blood flow (V).
Results: Among 79 patients enrolled in this study, asymptomatic thrombosis was detected in 56 patients (70.9%) by Doppler ultrasound imaging after the first course of Bev and there was no thrombus in 23 patients (29.1%). Of these 56 patients, 11 (19.6%) received anticoagulant therapy with warfarin, including eight after the first course and three after follow-up imaging. S + V was observed in four of 11 patients (36.4%), as well as V in two (18.2%), S + V + C in one (9.1%), E + S + V in one (9.1%), E + C in one (9.1%), E in one (9.1%), and C in one (9.1%). All patients resumed chemotherapy, including seven who resumed Bev. Improvement or stabilization of thrombi was achieved in ten patients (90.9%). Only one patient had symptomatic thromboembolism. Mild bleeding due to anticoagulant therapy occurred in six patients (54.5%), but there were no treatment-related severe adverse events or deaths. Severe thromboembolism was not observed in the other 68 patients.
Conclusion: Our anticoagulant protocol for asymptomatic thrombosis detected by Doppler ultrasound imaging was effective at preventing severe thromboembolism during continued treatment with Bev.
Keywords: venous thromboembolism, Bev, anticoagulant therapy, colorectal cancer
Introduction
Bevacizumab (Bev), a recombinant humanized monoclonal antibody directed against vascular endothelial growth factor (VEGF), is a key biological agent for metastatic colorectal cancer that has a positive impact on survival.1–5 A randomized controlled trial showed that continuing treatment with Bev after disease progression achieves significant improvement of overall survival and progression-free survival,5 suggesting that it could be a new second-line treatment strategy for metastatic colorectal cancer. On the other hand, adverse events such as arterial thromboembolism, venous thromboembolism (VTE), and pulmonary embolism (PE) have been demonstrated to increase when Bev is added to chemotherapy.1,3,6,7 A meta-analysis demonstrated that Bev is a risk factor for VTE, and indications for anticoagulant therapy were proposed.7 An implantable central venous access system (CVAS) is often used in cancer patients for chemotherapy or palliative care, but is associated with an increased risk of deep VTE. In addition, VTE is associated with severe PE.8–10 Although prophylactic anticoagulant therapy for severe thromboembolism has been investigated, routine prophylaxis for CVAS-associated VTE is not recommended, especially in patients with a risk of bleeding.11–13
We aimed to establish appropriate screening methods for symptomatic or asymptomatic VTE and indications for anticoagulant therapy in patients with CVAS. We previously reported the efficacy of Doppler ultrasound (DU) imaging for management of VTE in patients receiving chemotherapy combined with Bev.14 Our findings suggested that DU is useful for management of VTE associated with a subclavicular implantable CVAS in patients treated with Bev, while D-dimer or other baseline laboratory data were not useful as prognostic factors. In addition, we found that anticoagulant therapy was beneficial for progressive asymptomatic thrombosis, but the indications for such therapy need to be established to avoid severe thromboembolism including PE.
Accordingly, the objectives of the present study were to assess the validity of our indications for anticoagulant therapy in patients with asymptomatic thrombosis, and to investigate the efficacy and safety of our anticoagulant regimen based on DU findings.
Patients and methods
Study design
This was a prospective cohort study conducted at a single institution. Patients were enrolled from December 2007 and all patients provided written informed consent before commencing treatment. The study protocol was approved by our institutional review board. An outline of the study design (including the timing of DU) is shown in Figure 1; it was the same as in our previous study.14 DU was performed to assess the deep venous system in the upper extremities on the CVAS side. To detect incipient thrombosis during chemotherapy, DU was first performed after the first course of Bev. If asymptomatic thrombus was detected, follow-up evaluation was be performed after the third course of Bev. The location and dimensions of any thrombus were assessed, as well as blood flow and collateral flow. A radiologist at our center performed DU and evaluated the findings. Pretreatment DU was also performed between CVAS implantation and initiation of Bev in patients who consented to this additional examination.
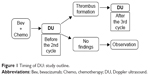  | Figure 1 Timing of DU: study outline. |
Patients
Patients could be enrolled if they conformed to the following criteria: histological confirmation of colorectal cancer; presence of advanced metastatic colorectal cancer; age ≤75 years; Eastern Cooperative Oncology Group performance status (PS) of 0 or 1; no history of thromboembolic events; no prior use of Bev; no risk factors for bleeding; and no hypertension or hypertension controlled by one or two agents, if present. All patients received the initial cycle of treatment in hospital, followed by additional cycles on an ambulatory basis. The complete blood count, international normalized ratio (INR), and D-dimer were measured every two weeks or before treatment in all patients.
Chemotherapy
The following chemotherapy regimens were employed: FOLFOX 4 + Bev (85 mg/m2 of oxaliplatin intravenously over 2 hours, 100 mg/m2 of L-leucovorin over 2 hours, and a bolus of 400 mg/m2 of 5-FU on day 1, followed by a 22-hour infusion of 600 mg/m2 of 5-FU on days 1 and 2, plus 5–10 mg/kg of Bev over 30–90 minutes on day 1 every 2 weeks) or FOLFIRI + Bev (150 mg/m2 of irinotecan intravenously over 1.5 hours, 200 mg/m2 of L-leucovorin over 2 hours, and a bolus of 400 mg/m2 of 5-FU on day 1, followed by a 46-hour infusion of 1,200 mg/m2 of 5-FU on days 1 and 2, plus 5 mg/kg of Bev over 30–90 minutes on day 1 every 2 weeks). Patients were treated until progression occurred or toxicity became unmanageable.
Indications for anticoagulant therapy in patients with asymptomatic thrombus
Prophylactic anticoagulant therapy was not permitted. However, anticoagulant therapy was permitted at the discretion of the attending physician after detection of asymptomatic thrombus by DU that might have the potential to cause PE. The indications for initiating anticoagulant therapy in patients with asymptomatic thrombosis were as follows: an enlarging thrombus (E); thrombus >40 mm in diameter (S); thrombus involving the superior vena cava (C); and thrombus causing decreased blood flow (V). All patients with one or more of these findings received anticoagulant therapy, except those who had an increased risk of bleeding.
Anticoagulant therapy
Warfarin was used for anticoagulant therapy with a target INR of 2 to 3.
Evaluation of toxicity and efficacy
All patient data were recorded and reviewed as electronic medical records. Adverse events were graded at baseline and biweekly during treatment using the National Cancer Institute Common Toxicity Criteria, version 3.0. Tumor response was assessed every 3 months by computed tomography according to the Response Evaluation Criteria In Solid Tumors (RECIST 1.0). Data on toxicity and tumor response were also reviewed from the electronic medical records and the images of each patient.
Results
Patient profile
Seventy-nine patients were enrolled in this study, and their baseline characteristics are shown in Table 1. Fifty-seven patients (72.2%) received Bev as first-line treatment, and almost all of the subjects had a good PS. Eighteen patients (22.8%) were receiving antihypertensive therapy at baseline, but no other risk factors for thromboembolism were identified before administration of Bev.
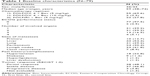  | Table 1 Baseline characteristics (N=79) |
Efficacy of DU
Evaluation of DU is summarized in Table 2. Among 44 patients who received pretreatment DU after CVAS implantation, 21 patients (47.7%) had mild asymptomatic thrombosis, but all patients started treatment without problems. Asymptomatic CVAS-related thrombosis was detected by DU after the first course of Bev in 56 patients (70.9%), while there was no evidence of thrombus in 23 patients (29.1%). Fourteen (60.9%) of the 23 patients without thrombus on pretreatment DU imaging were found to have developed thrombus by DU after the first course of Bev, while asymptomatic thrombus detected in 4 patients (19.0%) by pretreatment DU had disappeared when DU was done after the first course. According to our screening protocol, 56 patients with asymptomatic thrombosis received follow-up DU examination: thrombus resolved in five patients (8.9%), asymptomatic thrombus persisted in 50 patients (89.3%), and one patient (1.8%) experienced symptomatic thrombosis. Eleven (19.6%) of the 56 patients with asymptomatic thrombosis received anticoagulant therapy, including eight patients treated when thrombus was found by DU after the first course and three patients treated when it was detected by follow-up DU. The details of each patient are shown in Table 3, and their data are summarized in Table 4. The main indication for anticoagulant therapy was V (72.7%), followed by S (54.5%), and seven patients had two or more indications. The median interval between initiation of Bev and the start of anticoagulant therapy was 8 days. Bev could be resumed in seven of the eleven patients (63.6%). The median interval between commencement of anticoagulant therapy and resumption of Bev was 20 days.
  | Table 2 Results of DU imaging (N=79) |
When evaluating the response to anticoagulant therapy, improvement on DU was defined as improvement of at least one finding among E, S, C, or V, while progression meant the deterioration of at least one of these findings. The patients who did not fit into these two groups were considered to have stable thrombosis. Overall, thrombosis improved in seven patients (63.6%), while it progressed to become symptomatic in one patient (9.1%). In this patient, a large asymptomatic thrombus with V was detected after the first course of Bev and it rapidly became symptomatic despite immediate initiation of anticoagulant therapy. Overall, control of thrombosis by anticoagulant therapy was achieved in 90.9% of the patients (Table 5). Mild bleeding was the main treatment-related adverse event, being observed in 54.5% of the patients (Table 6). No severe treatment-related adverse events or deaths were observed.
  | Table 5 Efficacy of anticoagulant therapy (n=11) |
  | Table 6 Safety of anticoagulant therapy (n=11) |
The outcomes of all patients enrolled in this study are shown in Figure 2. All of the patients (11) receiving anticoagulant therapy resumed treatment, including seven who resumed Bev. The 68 patients who did not receive anticoagulant therapy, including 23 patients without thrombosis on DU after the first course of Bev, could also continue treatment including Bev without severe thrombosis. Accordingly, most patients with asymptomatic thrombosis detected by DU could continue chemotherapy including Bev by following our anticoagulant protocol.
Discussion
In patients receiving Bev plus chemotherapy,1–4,15–19 the incidence of thromboembolism ranges between 3% and 26%, with PE occurring in a few patients. Prophylactic anticoagulant therapy was not recommended or applied in any study, but there have been four studies that investigated anticoagulant therapy for maintenance of CVAS.1,2,4,16 The rarity of thromboembolic events suggests that there is no need for routine prophylactic anticoagulant therapy. According to the American Society of Clinical Oncology guideline, the major risk factors for VTE in cancer patients are the primary tumor site, advanced stage, and adenocarcinoma histology as cancer-related factors; indwelling venous access and chemotherapy or antiangiogenic agents as treatment-related factors; older age, a history of VTE, and poor PS as patient-related factors; and a platelet count >350,000/μL and hemoglobin <10 g/dL as biomarkers.20,21 The guideline does not recommend routine pharmacologic thromboprophylaxis for cancer outpatients receiving systemic chemotherapy, but suggests prophylaxis with low-molecular-weight heparin (LMWH) for high-risk patients on a case-by-case basis. An association between CVAS and VTE has been identified in some retrospective studies, ranging from 12% to 64%.22–26 In a prospective trial, the incidence of thrombosis was investigated in two groups assigned to receive LMWH or placebo for 6 weeks, with no significant difference being found (14.1% versus 18%).27 Another trial12 also showed no significant difference in the incidence of symptomatic thrombosis between warfarin and placebo (4.6% vs 4.0%). The American Society of Clinical Oncology recommends the following methods for the prevention of recurrent VTE in cancer patients.20 LMWH is preferred over unfractionated heparin for the initial 5 to 10 days of treatment in cancer patients with newly confirmed VTE, excluding those with severe renal impairment (creatinine clearance <30 mL/min). LMWH is preferred to vitamin K antagonists for long-term prevention over more than 6 months due to its better efficacy, but vitamin K antagonists are also recommended if LMWH is not available.
In Japan, enoxaparin sodium has been approved as an LMWH for prevention of VTE only in patients undergoing orthopedic surgery on the lower limb or invasive abdominal surgery with a high risk of VTE, but not in cancer patients. Therefore, unfractionated heparin is commenced first, followed by warfarin for at least 6 months with a target INR of 2 to 3. The appropriate treatment duration is unclear for cancer patients, so we continued anticoagulation during chemotherapy in patients without a risk of bleeding.
In our previous study, we prospectively assessed the effectiveness of DU for early identification of catheter-related thrombosis and changes of asymptomatic venous thrombosis during use of Bev.14 In addition, the purpose of that study was to detect Doppler diagnostic findings independent of clinical findings. In that study, prophylactic anticoagulant therapy for VTE was not given before the start of chemotherapy so as to evaluate asymptomatic thrombus formation. The higher-than-expected number of thrombi detected by DU were mostly asymptomatic. The results of sub-analyses suggested that detection of CVAS-associated thrombus in patients without a history of Bev does not mean that use of Bev is a risk factor for VTE. The findings that mainly affected the outcome of asymptomatic thrombosis were changes in thrombus size or decreased blood flow, and comparing each finding between initial and follow-up DU studies revealed that candidate risk factors for symptomatic VTE or PE were an enlarging or large thrombus (>40 mm in diameter) and decreased venous flow. Hence, we started to use these indications for prophylactic anticoagulant treatment of asymptomatic VTE after the study, but did not routinely give prophylaxis to patients treated with Bev in order to avoid an increased risk of bleeding.
The present study confirmed the validity of this anticoagulant strategy for management of CVAS-associated thromboembolism based on DU findings. As expected, asymptomatic thrombosis was detected frequently in patients receiving Bev, and the results revealed two important outcomes. First, most of the patients who needed anticoagulant therapy for VTE could continue chemotherapy including Bev without severe complications. Second, almost all patients without VTE at the first DU or progression of thrombosis during treatment could continue chemotherapy without new thromboembolism during subsequent treatment.
Thus, an anticoagulant strategy based on routine screening for thromboembolism using DU was demonstrated to be feasible for patients receiving chemotherapy combined with Bev, though this was not a randomized controlled study that compared patients receiving anticoagulant therapy and those without anticoagulant therapy.
Conclusion
Prophylactic anticoagulant therapy is not necessary for all patients in whom asymptomatic thrombosis is detected during treatment with Bev. Our anticoagulant protocol for managing asymptomatic thrombosis based on DU is a reasonable option for preventing severe thromboembolism while continuing Bev.
Acknowledgments
We are grateful to Dr Fujiwara for interpretation of radiological findings, and to the staff of the Ambulatory Treatment Center at the Cancer Institute Hospital for management of the patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in frst-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. | ||
Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. | ||
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. | ||
Giantonio BJ, Catalano PJ, Meropol NJ, et al; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. | ||
Bennouna J, Sastre J, Arnold D, et al; ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. | ||
Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. | ||
Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300(19):2277–2285. | ||
Lee AY, Levine MN, Butler G, et al. Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J Clin Oncol. 2006;24:1404–1408. | ||
Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21:3665–3675. | ||
Monreal M, Raventos A, Lerma R, et al. Pulmonary embolism in patients with upper extremity DVT associated to venous central lines – a prospective study. Thromb Haemost. 1994;72:548–550. | ||
Fagnani D, Franchi R, Porta C, et al; POLONORD Group. Thrombosis-related complications and mortality in cancer patients with central venous devices: an observational study on the effect of antithrombotic prophylaxis. Ann Oncol. 2007;18:551–555. | ||
Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23:4063–4069. | ||
Karthaus M, Kretzschmar A, Kröning H, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. 2006;17:289–296. | ||
Suenaga M, Mizunuma N, Kobayashi K, et al. Management of venous thromboembolism in colorectal cancer patients treated with bevacizumab. Med Oncol. 2010;27:807–814. | ||
Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. | ||
Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. | ||
Van Cutsem E, Rivera F, Berry S, et al; First BEAT investigators. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–1847. | ||
Kozloff M, Yood MU, Berlin J, et al; Investigators of the BRiTE study. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist. 2009;14:862–870. | ||
Cassidy J, Clarke S, Díaz-Rubio E, et al. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer. 2011;105:58–64. | ||
Lyman GH, Khorana AA, Kuderer NM, et al; American Society of Clinical Oncology Clinical Practice. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–2204. | ||
Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. | ||
Newman KA, Reed WP, Schimpff SC, Bustamante CI, Wade JC. Hickman catheters in association with intensive cancer chemotherapy. Support Care Cancer. 1993;1:92–97. | ||
Drakos PE, Nagler A, Or R, Gillis S, Slavin S, Eldor A. Low molecular weight heparin for Hickman catheter – induced thrombosis in thrombocytopenic patients undergoing bone marrow transplantation. Cancer. 1992;70:1895–1898. | ||
Lokich JJ, Becker B. Subclavian vein thrombosis in patients treated with infusion chemotherapy for advanced malignancy. Cancer. 1983;52:1586–1589. | ||
Köksoy C, Kuzu A, Erden I, Akkaya A. The risk factors in central venous catheter-related thrombosis. Aust NZ J Surg. 1995;65:796–798. | ||
Cortelezzi A, Moia M, Falanga A, et al; CATHEM Study Group. Incidence of thrombotic complications in patients with haematological malignancies with central venous catheters: a prospective multicenter study. Br J Haematol. 2005;129:811–817. | ||
Verso M, Agnelli G, Bertoglio S, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. J Clin Oncol. 2005;23:4057–4062. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.