Back to Journals » OncoTargets and Therapy » Volume 12
Anticancer activity of 1, 25-(OH)2D3 against human breast cancer cell lines by targeting Ras/MEK/ERK pathway
Authors Zheng W, Cao L, Ouyang L, Zhang Q, Duan B, Zhou W, Chen S, Peng W, Xie Y, Fan Q, Gong D
Received 10 October 2018
Accepted for publication 4 December 2018
Published 22 January 2019 Volume 2019:12 Pages 721—732
DOI https://doi.org/10.2147/OTT.S190432
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Wei Zheng,1 Lin Cao,2 Linna Ouyang,1 Qian Zhang,3 Bofeng Duan,1 Wei Zhou,4 Shan Chen,5 Wei Peng,1 Yi Xie,1 Qing Fan,6 Daoxing Gong7
1Department of Thyroid and Breast Surgery, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong 518112, China; 2Department of Breast Surgery, Maternal and Child Health Care Hospital of Hunan Province, Changsha, Hunan 410008, China; 3School of Clinical Medicine, Xiangnan University, Chenzhou, Hunan 423000, China; 4Department of Medical Examination, Zhuzhou Central Hospital, Zhuzhou, Hunan 412007, China; 5Department of General Surgery, The Third Hospital of Changsha, Changsha, Hunan 410013, China; 6Department of Gastrointestinal and Breast and Thyroid Surgery, Changsha Hospital of Traditional Chinese Medicine (Changsha Eighth Hospital), Changsha, Hunan 410100, China; 7Department of Surgery, The Medical School, University of South China, Hengyang, Hunan 421000, China
Purpose: Breast cancer is the most common cancer among women with ~1.67 million cases diagnosed annually worldwide, and ~1 in 37 women succumbed to breast cancer. Over the past decades, new therapeutic strategy has substantially improved the curative effect for women with breast cancer. However, the currently available ER-targeted and HER-2-based therapies are not effective for triple-negative breast cancer patients, which account for ~15% of total breast cancer cases.
Materials and methods: We reported that 1,25-(OH)2D3, a biologically active form of vitamin D3, exhibited a strong anticancer effects on the proliferation, migration, invasion, cell cycle arrest, and apoptosis of both ER-positive (MCF-7) and ER-negative breast cancer cells (MDA-MB-453).
Results: The anticancer effect of 1,25-(OH)2D3 was more potent compared to the classical chemotherapeutics tamoxifen in MDA-MB-453 cells. Furthermore, we also found that 1,25-(OH)2D3 decreased the expression of Ras and resulted in decrease of the phosphorylation of downstream proteins MEK and ERK1/2, indicating that 1,25-(OH)2D3 plays its anticancer roles through targeting the Ras/MEK/ERK signaling pathway. In addition, Ras overexpression abrogated 1,25-(OH)2D3-induced G0/G1 cell cycle arrest and apoptosis of breast cancer cells, as well as the suppression of proliferation, migration, and invasion. Our study suggested that 1,25-(OH)2D3 suppressed breast cancer tumorigenesis by targeting the Ras/MEK/ERK signaling pathway.
Conclusion: 1,25-(OH)2D3 might serve as a promising supplement for breast cancer drug therapy, especially for the ER-negative breast cancer and drug-resistant breast cancer.
Keywords: breast cancer, 1,25-(OH)2D3, ER-negative, cell apoptosis, cell proliferation
Introduction
Breast cancer is an severe public health problem and one of the leading causes of cancer-related death in women.1 There has been a 20% increase in the number of reported breast cancer patients worldwide, which resulted in 522,000 deaths since 2008 according to recent epidemiologic studies.2,3 Initiation and progression of breast cancer occurrence have been attributed to both genetic and environmental factors.4 These known prominent risk factors for the onset of breast cancer include age, lifestyle, fertility status, exposure to radiation, administration of estrogen medications, and diet, as well as certain hereditary mutations, which also play important roles in breast cancer.3,5 Breast cancer is a heterogeneous disease that could be divided into multiple clinical subtypes based on expression of tumor histological markers, including the estrogen receptors (ERs), progesterone receptors (PRs), and human epidermal growth factor-2 (HER-2).6,7 Approximately >60% of total breast cancer cases were reported to be ER, PR, and HER-2 positive.8 Dysregulation of ER expression has been observed in 60%–70% of breast cancer patients.9,10 Tamoxifen (TAM) is a nonsteroidal triphenylethylene derivative and known to compete with estrogen to inhibit ER activity associated with tumor cell growth. In clinics, TAM has been widely administrated to patients with early localized breast cancer, which could be removed by surgical resection and those with metastatic breast cancer as a mainstay hormone therapy to prevent the relapse of ER-positive cancers.11,12 However, ER-negative breast cancer is usually associated with worse short-term outcomes than ER-positive cancer and accounts for 20%–30% of all patients with breast cancers, which is especially common in women with breast cancer diagnosed at young age and those in African ancestry.13 In consideration of limitations in current therapeutic modalities for ER-negative tumors, it has been pressingly needed to search for new prognostic tools and therapy targets.
Targeted therapy, also known as molecularly targeted therapy, refers to the modalities of cancer treatments featured by the suppression of malignant cell activity and cancer progression by targeting specific bioactive macromolecules especially functional proteins, but not simply killing all actively dividing cell as traditional chemotherapy.14,15 A number of target therapy modalities such as monoclonal antibodies, tyrosine kinase inhibitors, cyclin-dependent kinase inhibitors, mammalian target of rapamycin inhibitors, and poly ADP-ribose polymerase inhibitors have been successfully applied or showed great potential as novel effective treatments for patients with breast cancer.16,17 The roles of growth factors and mitogens in regulating the gene expression and cell apoptosis and differentiation were proved to be mediated by the Ras/MEK/ERK signaling cascade.18 This pathway has been reported to be frequently activated in various human cancer types such as breast and prostate cancers. Thus, these major components of the Ras/MEK/ERK pathway are ideal and important candidate targets for therapeutic intervention of breast cancer development. As expected, specific inhibitors targeting Ras, Raf, MEK, and other downstream proteins of the Ras/MEK/ERK pathway have been developed for antibreast cancer drugs, and some of them are currently being testing by clinical trials.19
1,25-Dihydroxy vitamin D3 (1,25-(OH)2D3), a biologically active form of vitamin D3, is one of the major controlling hormones of intestinal calcium absorption.20 In recent investigations, multiple new biological effects of 1,25-(OH)2D3 has also been disclosed, including the inhibition of cell proliferation and induction of cell apoptosis, which are closely associated with cancer initiation and development.21,22 However, little is known about the potential roles of 1,25-(OH)2D3 on malignant cell functions.
In this study, we investigate the effects of 1,25-(OH)2D3 on the proliferation, apoptosis, invasion, and migration of human breast cancer cell lines MCF-7 (ER+ vitamin D receptor [VDR+]) and MDA-MB-453 (ER− VDR+). Also, the underlying molecular mechanisms were further explored with a focus on the Ras/MEK/ERK pathway. Collectively, our results proved the inhibitory roles of 1,25-(OH)2D3 on the proliferation, invasion, and migration of both the ER-positive and the ER-negative human breast cancer cell lines, which suggested that 1,25-(OH)2D3 could serve as a promising supplement for breast cancer therapy and provided a theoretical basis for the extensive application of 1,25-(OH)2D3 in clinical practice.
Materials and methods
Cell culture and treatment
Breast cancer cell lines MCF-7 (ATCC® HTB-22) and MDA-MB-453 (ATCC® HTB-131), purchased from the American Type Culture Collection, were grown in a monolayer culture in the high glucose DMEM (#11995065; Thermo Fishier Scientific) with 10% (v/v) FBS (Gibco®) and 1% (v/v) Pen-Strep (104 U/mL penicillin G sodium, 10 mg/mL streptomycin sulfate) at 37°C with supply of 5% CO2. Bovine insulin was added for MCF-7 cultivation at concentration of 2×10−6 mol/L. Both breast cancer cells were treated with 1,25-(OH)2D3 (#NIST2972A; Sigma) at different concentrations according to designated experimental requirements. Plasmids were transfected into cells using Lipofectamine 2000 (Thermo Fisher Scientific) following the manufacturer’s instructions.
Cell proliferation assay
Cultured MCF-7 and MDA-MB-453 cells were seeded into 96-well plates, which were then treated with 0.1 μm TAM or 1,25-(OH)2D3 at different concentrations (10−7, 10−8, and 10−9 mol/L) for 48 hours. The proliferation rates of breast cancer cells were finally analyzed using cell counting kit-8 (Dojindo, Japan) according to the manufacturer’s instructions. The optical densities were measured at 450 nm for evaluation of breast cancer proliferation. This assay was biologically repeated for more than three times.
Scratch wound assay
MCF-7 and MDA-MB-453 cells were grown in 35 mm dishes to 100% confluence, which were then scratched using a sterile pipette tip to form a wound. The cells were then treated with 0.1 μm TAM or 1,25-(OH)2D3 concentration gradient with absence or presence of combined treatment with ERK agonist (U46619) for 24 hours. Cell images were finally recorded using a photomicroscope for cell migration assessments. At least three biological repeats were performed for statistical analysis.
Cell invasion assay
Cell invasion assay was performed using a polyethylene terephthalate membrane (8 μm pore size) with Millicell hanging cell culture inserts and BD Matrigel (Millipore). The MCF-7 and MDA-MB-453 cells loaded into the upper chamber were treated with 0.1 μm TAM or with 1,25-(OH)2D3 in the absence or presence of the ERK agonist (U46619). Conditioned medium containing 10% (v/v) FBS was added to each well, and breast cancer cells were allowed to invade by penetrating through the membrane during 24-hour incubation at 37°C. Cells that had migrated through the filter pores were fixed with methanol, stained with crystal violet, and the invasive potential of breast cancer cells was evaluated microscopically by counting the number of invading cells. More than three biological replicates were done for cell invasion evaluation.
Cell cycle analysis
Cell cycle progression of breast cancer cells after designated treatments was analyzed by quantification of DNA content. Briefly, cultured breast cancer cells were harvested and fixed in 70% ethanol for 24–48 hours at 4°C, treated with ribonuclease to remove RNA contamination, and DNA was stained with propidium iodide (PI). Fluorescence of PI-stained cells was measured by flow cytometry. At least three biological repeats were performed for cell cycle progression analysis.
Apoptosis analysis
Cultured breast cancer cells were harvested and stained with Annexin V FITC and PI (#V13242; Thermo Fishier Scientific) according to the manufacturer’s protocol. Percentages of apoptotic cells in each group were detected by flow cytometry for calculation of the breast cancer cell apoptosis rates for three biological repeats.
Western blotting
Breast cancer cells were collected after specified treatments and lysed in RIPA buffer supplemented with protease inhibitor cocktail (Roche) for 30 minutes on ice. The protein concentration was determined via the BCA protein assay. Approximately 30 μg proteins of each group were boiled at 100°C for 5 minutes, separated by SDS-PAGE electrophoresis, and transferred into the PVDF membrane. These membranes were then blocked with 5% skim milk, incubated with first antibody at 4°C overnight, washed with tris-HCl buffer solution and tween-80 (TBST), incubated with the secondary antibody at room temperature for 1 hour, followed by washing by TBST. ECL luminescent reagent was added and the resultant signals were recorded on X-ray film in darkroom. The glyceraldehyde-3-phosphate dehydrogenase antibody was used as loading control.
Plasmid construction
For the overexpression of Ras gene in breast cancer cells, full-length Homo sapiens Ras genomic sequences based in NCBI were cloned and ligated onto pcDNA3.0 (Addgene, Watertown, MA, USA). The recombinant constructs were sequence verified through DNA sequencing by the Thermo Fishier Scientific.
Statistical analysis
Statistical analysis in this study was performed using the GraphPad Prism software. Data were expressed as mean ± SD and subjected to Student’s t-test for evaluation of the significance of differences between two groups. Each assay was biologically repeated for at least three times. Significant differences were defined by a P-value of <0.05, <0.01, or <0.001.
Results
1,25-(OH)2D3 inhibited breast cancer cell proliferation, migration, and invasion
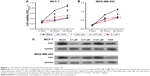
To investigate the influence of 1,25-(OH)2D3 on breast cancer cell growth and motility, we first used ER-positive breast cancer MCF-7 cells and ER-negative breast cancer DA-MB-453 cells to perform cell growth assay. Cells were treated with different concentrations of 1,25-(OH)2D3 for 48 hours, and the 0.1 μm TAM group was used as positive control. We showed that 1,25-(OH)2D3 could significantly inhibit the proliferation of both MCF-7 cells and MDA-MB-231 cells (Figure 1A and B). At concentrations of ≥10−7 mol/L, 1,25-(OH)2D3 inhibited proliferation of MDA-MB-453 cells more potently than the classical antiestrogen TAM (Figure 1B). The IC50 concentrations of 1,25-(OH)2D3 on MCF-7 and MDA-MB-231 cells were found to be 0.008 and 0.102, respectively. By Western blotting, we found that the expression level of Ki67, the proliferation marker, was greatly suppressed by 1,25-(OH)2D3 treatment in both breast cancer cell lines (Figure 1C).
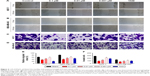
To evaluate the potential antimetastatic effects of 1,25-(OH)2D3, we analyzed its ability of inhibiting the migration and invasion of both MCF-7 cells and MDA-MB-453 cells. It was demonstrated that 1,25-(OH)2D3 inhibited the migration (Figure 2A and B) and invasion (Figure 2C and D) of MCF-7 cells, while its inhibitory effect against MDA-MB-453 cells was much more potent than TAM (Figure 2A–F). Also, we found that the effects of 1,25-(OH)2D3 on breast cancer cell proliferation, migration, and invasion were dose dependent.
1,25-(OH)2D3 induced cell cycle arrest and apoptosis of breast cancer cells
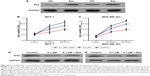
To further investigate the inhibition of breast cancer cells proliferation by 1,25-(OH)2D3, we analyzed its effect on the cell cycle progression of breast cancer cell lines. Cell population in each phase of cell cycle was determined with PI staining, followed by flow cytometry after treatments with 1,25-(OH)2D3 at different concentration for 48 and 72 hours. We showed that 1,25-(OH)2D3 increased the population of both MCF-7 cells and MDA-MB-453 cells in G0/G1 phase, which was accompanied by proportional decrease of breast cancer cells in S and G2/M phases (Figure 3A–C). Moreover, we demonstrated that 1,25-(OH)2D3 induced the apoptosis of both MCF-7 cells and MDA-MB-453 cells (Figure 3D–F). The effects of 1,25-(OH)2D3 on cell cycle arrest and apoptosis of both MCF-7 and MDA-MB-453 cells were more remarkable than cells treated with TAM (Figure 3A–F). The effects of 1,25-(OH)2D3 on breast cancer cell cycle progression and apoptosis were also dose and time dependent in MDA-MB-453 cells.
1,25-(OH)2D3 inhibited Ras expression and MEK and ERK phosphorylation in both ER-positive and ER-negative breast cancer cells
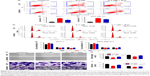
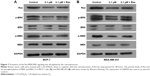
To illuminate the molecular mechanisms involved in the potential roles of 1,25-(OH)2D3 on breast cancer cells, we tested Ras protein abundance, and phosphorylation of MEK and ERK1/2 proteins in both MCF-7 cells and MDA-MB-453 cells after 1,25-(OH)2D3 treatment for 48 hours. Our Western blot assay revealed that 1,25-(OH)2D3 strongly decreased Ras protein abundance and the phosphorylation of MEK and ERK1/2 in both breast cancer cells (Figure 4). These data indicated that the anticancer effect of 1,25-(OH)2D3 was likely mediated by suppressing the activation of the Ras/MEK/ERK signal pathway.
To explore the potential functions of the Ras/MEK/ERK signal pathway in breast cancer cell function regulation by 1,25-(OH)2D3, both MCF-7 cells and MD-MB-453 cells were treated with 1,25-(OH)2D3 alone or in combination with overexpression of Ras gene, followed by determination of breast cancer cell proliferation, migration, invasion, cell cycle progression, and apoptosis. We revealed that the alterations of these cellular processes in both MCF-7 cells and MDA-MB-453 cells induced by treatment with 1,25-(OH)2D3 were recovered to a certain extent by overexpression of Ras gene (Figures 5 and 6). The downregulation of Ki67 protein expression in breast cancer cells by 1,25-(OH)2D3 was also repressed by Ras gene overexpression (Figure 5D and E). Also, the phosphorylation of MEK and ERK in breast cancer cells treated with 1,25-(OH)2D3 was significantly upregulated by Ras gene overexpression (Figure 7). Thus, our data strongly indicated that the activity of Ras/MEK/ERK signal pathway was a major mechanism underlying 1,25-(OH)2D3-induced anticancer effect in both ER-positive and the ER-negative breast cancer cells.
Discussion
Breast cancer remains a leading cause of cancer morbidity and mortality among women all over the world.23 Previous statistics showed that each year ~2,400 deaths of women with breast cancer occurred at ages below 45 years, and the majority of breast cancer-related deaths happened in women older than 45 years.24 The survival rate for women with nonmetastatic breast cancer was reported to be 98.5%, but there is a significant drop of survival rate for patients with metastatic breast cancer, which ranges from 84% to 24%.25 TAM is known to compete with estrogen to inhibit the ER activity associated with tumor cell activity and has been approved for treatment of postmenopausal women suffering from advanced breast cancer by the US Food and Drug Administration in 1977 and later for postsurgery adjuvant treatment to eradicate micrometastasis derived from primary breast cancer.26 The antiestrogenic activity of TAM mediated by ER has been well established and was the main reason for TAM treatment in ER-positive breast cancer; however, recent clinical studies applying TAM in treating patients with ER-negative breast cancers failed to produce significant benefits in terms of mortality reduction or inhibition of breast cancer recurrence.27,28 Thus, it is pressingly needed to develop novel effective therapeutic strategies for female patients with ER-negative and triple-negative breast cancers, the latter being an aggressive disease with worse clinical outcomes.
In this study, we found that TAM exhibited significant anticancer effects on both ER-positive and ER-negative breast cancer cells by inhibiting cell viability, proliferation, migration, invasion, and cell cycle progression, while promoting breast cancer apoptosis. However, the therapeutic efficacy of TAM on ER-negative breast cancer cells was found weaker than in the ER-positive ones, indicating that TAM alone was not an ideal chemotherapeutic strategy for treating ER-negative breast cancer. Thus, we investigated the functions of 1,25-(OH)2D3, one biologically active form of vitamin D3, in regulating breast cancer cell activities associated with tumorigenesis and metastasis. Hopefully, we showed that 1,25-(OH)2D3 effectively inhibited the growth, proliferation, migration, and invasion capabilities of both MCF-7 cells and MDA-MB-453 cells in the dose-dependent manner, companied by induced G0/G1 arrest and apoptosis. These cellular assays showed that 1,25-(OH)2D3 possesses significant inhibitory effects on breast cancer cell activity.
The Ras/MEK/ERK mitogen-activated protein kinase cascade has been established as a key intracellular signaling pathway responsible for regulating diverse cellular functions including cell proliferation, survival, apoptosis, motility, transcription, metabolism, and differentiation, which are all closely associated with tumorigenesis.29 Multiple components of this pathway were reported to be mutated or aberrantly expressed in human breast cancer cells and linked with poor prognosis of breast cancer patients. Moreover, the Ras/MEK/ERK pathway has also been associated with chemotherapeutic drug resistance in breast cancer.30 Thus, it is reasonable to speculate that the Ras/MEK/ERK signaling pathway would be applied as an ideal target for therapeutic intervention of breast cancer progression.19 In the present study, we found that 1,25-(OH)2D3 depressed Ras expression level and the phosphorylation of MEK and ERK1/2 in both ER-positive and ER-negative breast cancer cells, suggesting the role of Ras/MEK/ERK signaling during breast cancer inhibition by 1,25-(OH)2D3. More importantly, we proved that the expression of Ki67 gene and the phosphorylation of MEK and ERK in both breast cancer cell lines treated with 1,25-(OH)2D3 were significantly recovered by overexpression of Ras gene. Consistent with these molecular alterations, the alterations of breast cancer cell functions including growth, proliferation, migration, invasion, cell cycle progression, and apoptosis caused by 1,25-(OH)2D3 were also remarkable suppression by Ras gene overexpression, which further validated that 1,25-(OH)2D3 performs its inhibitory roles on breast cancer progression through modulation of the Ras/MEK/ERK signaling pathway.
Conclusion
We have shown that 1,25-(OH)2D3 possesses remarkable anticancer effects on both ER-positive and ER-negative breast cancer cells. Furthermore, our study identified the depressed activation of the Ras/MEK/ERK pathway by 1,25-(OH)2D3 as one key underlying mechanism. Our findings provided a rationale for preclinical and clinical evaluation of 1,25-(OH)2D3 alone or in combination with other chemotherapeutic drugs for breast cancer therapy, especially for the ER-negative breast cancer.
Acknowledgments
This research is supported by the Project of Science and Technology Department of Hunan Province (2013SK3189), Scientific Research Fund of Hunan Provincial Education Department (No 15B225), and Scientific Research Fund of Hunan Provincial Health and Family Planning Commission (No C2017014).
Disclosure
The authors report no conflicts of interest in this work.
References
Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50(1):33. | ||
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Desantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. | ||
Guo J, Sueta A, Nakamura K, et al. Genetic and environmental factors and serum hormones, and risk of estrogen receptor-positive breast cancer in pre- and postmenopausal Japanese women. Oncotarget. 2017;8(39):65759–65769. | ||
Bertrand KA, Bethea TN, Adams-Campbell LL, Rosenberg L, Palmer JR. Differential patterns of risk factors for early-onset breast cancer by ER status in African American women. Cancer Epidemiol Biomark Prevent. 2017;26(2):270–277. | ||
Hergueta-Redondo M, Palacios J, Cano A, Moreno-Bueno G. “New” molecular taxonomy in breast cancer. Clin Transl Oncol. 2008;10(12):777–785. | ||
Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. | ||
Mh L, Hou CL, Wang C, Sun AJ. HER-2, ER, PR status concordance in primary breast cancer and corresponding metastatic lesion in lymph node in Chinese women. Pathol Res Pract. 2016;212(4):252–257. | ||
McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296(5573):1642–1644. | ||
Maruani DM, Spiegel TN, Harris EN, et al. Estrogenic regulation of S6K1 expression creates a positive regulatory loop in control of breast cancer cell proliferation. Oncogene. 2012;31(49):5073–5080. | ||
Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609–1618. | ||
Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205–213. | ||
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. | ||
Murthy RK, Chavez-Macgregor M, Hortobagyi GN. Adjuvant HER2-targeted therapy update in breast cancer: escalation and de-escalation of therapy in 2018. Curr Breast Cancer Rep. 2018;10(4):296–306. | ||
Sobhani N, Ianza A, D’Angelo A, et al. Current status of fibroblast growth factor receptor-targeted therapies in breast cancer. Cells. 2018;7(7):76. | ||
Larionov AA. Current therapies for human epidermal growth factor receptor 2-positive metastatic breast cancer patients. Front Oncol. 2018;8:89. | ||
Mina A, Yoder R, Sharma P. Targeting the androgen receptor in triple-negative breast cancer: current perspectives. Onco Targets Ther. 2017;10:4675–4685. | ||
Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18(2):189–218. | ||
McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773(8):1263–1284. | ||
Christakos S. Recent advances in our understanding of 1,25-dihydroxyvitamin D(3) regulation of intestinal calcium absorption. Arch Biochem Biophys. 2012;523(1):73–76. | ||
Cove-Smith A, Hendry BM. The regulation of mesangial cell proliferation. Nephron Exp Nephrol. 2008;108(4):e74–e79. | ||
Zhang CJ, Zhao D, Yin X, et al. Effects of 1,25(OH)2D3 on proliferation and apoptosis of human glomerular mesangial cells. Am J Transl Res. 2016;8(6):2659–2666. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Sirkisoon SR, Carpenter RL, Rimkus T, Miller L, Metheny-Barlow L, Hw L. EGFR and HER2 signaling in breast cancer brain metastasis. Front Biosci. 2016;8:245–263. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Manna S, Holz MK. Tamoxifen action in ER-negative breast cancer. Sign Transduct Insights. 2016;5:1–7. | ||
Naugler C. Estrogen receptor testing and 10-year mortality from breast cancer: a model for determining testing strategy. J Pathol Inform. 2012;3(1):19. | ||
Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. | ||
Lin Z, Zhang C, Zhang M, et al. Targeting cadherin-17 inactivates Ras/Raf/MEK/ERK signaling and inhibits cell proliferation in gastric cancer. PLoS One. 2014;9(1):e85296. | ||
McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46(1):249–279. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.