Back to Journals » Cancer Management and Research » Volume 12
Anticancer Effects of ACT001 via NF-κB Suppression in Murine Triple-Negative Breast Cancer Cell Line 4T1
Authors Liu Y, Wang L, Liu J, Xie X, Hu H, Luo F
Received 4 January 2020
Accepted for publication 19 May 2020
Published 26 June 2020 Volume 2020:12 Pages 5131—5139
DOI https://doi.org/10.2147/CMAR.S244748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Seema Singh
Yanyang Liu,* Li Wang,* Jiewei Liu, Xiaoxiao Xie, Haoyue Hu, Feng Luo
Lung Cancer Center, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feng Luo Email [email protected]
Purpose: ACT001 is a novel sesquiterpene lactone derivative with anticancer effects, including the reversal of tamoxifen resistance in estrogen receptor-positive breast cancer cells. However, few studies have investigated the anticancer effects of ACT001 in triple-negative breast cancer (TNBC), a highly aggressive cancer with a poor prognosis. This study aimed to investigate the effects of ACT001 on TNBC and the potential mechanism underlying these effects.
Materials and Methods: The anticancer effects of ACT001 on the murine TNBC cell line 4T1 were evaluated by Cell Counting Kit-8 assay, animal experiments, TUNEL staining, flow cytometry, immunofluorescence, enzyme-linked immunosorbent assay, and Western blotting analysis.
Results: ACT001 induced apoptosis in 4T1 cells by upregulating B cell lymphoma 2-associated X protein expression. Moreover, ACT001 markedly decreased levels of secretory granulocyte-macrophage colony stimulating factor (GM-CSF) in 4T1 tumors, decreased the number of myeloid-derived suppressor cells (MDSCs), and reduced angiogenesis. Furthermore, GM-CSF promoted angiogenesis and the proliferation of MDSCs in a dose-dependent manner. Finally, ACT001 suppressed phospho-NF-κB and IκB-α levels in 4T1 cells, thereby further decreasing GM-CSF levels.
Conclusion: Our results suggest that ACT001 exerts its anticancer effects by inducing apoptosis in murine TNBC cell line 4T1 and regulates the tumor microenvironment by attenuating angiogenesis and accumulation of MDSCs in 4T1 tumors. The underlying mechanism may involve the suppression of NF-κB activity.
Keywords: ACT001, triple-negative breast cancer, myeloid-derived suppressor cells, angiogenesis, NF-κB
Introduction
Breast cancer is one of the most common malignancies with a high incidence among women worldwide. Triple-negative breast cancer (TNBC) accounts for approximately 10–24% of all breast cancer cases.1 This molecular subtype is clinically characterized by lack of expression of the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2). TNBC is common among younger women, especially Black women. It is highly aggressive, with a higher propensity to metastases and a shorter survival period compared with other breast cancer subtypes. The median survival time of patients with metastatic TNBC is only about 18 months.2
Owing to the unavailability of endocrine and HER2-targeted therapies, chemotherapy remains the first-line treatment for TNBC. Although TNBC has a higher pathologic complete response rate after chemotherapy, its post-chemotherapy relapse rate is high. Along with chemotherapy-mediated apoptosis induction in tumor cells, regulation of the tumor microenvironment via anti-PD-1 immunotherapy and anti-angiogenic therapy has potential for treating TNBC; however, more data are required in support of these treatment strategies.3 Hence, novel compounds are required to be developed for treating TNBC.
ACT001, a novel sesquiterpene lactone derivative with high hydrophilicity, is the fumarate salt of dimethylaminomicheliolide (DMAMCL).4 The molecular formula of ACT001 is C17H27NO3·C4H4O4, and its molecular weight is 409.47 Da. ACT001 has been reported to induce apoptosis in glioma cells and reverse tamoxifen resistance in human breast cancer cell lines by inhibiting NF-κB activation.5,6 This evidence suggests that ACT001 may have considerable anticancer potential. However, whether ACT001 exerts anticancer effects in more aggressive breast cancers, including TNBC, remains unclear, as does its mechanism of action.
Murine breast cancer cell line 4T1, a highly metastatic phenotype of breast cancer similar to human TNBC, has been widely used in TNBC research.7 In our study, we investigated the anticancer activity of ACT001 in 4T1 cells and explored its underlying molecular mechanism.
Materials and Methods
Cell Lines and Culture
Murine TNBC cell line 4T1 and human umbilical vein endothelial cells (HUVECs) were obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). These cells were maintained in DMEM (Life Technologies, Bedford, MA, USA) containing 10% fetal bovine serum (FBS) and 1% antibiotic solution (penicillin 100 U/mL, streptomycin 100 ug/mL), and cultured in a humid chamber at 37 °C with 5% CO2.
Cell Counting Kit-8 (CCK-8) Assay
Cells were seeded in 96-well plates at a density of 3000 cells/well overnight. After treatment with 20, 40, 60 and 80 μM ACT001 (provided by Accendatech, Tianjin, China), respectively, for 72 h, 10 μL CCK-8 (Dojindo Laboratories, Kumamoto, Japan) was added, followed by incubation at 37 °C for 2 h. The Optical density (OD) values were measured at 450 nm on a scanning multi-well spectrophotometer (Bio-Rad Model 550, CA, USA). The cell inhibitory rate was calculated by the following equation: cell inhibitory rate = ([OD control group− OD experimental group]/OD control group) × 100%. All experiments were performed in triplicate with three independent repetitions.
Murine Breast Tumor Model and Treatment
BALB/c mice (female, 5 weeks old; Bai Ou Bioscience, Chengdu, Sichuan, China) were maintained in the laboratory for animal experiments at Sichuan University under specific pathogen-free conditions with animal food and water. Mice were injected subcutaneously in the right axillary fossa with 4T1 cells (5×105/0.1 mL). The tumor volume (mm3) was calculated as length x width2/2. When tumor volumes reached about 70–100 mm3, mice were randomly assigned to treatment and control groups. For mice in the treatment group, 100 mg/kg ACT001 dissolved in distilled water was administered orally once per day; the control group was given the same volume of sterile water. After 18 d, mice were sacrificed and the effects of ACT001 on tumor growth (n = 5/group) and survival time (n= 8/group) were evaluated. Experimental procedures and protocols were approved by the Animal Ethics Committee of Sichuan University. All experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Flow Cytometry
Part A
Murine 4T1 tumors were washed with ice-cold phosphate-buffered saline (PBS; pH 7.4), and then dissociated by mincing the tissue with a scalpel, followed by the addition of DMEM containing 1 mg/mL collagenase I + collagenase IV, and incubated for 2 h at 37 °C. The dissociated tumor tissue was then washed with ice-cold PBS and filtered through a 70-μm cell strainer (BD Bioscience, Franklin Lakes, NJ, USA). The cell suspension was then centrifuged at 1500 rpm for 5 min at 4 °C. The resuspended cells were stained with fluorochrome-labeled antibodies targeting murine CD11b-PE-CY7, F4/80-PE (eBioscience, San Diego, CA, USA), and Gr-1-APC (Biolegend, San Diego, CA, USA) or an appropriate isotype control antibody (rat IgG2b kappa isotype control (eB149/10H5) PE-CY7, rat IgG2a kappa isotype control (eBR2a) PE (eBioscience), or rat IgG2b-APC kappa isotype control (Biolegend)), and then analyzed by flow cytometry (Beckman Coulter, Indianapolis, IN, USA).
Part B
Isolation of MDSCs by fluorescence-activated cell sorting. Femurs and tibias were isolated from 4T1 tumor-bearing female BALB/c mice, and muscle was removed. Both ends of bones were cut with scissors and then flushed with 5 mL of RPMI 1640 using a 25-gauge needle. Bone marrow cells were seeded at a density of 2 × 105 cells/cm2 in DMEM containing 10% FBS and 1% antibiotic mixture (penicillin 100 U/mL, streptomycin 100 ug/mL).6 MDSCs in bone marrow cells were stained using anti-CD11b and anti-Gr-1 (eBioscience), and the stained cells were isolated using flow cytometry (Beckman Coulter).
MDSCs were cultured in DMEM complete medium with or without the addition of 100 ng/mL mouse granulocyte-macrophage colony stimulating factor (GM-CSF) (GenScript, Nanjing, Jiangsu, China) for 4 d. Cells were then stained with CD11b-PE-CY7 (eBioscience) and Gr-1-APC (Biolegend), and analyzed by flow cytometry (Beckman Coulter).
Histology, Immunohistochemistry, and Immunofluorescence
Part A
The murine 4T1 tumors were embedded in paraffin. Then, paraffin sections (thickness 5 μm) were been deparaffinized in xylene for 15 min, rehydrated with a descending alcohol series (100, 95, 90, 75, and 70%), and treated with EDTA buffer for 10 min. Slides were subsequently covered with 3% hydrogen peroxide to inhibit endogenous peroxidase activity, and goat serum (Beyotime Biotechnology, Shanghai, China) to block non-specific binding sites. Sections were incubated with anti-Arg-1 and anti-iNOS (Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C, followed by incubation for 20 min at room temperature with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Beyotime Biotechnology). Chromogen detection was performed using 3,3ʹ-diaminobenzidine. Following counterstaining with hematoxylin for 3 min at room temperature, positive cells were counted under a microscope at 200x magnification.
Murine 4T1 tumor paraffin sections were stained using a DeadEnd™ Fluorometric TUNEL Kit (Promega, Madison, WI, USA) for histopathological examination to evaluate the apoptotic cells.
The lungs, hearts, livers and kidneys of 4T1 tumor-bearing mice treated with sterile water or ACT001 were collected. Paraffin sections (thickness 5 μm) were stained with hematoxylin and eosin using routine methods for histopathological examination to evaluate metastases and the toxicity of ACT001 to organs.
Part B
Frozen sections (thickness 5 μm) of 4T1 tumors were used to detect expression of CD31. Sections were incubated at 4 °C overnight with primary antibody (anti-mouse CD31; eBioscience) before being incubated with appropriate secondary antibodies (ZSGB-BIO, Beijing, China) for immunofluorescence staining. Nuclei were highlighted using 4′,6-diamidino-2-phenylindole (Sino Biological Inc, Beijing, China) for 5 min. Sections were viewed and digitally photographed using a fluorescence microscope (Olympus UIS2, Tokyo, Japan). CD31+ cells were assessed in five randomly fields (n = 5) at 200x magnification for statistical analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
Murine 4T1 tumors were rapidly frozen at −80 °C, and then homogenized in RIPA buffer (Beyotime Biotechnology), followed by centrifugation at 13,300 rpm for 15 min at 4 °C. A BCA Protein Assay Kit (Beyotime Biotechnology) was used to test the protein concentrations of samples. Levels of cytokine GM-CSF were assessed using a mouse ELISA kit (Neobioscience, Shenzhen, China) according to the manufacturer’s protocol, and measured at 450 nm using a microplate reader (Benchmark Electronics, Angleton, TX, USA).8
Tube Formation Assay
HUVECs (2×104 cells/well) were seeded into a 96-well plate (pre-coated with 100 μL Matrigel, BD Biosciences) and cultured with 100 ng/mL human GM-CSF (GenScript). Formation of tube-like structures was monitored by microscopic observation.
Real-Time Quantitative Reverse Transcription-PCR (RT-qPCR)
The RT-qPCR analysis was performed using TAKARA SYBR Premix EXTaqTM (TAKARA BIOTECHNOLOGY, Dalian, China). The reaction mixtures containing SYBR Green were composed following the manufacturer’s protocol. The primer sequences used were as follows.
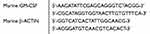 |
|
Cell Transfection
Small interfering RNA (siRNA) sequences were provided by GenePharma (Shanghai, China). The sequences of the siRNAs used were as follows.
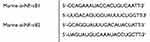 |
|
Murine 4T1 cells were seeded into six-well plates so that a confluence of 70–90% would be reached in 24 h for transfection. Lipo2000 (Thermo Fisher Scientific, Waltham, MA USA) was mixed directly with the appropriate si-NF-κB1 or si-NF-κB2 in DMEM without FBS or antibiotic. The lipoplexes were incubated for 20 min at room temperature. Then, the complete growing medium containing FBS in the plates was replaced with DMEM containing the transfection complex. After incubation at 37 °C for 4 h, the medium was replaced with complete growing medium and cultured for 48 h.
Western Blotting
Total protein of murine 4T1 cells was extracted using RIPA lysis buffer (50 mM Tris, 150 mM sodium chloride, 1% NP-40, 0.25% deoxycholate) supplemented with protease and a phosphatase inhibitor cocktail (Beyotime Biotechnology). Equal amounts of protein were separated by 8–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Merck Millipore, Burlington, MA, USA) by electroblotting. The membranes were probed with primary antibodies against B cell lymphoma 2-associated X (BAX; 1:1000, Zen-bioscience, Chengdu, Sichuan, China), IκBα, phospho-IκBα, NF-κB, phospho-NF-κB (1:1000 Cell Signaling Technology). Blots were developed with HRP-conjugated secondary antibodies and chemiluminescent substrates on Kodak X-ray film. The density of electrophoresis bands was quantified for statistical analysis.
Statistical Analysis
For quantitative data analysis, results were plotted as mean ± SD values. Statistical analysis was performed using SPSS version 22.0 (IBM Software, New York, NY, USA). Statistical significance was determined using Student’s t-test. P < 0.05 was considered statistically significant.
Results
ACT001 Inhibited Murine TNBC 4T1 Tumor Growth
To investigate the role of ACT001 (Figure 1A) in TNBC 4T1 cells, we first performed CCK-8 assays to evaluate its cytotoxicity. As shown in Figure B, after ACT001 (20, 40, 60, and 80 μM) treatment for 72 h, the proliferation of 4T1 cells decreased in a dose-dependent manner, and the half-maximal inhibitory concentration was about 43.2 μM.
Then, murine 4T1 tumor-bearing mice were established. As expected, 100 mg/kg ACT001 treatment suppressed tumor growth significantly (P < 0.05) (Figure 1C and D). TUNEL staining revealed that the number of apoptotic cells had increased following ACT001 treatment, compared with the control group (Figure 1E). Moreover, the median survival duration of ACT001-treated TNBC 4T1 tumor-bearing mice was 53 d, whereas that of the control group was 42 d (Figure 1F). Through Western blotting analysis, we demonstrated that pro-apoptotic protein BAX was up-regulated in 4T1 cells after 60 μM ACT001 treatment and in 4T1 tumors after 100mg/kg ACT001 treatment (Figure 1G).
Furthermore, ACT001 decreased lung metastasis in murine 4T1 tumor-bearing mice. As shown in Supplementary Figure 1A, the numbers of metastatic nodules were 21.25 ± 0.85 in the 100 mg/kg ACT001 treatment group and 28.50 ± 2.60 in the control group. More importantly, hematoxylin and eosin staining revealed no significant toxicity-related changes in heart, liver, or kidney tissue in murine 4T1 tumor-bearing mice after treatment with100 mg/kg ACT001 (Supplementary Figure 1B).
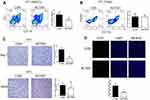
ACT001 Reduced the Accumulation of Myeloid-Derived Suppressor Cells (MDSCs) and Angiogenesis in Murine TNBC 4T1 Tumors
Owing to significant cytotoxicity of ACT001 in murine 4T1 cells, we investigated whether ACT001 suppression of murine 4T1 tumor growth was involved in the regulation of the tumor microenvironment. As shown in Figure 2A and B, flow cytometry analysis revealed that 100 mg/kg ACT001 treatment significantly decreased the numbers of MDSCs (CD11b+Gr-1+) in murine 4T1 tumors, with almost no effect on tumor-associated macrophages (CD11b+F4/80+). Furthermore, MDSCs contributed to tumor growth through immune suppression, partly by upregulating arginase 1 (Arg-1) and inducible nitric oxide synthase (iNOS). Immunohistochemical staining revealed a significant reduction in the number of Arg-1+ cells (P < 0.05) in the ACT001 treatment group, and the number of iNOS+ cells showed a decreasing trend (Figure 2C). Moreover, immunofluorescence staining of CD31 in murine 4T1 tumors revealed that ACT001 markedly decreased the number of microvessels in murine 4T1 tumors, indicating a reduction in tumor angiogenesis (Figure 2D).
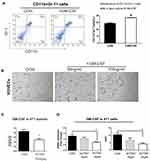
ACT001 Decreased GM-CSF Secretion via Suppressing NF-κB Activity in Murine TNBC 4T1 Tumors
GM-CSF contributes to tumor angiogenesis and the accumulation of MDSCs.9,10 Here, GM-CSF maintained the proportion of MDSCs isolated from the bone marrow of 4T1 tumor-bearing BALB/c mice cultured in vitro for 4 d (Figure 3A). Moreover, after co-culturing HUVECs with GM-CSF, primary capillary-like structures were observed (Figure 3B); these are closely associated with tumor angiogenesis. Based on changes in MDSCs and angiogenesis in vivo, we speculated that GM-CSF levels might be decreased in tumors in ACT001-treated 4T1 tumor-bearing mice. As expected, the ELISA results revealed a marked reduction in GM-CSF levels in murine 4T1 tumors in the ACT001 group (Figure 3C). Furthermore, treating 4T1 cells with ACT001 in vitro resulted in decreased GM-CSF expression and secretion (Figure 3D).
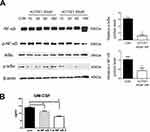
NF-κB activity is associated with cell proliferation, apoptosis, and the regulation of inflammation. ACT001 markedly suppressed NF-κB activity by attenuating phospho-IκBα and phospho-NF-κB levels in 4T1 cells (Figure 4A). Following suppression of NF-κB activity, secretory GM-CSF levels in murine 4T1 cells were markedly decreased (Figure 4B).
Discussion
This study shows that ACT001 exerts potent anticancer effects in the murine TNBC 4T1 cell line. TNBC is a highly aggressive molecular subtype of breast cancer. Conventional chemotherapy (eg, anthracyclines, taxane, and platinum-based agents) remain the mainstay of treatment for TNBC. Poly ADP-ribose polymerase inhibitors are approved as targeted treatments for TNBC. Furthermore, anti-angiogenic drugs such as bevacizumab, sunitinib, regorafenib, and immune checkpoint inhibitors (PD-1/PD-L1 inhibitors) are emerging as promising therapeutic agents for the treatment of metastatic TNBC.2
In this study, we investigated the anticancer effects of ACT001 in TNBC. ACT001 is a novel sesquiterpene lactone compound. Previous studies have reported that sesquiterpene lactones and their analogs, which include parthenolide, dimethylaminoparthenolide, and artesunate, inhibit tumor growth by activating apoptosis or inhibiting growth signals.11 As sustained growth and resistance to apoptosis are shared characteristics of cancers, we first investigated the impact of ACT001 on cell proliferation and cell apoptosis in the murine TNBC 4T1 cell line. Our results show that ACT001 suppressed the proliferation of 4T1 cells and upregulated pro-apoptotic protein BAX.
Immune evasion and tumor angiogenesis are other factors influencing TNBC growth.3,12,13 MDSCs have immunosuppressive functions and their population is increased in several cancers.14,15 In breast cancer, MDSC is associated with the clinical stage and patient prognosis, and modification of MDSCs can inhibit breast cancer growth.16,17 Furthermore, angiogenesis is essential for tumorigenesis. Suppression of tumor angiogenesis in breast cancer has been reported to inhibit tumor growth and prolongs patient survival.18,19 Our results show that ACT001 decreased the numbers of MDSCs and microvessels in the 4T1 tumor microenvironment.
Furthermore, GM-CSF upregulation in breast cancer is associated with a poor prognosis.20 GM-CSF mediates the growth, differentiation, survival, and functional activities of macrophages, granulocytes, and other leukocytes.21 Our results show that GM-CSF maintained the proliferation of MDSCs and promoted the formation of microvessels by HUVECs, consistent with previous reports.10,22,23 Moreover, ACT001 decreased GM-CSF levels in murine TNBC 4T1 in vitro and in vivo. These results indicate that ACT001-based reduction of GM-CSF levels may contribute to a reduction in MDSC accumulation and suppression of angiogenesis in murine TNBC 4T1 tumors.
NF-κB is closely associated with cell proliferation, apoptosis, and inflammation.24 NF-κB subunits (including p65) are aberrantly constitutively activated in TNBC. Activation of NF-κB promotes development and progression of breast cancer by downregulating pro-apoptotic genes, increasing the secretion of inflammatory cytokines, and mediating the accumulation of inflammatory cells.25 Hence, NF-κB is a valuable pharmacological target for breast cancer therapy. Here, ACT001 inactivated NF-κB, as evidenced by the decreased phospho-IκBα and phospho-NF-κB-p65 levels in murine TNBC 4T1 cells. Furthermore, we verified that the NF-κB pathway regulated secretory GM-CSF levels, which are potentially associated with the ACT001-mediated suppression of angiogenesis and reduction in MDSCs accumulation.
Conclusions
In summary, this study shows that ACT001 exerts potent anticancer effects against murine TNBC 4T1 tumors through growth inhibition, apoptosis induction, and the regulation of the tumor microenvironment, along with attenuation of MDSC accumulation and angiogenesis. The underlying mechanism may involve the suppression of NF-κB activity. Further studies of ACT001 may facilitate the development of novel drugs and treatment strategies for TNBC.
Acknowledgments
This study was supported by grants from Sichuan Health Planning Commission (No. 18PJ185), National Natural Science Foundation of China (grant no. 81802512), and full-time post-doctoral researcher and development foundation of Sichuan University (no. 2018SCU12034).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Elsawaf Z, Sinn HP. Triple-negative breast cancer: clinical and histological correlations. Breast Care. 2011;6(4):273–278. doi:10.1159/000331643
2. Vagia E, Mahalingam D, Cristofanilli M. The landscape of targeted therapies in TNBC. Cancers. 2020;12:4. doi:10.3390/cancers12040916
3. Diana A, Carlino F, Franzese E, et al. Early triple negative breast cancer: conventional treatment and emerging therapeutic landscapes. Cancers. 2020;12:4. doi:10.3390/cancers12040819
4. Xi X, Liu N, Wang Q, et al. Pharmacokinetics, tissue distribution and excretion of ACT001 in sprague-dawley rats and metabolism of ACT001. J Chromatogr B. 2019;1104:29–39. doi:10.1016/j.jchromb.2018.11.004
5. Xi X, Liu N, Wang Q, et al. ACT001, a novel PAI-1 inhibitor, exerts synergistic effects in combination with cisplatin by inhibiting PI3K/AKT pathway in glioma. Cell Death Dis. 2019;10(10):757. doi:10.1038/s41419-019-1986-2
6. Jin XH, Jia YS, Shi YH, et al. ACT001 can prevent and reverse tamoxifen resistance in human breast cancer cell lines by inhibiting NF-κB activation. J Cell Biochem. 2019;120(2):1386–1397. doi:10.1002/jcb.27146
7. Lee JO, Kang MJ, Byun WS, et al. Metformin overcomes resistance to cisplatin in triple-negative breast cancer (TNBC) cells by targeting RAD51. Breast Cancer Res. 2019;21(1):1–18. doi:10.1186/s13058-019-1204-2
8. Suarez I, Salmerongarcia A, Cabeza J, Capitanvallvey LF, Navas N. Development and use of specific ELISA methods for quantifying the biological activity of bevacizumab, cetuximab and trastuzumab in stability studies. J Chromatogr B. 2016;1032:155–164. doi:10.1016/j.jchromb.2016.05.045
9. Anzinger JJ, Chang J, Xu Q, et al. Murine bone marrow-derived macrophages differentiated with GM-CSF become foam cells by PI3Kγ-dependent fluid-phase pinocytosis of native LDL. J Lipid Res. 2011;12(1):30. doi:10.1194/jlr.M018887
10. Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b−Gr1− bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat. 2009;123(1):39–49. doi:10.1007/s10549-009-0622-8
11. Amorim MHR, Costa RMGD, Lopes C, Bastos M. Sesquiterpene lactones: adverse health effects and toxicity mechanisms. Crit Rev Toxicol. 2013;43(7):559–579. doi:10.3109/10408444.2013.813905
12. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–326. doi:10.1016/j.cell.2018.09.035
13. Marquardt G, Schulz TF, Schweighofer B. Angiogenesis in cancer. Nature. 2012;407(6801):249–257. doi:10.1038/35025220
14. Sanctis FD, Solito S, Ugel S, Molon B, Marigo I, Marigo I. MDSCs in cancer: conceiving new prognostic and therapeutic targets. Biochim Biophys Acta. 2015;1865(1):35–48. doi:10.1016/j.bbcan.2015.08.001
15. Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi:10.1007/s00262-008-0523-4
16. Shou D, Wen L, Song Z, Yin J, Sun Q, Gong W. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget. 2016;7(39):64505–64511. doi:10.18632/oncotarget.11352
17. Vilaleahey A, Oldford SA, Marignani PA, Wang J, Haidl ID, Marshall JS. Ranitidine modifies myeloid cell populations and inhibits breast tumor development and spread in mice. OncoImmunology. 2016;5:7. doi:10.1080/2162402X.2016.1151591
18. Zibara K, Awada Z, Dib L, et al. Anti-angiogenesis therapy and gap junction inhibition reduce MDA-MB-231 breast cancer cell invasion and metastasis in vitro and in vivo. Sci Rep. 2015;5(1):12598. doi:10.1038/srep12598
19. Bell R, Cameron D. Bevacizumab: the first anti-angiogenic agent approved for the treatment of metastatic breast cancer. Eur J Cancer Suppl. 2008;6(6):1–6. doi:10.1016/S1359-6349(08)70286-6
20. Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–620. doi:10.1016/j.ccr.2014.03.021
21. Aliper A, Friedenkorovkina VP, Buzdin A, Roumiantsev S, Zhavoronkov A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med. 2014;3(4):737–746. doi:10.1002/cam4.239
22. Valdembri D, Serini G, Vacca A, Ribatti D, Bussolino F. In vivo activation of JAK2/STAT-3 pathway during angiogenesis induced by GM-CSF. FASEB J. 2002;16(2):225–227. doi:10.1096/fj.01-0633fje
23. Finke JH, Ko JS, Rini BI, Rayman P, Ireland J, Cohen PA. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11(7):856–861. doi:10.1016/j.intimp.2011.01.030
24. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–324. doi:10.1038/nri.2017.142
25. Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Sledge GW. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17(7):3629–3639. doi:10.1128/MCB.17.7.3629
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.