Back to Journals » Journal of Inflammation Research » Volume 15
Anticipation of Relapse and Acute Graft-Versus-Host Disease after Allogeneic Peripheral Blood Stem Cell Transplantation: The Fundamental Role of Antigen-Presenting (Dendritic) Cells
Authors Nasif KA, Al Samghan AS, El-Sharkawy N, Abass AM, Elgezawy E, Khaled SAA , Elbadry MI , Thabet MM
Received 16 March 2022
Accepted for publication 4 June 2022
Published 30 June 2022 Volume 2022:15 Pages 3733—3747
DOI https://doi.org/10.2147/JIR.S366619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Video abstract presented by Safaa AA Khaled.
Views: 54
Khalid Ali Nasif,1,2 Awad S Al Samghan,3 Nahla El-Sharkawy,4 Amr M Abass,5 Ebtesam Elgezawy,6,7 Safaa AA Khaled,8 Mahmoud I Elbadry,9 Marwa M Thabet6
1Clinical Biochemistry Department, College of Medicine, King Khalid University, Abha, Saudi Arabia; 2Biochemistry Department, Faculty of Medicine, Minia University, Minia, Egypt; 3Family and Community Medicine Department, College of Medicine, King Khalid University, Abha, Saudi Arabia; 4Clinical Pathology Department, National Cancer Institute, Cairo University, Cairo, Egypt; 5Medical Physiology Department, College of Medicine, King Khalid University, Abha, Saudi Arabia; 6Clinical Pathology Department, Faculty of Medicine, Assiut University, Assiut, Egypt; 7Immunohematology Consultant, AMCH, Asir, Saudi Arabia; 8Department of Internal Medicine, Clinical Hematology Unit, AUH/Unit of Bone Marrow Transplantation, South Egypt Cancer Institute, Faculty of Medicine, Assiut University, Assiut, Egypt; 9Department of Internal Medicine, Division of Haematology, Faculty of Medicine, Sohag University, Sohag, Egypt
Correspondence: Safaa AA Khaled, Department of Internal Medicine, Clinical Hematology Unit, AUH/Unit of Bone Marrow Transplantation, South Egypt Cancer Institute, Faculty of Medicine, Assiut University, Assiut, Egypt, Email [email protected]; [email protected] Mahmoud I Elbadry, Department of Internal Medicine, Division of Haematology, Faculty of Medicine, Sohag University, Sohag, Egypt, Tel +20 10-6596-4083, Fax +20 93-460-9304, Email [email protected]; [email protected]
Background: Dendritic cells (DCs) are antigen-presenting cells. In humans two distinct lineages of DCs exist: DC1 and DC2. Efforts to explore the role of DCs in acute graft-versus-host disease (aGVHD) after allogeneic peripheral blood stem–cell transplantation (PBSCT) are gaining traction. However, further research is needed to identify particular lineages and their values in terms of developing an evidence-based aGVHD- or relapse-prevention strategy. We monitored DC counts and subsets in PBSC grafts while harvesting stem cells in recipients to elucidate their value in anticipating disease relapse or aGVHD.
Methods: We enrolled 29 participants. Using fluorescence-activated cell sorting, total counts/kg of CD34+, DCs, and DC subsets were analyzed in 29 PBSC-graft components using CMRF44, CD11c, and CD4 monoclonal antibodies (MoAbs).
Results: In the 29 grafts, we detected a significant positive correlation (P< 0.01) between DCs and both DC1 and DC2. Significantly higher counts (P< 0.01) of DCs and DC1 in those who had developed aGVHD (nine cases) were also observed. Relapsed cases (two) were also associated with higher counts of DCs and DC2. A significant positive correlation (P< 0.05), was recorded between DCs and DC1 counts and the day of myeloid engraftment, while this was not detected on the day of platelet engraftment. Myeloid engraftment transpired earlier in patients without aGVHD. Increased DC-graft numbers, particularly DC1 measured by CD11c Moabs, were associated with aGVHD. Recipients of higher numbers of CD4bright DCs had an increased risk of relapse after allogeneic PBSCT.
Conclusion: This study analyzed DCs in PBSC grafts, using novel specific MoAbs and flow cytometry. Our data showed that higher donor DC1 counts were linked to the incidence of aGVHD and DC2 with relapse. We propose a fundamental role for DC-graft monitoring in anticipating aGVHD and disease relapse.
Keywords: dendritic, cell, biomarker, acute GVHD, relapse, allogeneic PBSCT
Introduction
Dendritic cells (DCs) develop from a precursor in the bone marrow and circulate through the blood to the outermost body tissue, where they participate in antigen scrutiny and T-cell activation.1 Exact or approximate DC counts in various disease conditions are not well understood. The absence of a standardized method for estimating DC numbers in a certain disease restricts investigations. Some authors have established a technique appropriate for routine checking of DC counts in the blood after short-term culture by fluorescence-activated cell sorting utilizing the CMRF44 monoclonal antibody.2 CMRF44 antigens represent the phenotype, shape, and functional characteristics of DCs.3 Measuring CMRF44+ cells represent DC numbers in peripheral blood stem cell (PBSC) grafts. Because DCs are scarce, culturing mononuclear cells is needed to permit the utilization of DC-motivation antigens for positive recognition of DCs.2 Aripinati et al suggested that G-CSF– militarized PBSCs, including immunoeffector antigen-presenting cells that stimulate T cells, to yield Th2 cytokines. In humans, numerous DC lineages have been identified. Of these, DC1 induces differentiation of T lymphocytes to Th1 cells and DC2 augments T-cell differentiation to Th2 cells. There are other DC subtypes, such as plasmacytoid, inflammatory, and Langerhans DCs.4
The impact of DCs in regulating immunoreconstitution after transplantation likely represents the competing effect of donor DC1 versus DC2 in the graft and donor DCs that are differentiated from CD34+ cells by the effect of cytokines derived from DC1 or DC2.5 Recently, Wang et al demonstrated that blocking CD11c on human PB DCs inhibited CD4+ T-cell proliferation and differentiation into IFNγ-generating Th1 cells, which are essential in acute graft-versus-host disease (aGVHD) pathogenesis.6
Studies have demonstrated reduced DC numbers in the PB of recipients with moderate–severe aGVHD.7,8 On the contrary, others discovered no link between aGVHD and higher DC counts in the early stages of aGVHD.9,10 These disparities could be explained in part by the steroid treatment for aGVHD, which causes a rapid drop in circulating DCs. Another report found that CD4bright cells had similar phenotypes and counts as CD123bright type 2 DC precursors. After short culture, CD4bright cells differentiated into typical DCs.11
It has been proved that recipients transplanted with higher CD4bright DC counts have more reversions. Relapse is the main etiology of disease after autologousPBSC transplantation (aPBSCT).12 In light of what was previously mentioned, there is still a definite need to undertake studies designed to explore the roles of DCs post-HSCT. The current study was conducted to elucidate the value of DCs in predicting disease relapse and/or aGVHD in aPBSCT recipients.
Methods
Subject and Graft Selection
A total of 29 recipients were prospectively enrolled in the study. They underwent allogeneic PBSCT from 29 HLA-matched siblings. Their age was ≥18 years, and women represented >50% of the study sample, as shown in Supplemental Table S1. Willingness to participate in the study was an inclusion criterion.
Analysis of Grafts
Analysis of the allogeneic PBSC grafts (harvests) was done as follows.
Mobilization
The matched donors received G-CSF SC injections (10 μg/kg/day) for 5 consecutive days.
- This study was carried out on 29 allogeneic PBSC grafts (harvests) after short-term culture in RPMI1640/10% FBS at 37°C and a 5% carbon dioxide incubator. Heparinized blood samples (5 mL) were collected from harvests and handled within 4 hours. Routine complete blood counts were done using a Coulter STKS. Absolute mononuclear cell counts were determined (mononuclear cells/liter of blood). White blood–cell counts were adjusted between 3,000 and 10,000/mm3. The rest of the blood samples were diluted 1:1 with sterile PBS and underlaid with Ficoll–Hypaque before being centrifuged at 520 g for 15 minutes at 37°C. The mononuclear cells were mended from the low-density boundary and washed three times, then cultured in RPMI1640/10% FBS for 24 hours at 37°C and the 5% CO2 incubator. Trypan blue exclusion was used to assess cellular viability after culture.13
Panel of Monoclonal Antibodies Used
- CD4 FITC/CD34 PE (BD Biosciences)
- CD34 FITC/CD11c PE (BD Biosciences)
- CMRF44 FITC (received as a gift from Professor DNJ Hart (Director, Mater Medical Research Institute, Raymond Terrace, Brisbane, Australia)/CD34 PE (BD Biosciences)
- Isotype controls
Flow-Cytometry Analysis
Sample analysis was done using a Coulter Epics-XL-MCL flow cytometer:
- CD34 enumeration — ISHAGE protocol, CD34+ threshold ≥3×106 cells/kg body weight.
- Percentage and absolute CD34+ cell counts, DC counts, and their subsets were analyzed.
The graft components of all cases were analyzed for enumeration of DC numbers and DC subsets through monitoring of the absolute count/kilogram body weight of the parameters measured by flow cytometry: total nucleated cells, CD34+ cells, CMRF44+ DCs, CD11c+ DCs (DC1), and CD4bright DCs (DC2). All these parameters were correlated with one another and the end result of transplantation with regard to complications, especially aGVHD and relapse.14,15
Treatment, Observation, and Follow-Up
Prospective longitudinal observational follow-up of the recipients was carried out. For conditioning regimens, seven cases (24.1%) underwent minitransplantation (nonmyeloablative SCT), while 22 cases (75.9%) underwent myeloablative transplantation. All patients received primary prophylaxis for aGVHD.
GVHD Prophylaxis
All patients received GVHD prophylaxis (calcineurin inhibitor, methotrexate, mycophenolic acid, or post-HCT cyclophosphamide). In a majority, cyclosporine (CsA) and short-course methotrexate were given as GVHD prevention. Starting on day −4, CsA was given in an intravenous dose of 2.5 mg/kg twice per day for 4 hours, then switched to an oral dose of 5 mg/kg twice daily once the mucositis was cured. The dose was modified to obtain a target level of 200–400 ng/mL after CsA levels were evaluated. On day 1, the methotrexate dose was 10 mg/m2, and on days 3, 6, and 11, the dose was 7 mg/m2. Day 6 and day 11 dosages were skipped if the mucositis was severe (grade III/IV) or bilirubin was >20 mg/L.
Follow-Up
All patients (recipients) were monitored for 100 days after transplantation for incidence of aGVHD, relapse, and other complications. They were assessed by clinical examination, complete blood count, blood culture, and liver and renal function. aGVHD, relapse, and relapse-free survival were the outcomes of interest. The onset of aGVHD usually occurred during the first 2 months (60 days) following PBSCT, and systemic steroid therapy was the primary line of treatment for aGVHD grade II. Staging and clinical grading for GVHD severity and response criteria were used as previously described14,15 (as described in Supplementary File Tables S2 and S3).
The presence of any morphological evidence of leukemia at the level of bone marrow or extramedullary sites was used to determine leukemia relapse. This was further confirmed with minimal residual disease monitoring with flow cytometry or PCR. The period from the start of the conditioning regimen to event or final follow-up was used to assess event-free survival (EFS) (aGVHD or relapse or death). The period from the start of the conditioning regimen to death or last follow-up was defined as overall survival (OS). Recipients were then classified into three groups based on clinical outcome: group I (event-free), group II (aGVHD), and group III (disease relapse).
Statistical Analysis
Data were collected and analyzed with SPSS 18.0. GraphPad Prism V5 was used also. Descriptive data are presented as means ± SD if numeric and percentages if categoric. Correlations and relationships were evaluated using Spearman’s rank-correlation coefficient. Kaplan–Meier survival curves were plotted for estimating OS, EFS, and relapse-free survival. Figures were created using GraphPad Prism 5.02). In all tests, statistical significance was presumed at P<0.05.
Results
Kinetics and Components of the Harvests
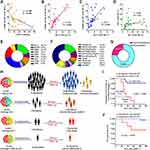
First, the kinetics and variable components of the grafts were examined. There were highly significant (P<0.001) positive correlations of CMRF44+ DC numbers (median 11.88) with both DC1 (median 5.63) and DC2 (median 4.3).Moreover, a significant negative association was noted between DC numbers and CD34+ cells. Interestingly, graft components showed comparable CMRF44 DC, DC1, and DC2 cell numbers in grafts from male and female donors (Table S4, Figure 1A–D). Supplementary Figure S1 shows graft components and their relationship to donor age and sex. Seven cases (24.1%) showed increased CMRF44+ DC numbers in the grafts. Four of those cases (13.8%) showed increased DC1 numbers, while two cases (6.9%) showed increased DC2 numbers. The last one case had neither increased DC1 nor DC2. Interestingly, all these cases developed complications after allogeneic PBSCT, particularly aGVHD, relapse, and higher fatality rate (Figure 1H–J).
Characteristics and Indications for Transplantation
As delineated in Figure 1E–G and Tables 1 and S4, the commonest underlying hematologic disorders were acute myeloid leukemia (AML), severe idiopathic aplastic anemia, and acute lymphoblastic leukemia (ALL), with variable cytogenetic abnormalities. A total of 21 cases (72.4%) underwent myeloablative transplantations. Table 1 shows treatments of all patients before allogeneic PBSCT, their cytogenetic profile, disease state at transplantation, and HLA-matched intensity.
 |
Table 1 Demographic characteristics, underlying hematologic disorders, treatment, response, cytogenetic profiles and HLA compatibility of the 29 recipients that underwent aPBSCT |
Associations among Graft-DC Counts, Subsets, and Postallogeneic PBSCT Complications
Considering development of complications after allogeneic PBSCT, particularly aGVHD and relapse, recipients were stratified into three groups based on clinical outcomes.
Group I
This was the event-free group (18 recipients, 62.1%). These recipients did not develop any complications during follow-up (0–100 days), either clinically or on laboratory results, and they were doing well.
Group II
This group comprised recipients (31%) who suffered from manifestations of aGVHD: grade I (4 recipients), grade II (3 recipients), and grades III and IV (one recipient each). Estimation of DC components in these cases revealed that DC numbers were increased in five cases, and increased DC1 numbers were recorded in four of those five (Figure 1H, Table 2). The numbers of DCs and DC1 reached higher levels with severity of GVHD in the recipient with grade IV aGVHD. This recipient died after 3 weeks. Conversely, the other cases did not show increased numbers of either DCs or DC1 in their grafts, despite clinical evidence of aGVHD. These 4 recipients had low-risk aGVHD (grade I, Table 2). From these results, we found that only group II had significantly (P<0.001) higher DC and DC1 counts (in their grafts) compared than event-free recipients (group I), (Tables 2 and S5, Figure 2A–K). Figure 2L is an example of aGVHD cases that showed increased CMRF44+ DCs and CD11c+ DCs (DC1, n=23, grade IV).
 |
Table 2 GVHD stage and grade, graft-DC components, medications, and outcomes of those who developed aGVHD |
Group III
This group comprised two recipients (6.9%) who developed relapse after aPBSCT. Higher doses of DCs and DC2 were recorded in the grafts of those cases (Figure 3A–C). Figure 3D and E is an example of relapsed cases that showed increased CMRF44+ and CD4bright DCs (DC2). Analysis of donor-related factors revealed that DC numbers had no significant relationship to age, sex, virological studies, or blood group.
Associations Between Graft Characteristics and Clinical Outcomes
For graft characteristics that could affect recipients’ clinical outcome, those who were transplanted with allografts containing higher CD34+cells counts fewer less complications and longer EFS after aPBSCT. Noteworthily, no significant differences were found in study groups I or II in respect if dose of CD34+cells/kg, or other variable components of the graft. There was no significant correlation between numbers of DC subsets (DC1 and DC2 and dose of CD34+ cells), but there was a significant negative correlation between numbers of CMRF44+ DCs and CD34+ cells (Table 3).
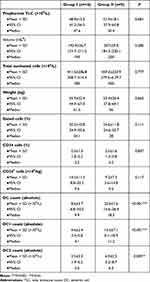 |
Table 3 Comparison between different variables of graft components in group II (cases with acute GVHD) versus group I (event-free group) |
It was found that a lesser dose of CD34+ donor cells was linked to aGVHD (two cases) and relapse (one case). A statistically significant association (positive) was found between DC numbers and day of myeloid engraftment, but no significant correlation was detected for day of platelet engraftment (P<0.05, Figure S2). Considering the occurrence of aGVHD, myeloid engraftment occurred earlier in group I (P<0.05) than group II, while no significant difference was found regarding platelet engraftment (Table 4). Multivariate analysis showed asignificant differences among groups I, II, and III when considered jointly for DCs, DC1, and D2. Wilks’s 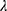 =0.094 (F22,32=3.281, P=0.001, partial
=0.094 (F22,32=3.281, P=0.001, partial 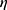 2=0.693). A separated ANOVA was conducted for each dependent variable, with each evaluated at α=0.025. There was a significant difference between group I and groups II and III for DCs, (F2,26=19.5, P<0.001, partial
2=0.693). A separated ANOVA was conducted for each dependent variable, with each evaluated at α=0.025. There was a significant difference between group I and groups II and III for DCs, (F2,26=19.5, P<0.001, partial 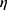 2=0.6, with group I (mean 8.62) DC numbers lower than groups I and II (means 20.6 and 23.3, respectively). There was a significant difference between group II and groups I and III for DC1 (F2,26=12.3, P<0.001, partial
2=0.6, with group I (mean 8.62) DC numbers lower than groups I and II (means 20.6 and 23.3, respectively). There was a significant difference between group II and groups I and III for DC1 (F2,26=12.3, P<0.001, partial 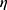 2=0.495), with group II (mean 13.3) DC1 numbers higher than groups I and III (means 4.6 and 5.3, respectively). There was a significant difference between group III and groups I and II for DC2 (F2,26=123.67, P<0.001, partial
2=0.495), with group II (mean 13.3) DC1 numbers higher than groups I and III (means 4.6 and 5.3, respectively). There was a significant difference between group III and groups I and II for DC2 (F2,26=123.67, P<0.001, partial 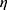 2=0.646), with group III (mean 18.4) DC1 numbers higher than groups I and II (means 3.5 and 6.9, respectively). There was no significant differences among the three groups for age or other graft components (Tables S6–S8).
2=0.646), with group III (mean 18.4) DC1 numbers higher than groups I and II (means 3.5 and 6.9, respectively). There was no significant differences among the three groups for age or other graft components (Tables S6–S8).
 |
Table 4 Associations between DC numbers and day of myeloid and platelet engraftments in allogeneic PBSCT recipients |
Associations Between Hospitalization Days and Graft-DC Counts and Subsets
A statistically significant association was detected among graft-DC numbers and days of hospitalization after aPBSCT, but a significant negative correlation was detected as regards CD34+ cell numbers and days of hospitalization (Figure 3F–I). EFS was affected by dose of CD34+ donor cells, and higher DC and lower doses of CD34+ cells shortened EFS (Figure 3J and K). Figure 4 shows estimated marginal means of DCs and a correlation matrix of variables of graft components and patients’ weight.
Associations Between Graft-DC Counts, Subsets, and Survival Rates
During the period of this study (0–100 days), the survival rate was 94.4% in the event-free group (group I). This decreased to 77.8% with increased numbers of DCs and DC1 in those who developed aGVHD (group II). High numbers of CD4bright DCs were correlated with reduced survival rate following aPBSCT (50%) among relapsed cases (group III, Figure 3L).
Discussion
DCs are a group of bone marrow–derived immunoeffector cells that play an important role in presenting antigens and their collaboration with T lymphocytes. As such, they are essential for cell-mediated immunity and starting the chief T-cell immunoreaction.16 Because of DC scarcity in harvests, their shorter survival in aPBSCT recipients, and the greater number of recipient DCs that are able to present alloantigens to donor T lymphocytes, the role of donor DCs and their subtypes in grafts has been neglected. Moreover, it is unclear if low DC counts after engraftment are linked to underprivileged consequences or if this is an indicator of humble immunological retrieval. Also, it seems unclear whether reduced PB DC numbers after HSCT reflect decreased DC synthesis, treatment effects (steroids) during this period, or enhanced DC migration to tissue impacted by infections and/or aGVHD. Indeed, the role of DC-count assays after HSCT as a predictive value in posttransplantation outcomes is still a mystery.17 This study focused on exploring DC numbers and subsets in allogeneic PBSCT grafts and investigating their predictive value in incidence of aGVHD and disease relapse. These objectives were examined in a two-phase study: firstly flow-cytometry analyses of grafts and secondly recipient treatment and follow-up.
As far as we know, this is the first time that recognized DC markers have been used for concurrent quantification, recognition, and segregation of two discrete DC subsets in freshly prepared blood samples. Flow-cytometry analysis has been used to enumerate CD11+ DCs (DC1) and CD4bright DCs (DC2) in PBSC grafts.17 This study reported significant negative correlations between DC counts and CD34+ cells. This could be explained by a previous report that normal DC counts were detected in patients grafted with CD34+-nominated cells, which concluded that CD34+-nominated cells had the capacity to repopulate the DC line.18 Interestingly, this study reported no effect of donor age or sex on CMRF44+ DC, DC1, or DC2 cell numbers or subsets in grafts.
Our results showed that in recipients with constant allogeneic retrieval, recipients received harvests of lower counts (CMRF44+ DCs, DC1, and DC2) and had lower incidence of aGVHD and relapse and better survival. Moreover, graft-DC count was found to be an independent effector of severity of aGVHD and fatality rate. Those findings were in accordance with Rajasekar et al, who concluded that a significant increase in DCs, particularly DC1, was linked with higher incidence of aGVHD.19–21 On the other hand, the current findings contradict with Reddy et al.22
This study proved that in allogeneic PBSCT recipients of grafts with high DC1 or DC2 counts are linked to a higher incidence of aGVHD or relapse, respectively. This suggests that assessment of DC numbers in the grafts could help identify patients who are at greater risk of aGVHD and losses, as well as allowing for a future evaluation plan and potential early therapeutic strategies or the development of an aGVHD-control strategy. These results support the findings of Waller et al, who found that the amount of donor DC2 was linked to a reduced graft-versus-leukemia effect and increased incidence of relapse following PBSCT, denoting a pivotal role for donor DCs in influencing immunoreactions after allogeneic PBSCT.19 In a previous study, recipients with fewer DCs following HSCT had a greater risk of aGVHD and disease relapse, despite the fact that their original grafts had more DCs than grafts administered to patients without aGVHD.20 Our results are consistent with earlier research showing that high CD4bright DC numbers in the marrow of donors12 or high pDC content in the PBSC graft21 are linked to higher relapse rate.
Fast and robust engraftment is the instantaneous apprehension after transplantation. It was found that higher quantities of CD34+ are constantly linked with swift engraftment; on the opponent little doses are recognized to yield late engraftment. A significant clarification can be obtained from the ISHAGE study. It showed that recognized swift engraftment occurred in all recipients who received >10–15×106/kg total CD34+ and sluggish engraftment in those received a dose of <2×106/kg body weight.22 The current study reported, delayed myeloid engraftment in recipients of grafts with high DC counts, without significant effect on platelet engraftment. In the current study recipients of allografts with higher DC counts had longer in hospital days, post PBSCT, on the contrary higher CD34+ cells was associated with shorter in hospital stay.
This study showed that recipients of allografts with higher CD34+ cells had lower incidence of complications and prolonged EFS. This was in accordance with others who concluded that patients of greater number of CD34+ cells had longer EFS; whereas those with less CD34+ cells had overdue hematopoietic engraftment and augmented disease from infection.12,22 The findings of this study provided strong scientific evidence that donor DCs could have a role in pathogenesis of aGVHD, and the applicability of this in advances in SC therapy.
Now the question is how donor DCs participate in aGVHD. The answer could be extracted from the current study and previous studies. DCs could eventually present antigens in either a tolerogenic or immunogenic way based on the degree of their maturation, which in turn is dependent on the circumstances in which they faced antigens. Therefore, DCs either generate a suitable immunoreaction to pathogens or avert autoimmunity.23 The net influence of DCs in modifying immunoreconstruction after transplantation possibly signifies the opposing effects of graft- donor DC1 versus DC2 and CD34+-differentiated donor DCs cells that are augmented by DC1 or DC2 cytokines.5
The role of APCs (DCs) in pathogenic donor T-cell development and differentiation is beginning to emerge, although the identification of DC types responsible for this abnormal T-cell differentiation remains puzzling. Gonçalves et al reported that greater pDC levels in the graft were linked to a higher risk of aGVHD, but pDC counts in the PB following transplantation had the reverse impact.24 Furthermore, Lee et al demonstrated that transplanting bone marrow deficient in MyD88 led in a slanted DC differentiation toward CD11c+ DCs that encompassed a majority of graft cells in GVHD hosts and resulted in GVHD and deterioration.25
It has been stated that G-CSF has a starring role in the differentiation of DCs. G-CSF injections of donors with 10–16 μg/kg/day for 5 days amplified PB DC2 numbers, whereas those of DC1 did not changed. G-CSF might increase DC2 numbers in the circulation by enhancing their production in the bone marrow, prolonging their survival, persuading mobilization, or reducing their movement out of the vascular enrollment into lymphoid organs.26 It is plausible that aPBSCT of G-CSF–mobilized PBSC does not result in devastating aGVHD, as the graft has mostly Th2-persuading DCs. Adoptive transmission of filtered DCs may bring immunoaberration after transplantation of HSCs or organ allografts. The significance of such reports has been highlighted by recent findings on progression of therapeutic approaches against cancers and transmittable agents using DCs. Some studies have proved that this method holds potential in preventing cancer progression or even causing cancer regression.25,26
Conclusion
This study aimed to monitor DC numbers and subsets during SC harvesting in PBSCT and their possible roles in prediction of incidence of aGVHD after PBSCT, represented by increased DC1 numbers in the grafts (harvests), and testing the hypothesis that larger doses of CD4bright DC (DC2) in PBSC grafts are associated with higher relapse rates after PBSCT. The results revealed that donor progenitor DCs, particularly DC1, can have a significant impact on engraftment associated with an increased incidence of aGVHD, while higher DC2 was linked to disease relapse. These results provide strong evidence that DCs can have an important role in the pathogenesis of aGVHD. Furthermore, we speculated on graft-DC monitoring as a predictive biomarker of aGVHD and/or relapse. These findings together with others suggest it is necessary to create a technique for accurate DC monitoring that can measure their numbers and subsets. We established a new technique that might facilitate quantitative measurements of DCs and subsets, and accordingly could simplify its usage as a biomarker in PBSCT allografts (harvests). The technique relies on exploiting modern flow-cytometry procedures and instruments using CD4 FITC/CD34 PE, CD34 FITC/CD11c PE, and CMRF44 FITC antibody panels. The specificity of the technique was primarily reliant on the specificity of the anti-DC antibody, whereas precision was founded on flow-cytometry capability in providing exact measurements.
Data Sharing
All data connected with and supporting the study results are within the manuscript and the Supplementary File.
Ethics
The research protocol, objectives, and methods were in accordance with the WMA Declaration of Helsinki. The ethics committee of the National Cancer Institute (NCI), Cairo University, Egypt granted approval for this study. The study had no impact on patients’ treatment, and no extra procedures were undertaken.
Patient Consent
Before inclusion in the study, written informed consent was obtained from all PBSCT recipients and their matched sibling donors who participated in the study voluntarily. Objectives, possible risks, and withdrawal rights were clarified to all participants before the study. Anonymity was addressed.
Acknowledgments
The authors wish to express deep gratitude to Professor DN Hart at the Mater Medical Research Institute, South Brisbane, Queensland, Australia for his CMRF44 FITC antibody gift. The researchers also thank all aPBSC recipients and their donors who participated in the study. In addition, they wish to thank all members of all contributing departments for their help and care during this study. The authors wish to acknowledge King Khalid University, Abha, Saudi Arabia and the Deanship of scientific research at King Khalid University for providing administrative and technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
No funding to declare.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi:10.1146/annurev-immunol-020711-074950
2. Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287.
3. Thomas R, Lipsky PE. Human peripheral blood dendritic cell subsets. Isolation and characterization of precursor and mature antigen-presenting cells. J Immunol. 1994;153:4016–4028.
4. Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. doi:10.1182/blood.V95.8.2484
5. Ryncarz RE, Anasetti C. Expression of CD86 on human marrow CD34+ cells identifies immunocompetent committed precursors of macrophages and dendritic cells. Blood. 1998;91:3892–3900. doi:10.1182/blood.V91.10.3892
6. Wang Q, Su X, He Y, et al. CD11c participates in triggering acute graft-versus-host disease during bone marrow transplantation. Immunology. 2021;164:148–160. doi:10.1111/imm.13350
7. Porta MD, Rigolin GM, Alessandrino EP, et al. Dendritic cell recovery after allogeneic stem-cell transplantation in acute leukemia: correlations with clinical and transplant characteristics. Eur J Haematol. 2004;72:18–25. doi:10.1046/j.0902-4441.2004.00172.x
8. Vakkila J, Thomson AW, Hovi L, Vettenranta K, Saarinen-Pihkala UM. Circulating dendritic cell subset levels after allogeneic stem cell transplantation in children correlate with time post transplant and severity of acute graft-versus-host disease. Bone Marrow Tran. 2005;35:501–507. doi:10.1038/sj.bmt.1704827
9. Talarn C, Urbano-Ispizua A, Martino R, et al. Kinetics of recovery of dendritic cell subsets after reduced-intensity conditioning allogeneic stem cell transplantation and clinical outcome. Haematologica. 2007;92:1655–1663. doi:10.3324/haematol.11076
10. Takebayashi M, Amakawa R, Tajima K, et al. Blood dendritic cells are decreased in acute graft-versus-host disease. Bone Marrow Tran. 2004;33:989–996. doi:10.1038/sj.bmt.1704406
11. Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi:10.1126/science.283.5405.1183
12. Waller EK, Rosenthal H, Jones TW, et al. Larger numbers of CD4 (bright) dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood. 2001;97:2948–2956. doi:10.1182/blood.V97.10.2948
13. Mannering SI, McKenzie JL, Hart DN. Optimisation of the conditions for generating human DC initiated antigen specific T lymphocyte lines in vitro. J Immunol Methods. 1998;219:69–83. doi:10.1016/S0022-1759(98)00125-2
14. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Tran. 1995;15:825–828.
15. Martin PJ, Rizzo JD, Wingard JR, et al. First-and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Tran. 2012;18:1150–1163. doi:10.1016/j.bbmt.2012.04.005
16. Kadowaki N. Dendritic cells—A conductor of T cell differentiation. Allergol Int. 2007;56:193–199. doi:10.2332/allergolint.R-07-146
17. Willmann K, Dunne JF. A flow cytometric immune function assay for human peripheral blood dendritic cells. J Leukoc Biol. 2000;67:536–544. doi:10.1002/jlb.67.4.536
18. López JA, Crosbie G, Kelly C, et al. Monitoring and isolation of blood dendritic cells from apheresis products in healthy individuals: a platform for cancer immunotherapy. J Immunol Methods. 2002;267:199–212. doi:10.1016/S0022-1759(02)00185-0
19. Waller EK, Rosenthal H, Sagar L. DC2 effect on survival following allogeneic bone marrow transplantation. Oncology. 2002;16:19–26.
20. Reddy V, Iturraspe JA, Tzolas AC, Meier-Kriesche HU, Schold J, Wingard JR. Low dendritic cell count after allogeneic hematopoietic stem cell transplantation predicts relapse, death, and acute graft-versus-host disease. Blood. 2004;103:4330–4335. doi:10.1182/blood-2003-09-3325
21. Rajasekar R, Lakshmi KM, George B, et al. Dendritic cell count in the graft predicts relapse in patients with hematologic malignancies undergoing an HLA-matched related allogeneic peripheral blood stem cell transplant. Biol Blood Marrow Tran. 2010;16:854–860. doi:10.1016/j.bbmt.2010.01.013
22. McQuaker I, Haynes A, Stainer C, Byrne J, Russell N. Mobilisation of peripheral blood stem cells with IVE and G-CSF improves CD34+ cell yields and engraftment in patients with non-Hodgkin’s lymphomas and Hodgkin’s disease. Bone Marrow Tran. 1999;24:715–722. doi:10.1038/sj.bmt.1701985
23. Gluckman J-C, Canque B, Rosenzwajg M. Dendritic cells: a complex simplicity. Transplantation. 2002;73:S3–S6. doi:10.1097/00007890-200201151-00004
24. Gonçalves MV, Yamamoto M, Kimura EY, et al. Low counts of plasmacytoid dendritic cells after engraftment are associated with high early mortality after allogeneic stem cell transplantation. Biol Blood Marrow Tran. 2015;21:1223–1229. doi:10.1016/j.bbmt.2015.03.010
25. Lee YK, Ju JM, Shon WJ, et al. Skewed dendritic cell differentiation of MyD88-deficient donor bone marrow cells, instead of massive expansion as myeloid-derived suppressor cells, aggravates GVHD. Immune Netw. 2018;18(6):e44. PMID: 30619630; PMCID: PMC6312895. doi:10.4110/in.2018.18.e44.
26. Hock B, Haring L, Ebbett A, Patton W, McKenzie J. Differential effects of G-CSF mobilisation on dendritic cell subsets in normal allogeneic donors and patients undergoing autologous transplantation. Bone Marrow Tran. 2002;30:733–740. doi:10.1038/sj.bmt.1703734
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.