Back to Journals » Infection and Drug Resistance » Volume 15
Antibiotics Resistance Prevalence of Helicobacter pylori Strains in Northwest China
Authors Xu H , Yun J, Li R, Ma X, Gou L, Che T, Zhang D
Received 24 July 2022
Accepted for publication 10 September 2022
Published 20 September 2022 Volume 2022:15 Pages 5519—5528
DOI https://doi.org/10.2147/IDR.S383444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Huimei Xu,1 Jianwei Yun,1 Ruiying Li,1 Xueni Ma,1 Lingzhu Gou,1 Tuanjie Che,2 Dekui Zhang1,3
1Department of Gastroenterology, Lanzhou University Second Hospital, Lanzhou, People’s Republic of China; 2Department of Center of Genomics, Key Laboratory of Functional Genomics and Molecular Diagnosis of Gansu Province, Lanzhou, People’s Republic of China; 3Department of Gastroenterology, Key Laboratory of Digestive Diseases of Lanzhou University Second Hospital, Lanzhou, People’s Republic of China
Correspondence: Dekui Zhang; Tuanjie Che, Lanzhou University Second Hospital, Cuiying Number 82, Chengguan District, Lanzhou, People’s Republic of China, Tel/Fax +86 17797634824, Email [email protected]; [email protected]
Purpose: This study aims to estimate the resistance rate of Helicobacter pylori (HP) to commonly used antibiotics and analyze the potential influencing factors in northwest regions of China.
Patients and Methods: HP-positive patients visiting the outpatient department of multiple hospitals were enrolled in the study. Then, gastric mucosal biopsy specimens were collected for HP isolation, culture, and investigation of the resistance rate of HP to amoxicillin, metronidazole, tetracycline, levofloxacin, and clarithromycin by Epsilometer test (E-test) antibiotic susceptibility testing. In addition, multi-drug resistance, the influence of HP eradication history, age, and region of residence on drug resistance rate were analyzed.
Results: In total, 198 HP clinical strains were successfully isolated and cultured. The resistance rates of amoxicillin, metronidazole, tetracycline, levofloxacin, and clarithromycin were 16.16%, 85.86%, 7.58%, 46.46%, and 55.05%, respectively. The multi-drug resistance rates demonstrated that dual and triple resistances were 30.30% and 22.73%, respectively. The quadruple resistance rate reached 9.60%. Our results revealed that the prior eradication history of HP significantly increased levofloxacin and clarithromycin resistance. Metronidazole and levofloxacin resistances significantly differed among different age groups, which presented an upward trend with increasing age. Drug resistance rates varied with geographic regions, especially amoxicillin and clarithromycin resistance, which were highest in Hexi Corridor and Longnan regions.
Conclusion: The current situation of HP resistance to common antibiotics is severe. Tetracycline is the most sensitive antibiotic, followed by amoxicillin, the first choice for HP eradication. However, the eradication failure of HP may lead to an increase in the resistance rate. Therefore, it is necessary to strengthen the standardized diagnosis and treatment of HP to improve the primary eradication rate.
Keywords: Helicobacter pylori, antibiotic, drug resistance, eradication
Introduction
HP, a gram-negative, microaerophilic bacterial pathogen, infects about 50% population worldwide, and HP infection is more frequent in developing countries.1 China’s northwest regions (Gansu province) experience a high prevalence of HP infection and a high incidence of gastric cancer. The eradication rate of HP eradication has generally decreased as resistance to the most commonly used antibiotics has increased. The most recent guidelines of the Maastricht V/Florence Consensus Report2 and the Fifth Chinese Consensus Report3 have recommended bismuth-containing quadruple regimen (a combination of PPI, bismuth, and two antibiotics) as the first-line treatment for HP. Commonly used antibiotics include amoxicillin, clarithromycin, levofloxacin, tetracycline, metronidazole, and furazolidone. Multiple studies focusing on drug resistance rate revealed a high level of HP resistance to clarithromycin, levofloxacin, and metronidazole in most countries and areas. Therefore, these antibiotics are unrecommended for treatment-naive patients.4–7 The HP eradication regimens should be standardized according to local resistance patterns and eradication history.8 There are significant differences in the HP resistance rate between regions, and the HP resistance rate within the same region can dramatically change over time.5 In September 2020, China released the drug resistance map of HP, aiming to dynamically display the resistance rate in different regions and populations and provide a reference for the treatment schemes to eradicate HP. However, the lack of monitoring data and dynamic updates of HP resistance to common antibiotics in northwest China has challenged the empirical treatment of HP infection. Based on this, we collected gastric mucosal specimens of HP-positive patients from multicenter hospitals in Gansu province. We carried out an antibiotic susceptibility test to analyze the current status of HP resistance in Gansu province to guide the selection of empirical eradication therapy for HP.
Materials and Methods
Subjects
From September 2018 to December 2020, a total of 320 patients attending the gastroenterology outpatient departments of ten hospitals in Gansu province were initially screened and enrolled, who were confirmed as being infected with HP by 13C-/14C- urea breath test (UBT). Multicenter hospitals included Lanzhou University Second Hospital, Wuwei Tumor Hospital of Gansu, The Third People’s Hospital of Gansu Province, The 940th Hospital of Joint Logistics Support Force of People’s Liberation Army, Affiliated Hospital of Gansu University of Traditional Chinese Medicine, The Second People’s Hospital of Tianshui, People’s Hospital of Dingxi, The Second People’s Hospital of Dingxi, The Second People’s Hospital of Lanzhou, and People’s Hospital of Jiuquan City.
Inclusion criteria were as follows: (1) 18–75 years old, both genders; (2) 13C-/14C-UBT was positive; (3) No medication history of antibiotics, PPI, bismuth, gastric mucosal protective agents, aspirin, anti-HP traditional Chinese medicine within four weeks; (4) Voluntary participation in the project and signed informed consent. Exclusion criteria included the following: (1) patients with gastrointestinal malignancy, massive bleeding of ulcers, Zollinger-Ellison syndrome, and other diseases; (2) patients with a history of gastrointestinal surgery, such as subtotal gastrectomy, gastroplasty, and vagotomy; (3) patients complicated with serious diseases of heart, liver, lung, kidney, blood, and other systems; (4) pregnant or lactating women. All patients were subjected to gastroscopy, and mucosa biopsy specimens from gastric antrum were taken for HP isolation, culture, and drug sensitivity test.
Culture and Identification of HP
Gastric mucosa biopsy specimens were collected and immersed in Brain Heart Infusion (BHI) broth (Solaibao Biological Technology, Beijing, China) containing 20% glycerin, then immediately stored at –80 °C. When we isolated and cultured HP strains, the specimens were thawed at room temperature, and homogenates were prepared using a sterile tissue homogenizer. The tissue homogenate was inoculated onto Columbia Blood Agar (Solaibao Biological Technology, Beijing, China) plates containing 5% sterile defibrinated sheep blood (Ruite Biotechnology, Guangzhou, China) and HP selective antibiotics additives (including vancomycin hydrochloride, soluble amphotericin B, cefsulodin and trimethoprim, purchased from Solaibao Biological Technology, Beijing, China) for the selective cultivation of HP isolates. The plates were incubated in a Tri-Gas Incubator (Heal Force, Shanghai Lishen Scientific Equipment Co.Ltd., Shanghai, China) with 85% nitrogen gas, 10% carbon dioxide, and 5% oxygen gas for 3–5 days at 37 °C under microaerophilic conditions to observe the growth of HP colonies. HP was identified by colony morphology, Gram staining (Gram staining Kit, Zhuhai Besso Biotechnology Co. Ltd., Zhuhai, China), and positive test (oxidase, catalase, and rapid urease test).
Antimicrobial Susceptibility Testing Using the E-Test Method
The antimicrobial susceptibility of the HP isolates was determined using the E-test method (E-test gradient strips, Liofilmchem, Italy). The bacterial suspensions were prepared by placing HP colonies in 1 mL of sterile 0.9% saline with a sterile cotton swab and adjusted to a turbidity of 2 McFarland (approximately 6×108 CFU/mL (CFU: colony forming units)) using an electronic turbidimeter (DensiCHEK, BioMérieux, France). Then, 200 µL bacterial suspensions were evenly inoculated on the Columbia Blood Agar (Solaibao Biological Technology, Beijing, China) plates containing 5% sterile defibrinated sheep blood (Ruite Biotechnology, Guangzhou, China). Subsequently, the E-test gradient strips (Liofilmchem, Italy) of five antibiotics were applied to the agar surface, and the plates were incubated at 37 °C under microaerophilic conditions for 72 h. The minimum inhibitory concentration (MIC) values for amoxicillin, metronidazole, tetracycline, levofloxacin, and clarithromycin were determined as the lowest concentration of test antibiotics completely inhibited HP growth. According to the criteria of antimicrobial resistance breakpoints established by the Clinical and Laboratory Standards Institute (CLSI) and Europe Committee on Antimicrobial Susceptibility Testing (EUCAST), in conjunction with the MICs value in the previous literature, the resistance MIC values were defined as amoxicillin>0.125 µg/mL, metronidazole>8 µg/mL, tetracycline>1 µg/mL, levofloxacin>1 µg/mL, clarithromycin>0.5 µg/mL in this study.9–11 The standard HP strains 26695 and SS1 (gifted from the Key Laboratory of the Digestive System Tumors of Gansu Province, Lanzhou, Gansu, China) were used as the controls for the susceptibility test.
Statistical Analysis
Statistical data analysis and graphing were performed using SPSS 21.0 statistical software and Prism7 (GraphPad software). The quantitative data with a normal distribution were presented as mean ± standard deviation (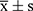 ), and an independent-samples t-test was used to compare the groups. In addition, count data were expressed by the number of cases and composition ratio (%), the chi-square test was performed for comparison between the groups, and P<0.05 indicated a statistically significant difference.
), and an independent-samples t-test was used to compare the groups. In addition, count data were expressed by the number of cases and composition ratio (%), the chi-square test was performed for comparison between the groups, and P<0.05 indicated a statistically significant difference.
Results
Baseline Characteristics
In total, 320 patients with HP infection meeting the inclusion criteria were included in this study. Gastric mucosal specimens were collected. We successfully isolated and cultured HP strains from 198 patients, including 132 males (66.67%) and 66 females (33.33%), aged from 23 to 73 years (48.29 ± 10.77). The results of endoscopic diagnosis included 44 cases of gastric ulcer, 16 of duodenal ulcer, 10 of compound ulcer, 3 of gastric cancer, and 125 of chronic gastritis. In addition, there were 150 patients (75.76%) without HP eradication history and 48 (24.24%) with HP eradication history. In addition, according to the unique topography of Gansu province, the regions where the objects were located were grouped as follows: Hexi Corridor (30 cases), Longnan Montane (11 cases), Longzhong and East Loess Plateau (150 cases), and Gannan Plateau (7 cases). Baseline data were compared based on HP antibiotic resistance, baseline and demographic characteristics summarized in Table 1.
 |
Table 1 Comparison of Baseline Data on Antibiotic Resistance |
HP Resistance to Five Commonly Used Antibiotics
A total of 198 HP isolates were successfully cultured and carried out sensitivity tests in vitro. The antimicrobial susceptibility test demonstrated that the resistance rate of HP to tetracycline was lower (7.58%, 15/198), followed by amoxicillin (16.16%). On the other hand, the resistance rates to levofloxacin and clarithromycin were higher, reached to 46.46% and 55.05%, respectively. Metronidazole resistance was the highest (85.86%). The results were illustrated in Table 2.
 |
Table 2 The Resistance Rate of HP to Antibiotics |
Multiple Antibiotic Resistance Rate of HP
Among these 198 clinical isolates, 15 (7.58%) were susceptible to all five antibiotics, while 7 (3.54%) were resistant to all antibiotics, and 52 (26.26%) were resistant to a single antibiotic. The total dual resistance rate was 30.30% (60/198). Metronidazole + clarithromycin was the highest (15.66%), followed by metronidazole + levofloxacin (12.63%). Moreover, Triple drug resistance was 22.73% (45/198), and the combination of metronidazole, levofloxacin, and clarithromycin was the highest (18.69%). The quadruple drug resistance rate reached 9.60% (19/198). The multiple drug resistance patterns were displayed in Table 3.
 |
Table 3 Multiple-Drug Resistance of HP |
Influence of HP Eradication History on Resistance Rate
A total of 198 subjects were followed up, with 150 naive patients without eradication history (a primary infection) and 48 with a history of HP eradication (a secondary infection). The study demonstrated that patients with a previous history of eradication significantly increased the resistance rates for levofloxacin and clarithromycin (P < 0.05). However, the resistance rates for the other three antibiotics were disassociated with eradication history, as displayed in Table 4 and Figure 1.
 |
Table 4 Influence of HP Eradication History on Resistance Rate |
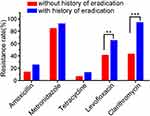 |
Figure 1 Influence of HP eradication history on resistance rate. Note: **P<0.01; ***P<0.001. |
Resistance of HP in Different Age Groups
The subjects were divided into five age groups ranging from 23 to 73 according to the per 10 years of age. Only one patient of 73 years was incorporated into the 60~ years old group. Specific age groups and the corresponding resistance rates were presented in Table 5. HP resistance rates to metronidazole and levofloxacin were significantly different in various age groups (P < 0.05), with an overall increasing trend with age. However, there was no significant difference in resistance rates for amoxicillin, tetracycline, and clarithromycin in different age groups (Figure 2).
 |
Table 5 The Resistance Rate of HP in Different Age Groups |
 |
Figure 2 The resistance rate of HP in different age groups. |
HP Resistance in Different Areas of Gansu Province
The resistance rates for amoxicillin and clarithromycin were significantly different among regions (P < 0.05), which were higher in Hexi Corridor and Longnan region. Tetracycline resistance was generally lower in different regions. A higher resistance rate was from Hexi Corridor (16.67%). Metronidazole demonstrated the highest resistance rate in all regions. The resistance rate for levofloxacin was higher in Hexi Corridor and Longnan Montane (Table 6 and Figure 3).
 |
Table 6 HP Resistance Rate in Different Areas of Gansu Province |
 |
Figure 3 HP resistance to antibiotics in different areas of Gansu Province. |
Discussion
HP infection is one of the most common chronic bacterial infections worldwide. Recently, the overall prevalence of HP infection has steadily declined. However, the infection rate in some developing countries remains elevated, related to the social and economic levels and health or environmental conditions.7 The prevalence of HP infection in China has reached 40–60%.12,13 HP has been defined as a class I carcinogen of gastric cancer. Successful eradication of HP can reduce the risk of gastric cancer and prevent HP-related gastrointestinal diseases, such as gastritis and peptic ulcer.14,15 Therefore, the diagnosis and treatment of HP have received increasing attention. At present, eradication treatment of HP is facing significant challenges due to the increasing drug resistance rate, poor patient compliance, and other factors.
HP resistance to commonly used antibiotics is severe, and clarithromycin resistance is generally high in most regions. In 2017, WHO listed HP as a “priority pathogen”.16 The Maastricht V consensus suggested that clarithromycin-based triple therapy should not be an empirical eradication regimen if the prevalence of clarithromycin resistance reaches 15%.2 In our study, the HP resistance rate to clarithromycin in Gansu province was 55.05%, much higher than the threshold. The fifth Chinese National Consensus Report on the management of HP infection recommended that seven bismuth-based quadruple therapies as the first-line treatment for HP, reached more than 85% eradication rate.3 However, a prospective multicenter study in Gansu suggested that the eradication rate of quadruple therapy combined with amoxicillin, clarithromycin, bismuth, and PPI was even lower than 50%,17 which was considered to be closely related to the high clarithromycin resistance. In addition, HP resistance to levofloxacin has increased yearly, and our study revealed that levofloxacin resistance was 46.46% in the northwest region of China, which may be associated with the widespread use in gastrointestinal, urinary, and respiratory infections. The resistance rates of clarithromycin and levofloxacin are more than 15% in most countries and regions, which is the limited resistance value for empirical treatment regimens based on these two antibiotics. Using clarithromycin and levofloxacin in eradication treatment is unrecommended unless prior antibiotic sensitivity is determined. This emphasizes the importance of continuously monitoring regional resistance rates in determining treatment regimens.2,6
Furthermore, the rate of metronidazole resistance is generally high worldwide, with a resistance rate of 85.86% in China northwest in our study. The possible reasons leading to a high prevalence of metronidazole resistance include non-standardized anti-HP treatment and long-term extensive use against anaerobic bacteria involved in various oral diseases, gynecological infections (especially vaginosis), and surgical anti-infection treatment. The study suggested that the previous history of nitroimidazole had no significant effect on the eradication rate of the bismuth-based quadruple therapy. However, repeated metronidazole application requires optimized dose. The metronidazole dosage could increase to 1600 mg/day, which may overcome drug resistance to a certain extent and achieve good efficacy. Notably, metronidazole should not be used again for HP eradication if the initial treatment optimized the dose.3,18
Combined with available data on HP resistance patterns in China, this trend of HP resistance to antibiotics in the northwest (Gansu) was consistent with that in most regions. The resistance rate for metronidazole was the highest, followed by clarithromycin and levofloxacin, while HP resistance rates to amoxicillin and tetracycline were relatively low. Moreover, the prevalence of multiple antibiotic resistance of HP gradually increased around the world, one of the major causes of HP eradication failure.19 However, there were still significant differences in drug resistance rates in different regions. A multicenter study in China4 depicted that the overall resistance rate for clarithromycin was 47.24%, and levofloxacin resistance was 41.40%. In addition, the overall resistance rates for amoxicillin, tetracycline, and furazolidone were 3.23%, 1.65%, and 2.05%, respectively. However, the overall resistance rate for amoxicillin in northwest China reached 15.89%, consistent with our findings. The higher resistance rate for amoxicillin in the area may be partly due to numerous non-standard amoxicillin use in recent years. Furthermore, HP eradication history significantly impacted drug resistance rate, especially clarithromycin and levofloxacin. The secondary resistance rates were significantly higher than the primary resistance. Therefore, the local resistance patterns and previous eradication history should be considered comprehensively when selecting second-line regimens for HP rescue therapy. Repeated antibiotic use is not recommended.
In this study, we found that the tetracycline resistance rate was the lowest in the northwest China, and the antibiotic was available in Gansu province, which could be the preferred antibiotic for HP eradication in this area. Moreover, the HP resistance rate to furazolidone is generally low in different regions, which can be used to treat refractory HP infection and attain better efficacy.20 It is noteworthy that adverse events and patients’ compliance with tetracycline and furazolidone. As for some patients allergic to penicillin, the bismuth-based quadruple regimen containing tetracycline, furazolidone, or metronidazole with an optimized dose, should be adopted to eradicate HP and overcome high resistance to levofloxacin and clarithromycin.21,22 Individualized treatment based on antibiotics sensitivity can effectively improve the eradication rate of HP as first-line treatment and rescue therapy. Additionally, a previously proven effective empiric therapy could also attain a high eradication rate of HP.23 Therefore, continuous surveillance of local antibiotic resistance patterns is particularly important for empiric treatment.
There are still some limitations in this study. First, we included a small sample size, especially the low proportion of patients in different age groups and regions. Thus, we cannot adequately assess the differences in HP resistance rate among various populations. Second, the enrolled subjects were all from Gansu province, the study lacks resistance data from other provinces in northwest China. In the future, it is necessary to expand the sample size from different regions, age distributions and other influencing factors, to further explore and monitor the HP resistance in northwest China more comprehensively.
Conclusion
To summarize, the situation with HP resistance in northwest China is challenging. The prevalence of clarithromycin and levofloxacin resistance rates increase gradually, empirical triple therapies containing these two antibiotics are unrecommended unless susceptibility-guided therapy is carried out. In contrast, tetracycline and amoxicillin resistance rates are relatively low, making them the preferred treatment for HP eradication. Therefore, eradication regimens of HP infection should be selected according to individual susceptibility tests or local resistance status to reduce the secondary resistance rate and improve the eradication rate of HP.
Ethics Approval
This study was reviewed and approved by the Medical Ethics Committee of Lanzhou University Second Hospital (2019A-178). This study complies with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Key R & D Program of Gansu Province [grant number 20YF8FA078]; Natural Science Foundation of Gansu Province [grant number 21JR1RA131]; Key Talent Project of Gansu Province [grant number 2022RCXM071]; Lanzhou Chengguan District Science and Technology Project [grant number 2018SHFZ0037].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kato M, Toda A, Yamamoto-Honda R, Arase Y, Sone H. Association between Helicobacter pylori infection, eradication and diabetes mellitus. J Diabetes Investig. 2019;10(5):1341–1346. doi:10.1111/jdi.13011
2. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-The Maastricht V/florence consensus report. Gut. 2017;66(1):6–30. doi:10.1136/gutjnl-2016-312288
3. Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management ofHelicobacter pyloriinfection. Helicobacter. 2018;23(2):e12475. doi:10.1111/hel.12475
4. Zhong Z, Zhang Z, Wang J, et al. A retrospective study of the antibiotic-resistant phenotypes and genotypes of Helicobacter pylori strains in China. Am J Cancer Res. 2021;11(10):5027–5037.
5. Megraud F, Bruyndonckx R, Coenen S, et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021;70(10):1815–1822. doi:10.1136/gutjnl-2021-324032
6. Gökçekuyu BM, Yılmaz Ö, Soytürk M, Ellidokuz H, Akpınar H, Şimşek İ. Unacceptable antibiotic resistance rates for Helicobacter pylori in Turkey: something must change. Turk J Gastroenterol. 2021;32(3):269–275. doi:10.5152/tjg.2021.20210
7. Bujanda L, Nyssen OP, Vaira D, et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013–2020: results of the European Registry on H. pylori management (HP-EuReg). Antibiotics. 2021;10(9):1058. doi:10.3390/antibiotics10091058
8. Zagari RM, Frazzoni L, Marasco G, Fuccio L, Bazzoli F. Treatment of Helicobacter pylori infection: a clinical practice update. Minerva Med. 2021;112(2):281–287. doi:10.23736/S0026-4806.20.06810-X
9. Hsieh MT, Chang WL, Wu CT, et al. Optimizing the MIC breakpoints of amoxicillin and tetracycline for antibiotic selection in the rescue therapy of H. pylori with bismuth quadruple regimen. Eur J Clin Pharmacol. 2020;76(11):1581–1589. doi:10.1007/s00228-020-02938-5
10. Alarcon T, Urruzuno P, Martinez MJ, et al. Antimicrobial susceptibility of 6 antimicrobial agents in Helicobacter pylori clinical isolates by using EUCAST breakpoints compared with previously used breakpoints. Enferm Infecc Microbiol Clin. 2017;35(5):278–282. doi:10.1016/j.eimc.2016.02.010
11. Jung DH, Kim JH, Jeong SJ, et al. Peptide nucleic acid probe-based analysis as a new detection method for clarithromycin resistance in Helicobacter pylori. Gut Liver. 2018;12(6):641–647. doi:10.5009/gnl18111
12. Xie C, Lu N. Review: clinical management of Helicobacter pylori infection in China. Helicobacter. 2015;20(1):1–10. doi:10.1111/hel.12178
13. Tian L, Yao Y, Yin L, et al. Direct detection of antibiotic resistance in Chinese Helicobacter pylori clinical isolates by sequencing-based approach. J Healthc Eng. 2022;6436256. doi:10.1155/2022/6436256
14. Lee Y, Chiang T, Chou C, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150(5):1113–1124.e1115. doi:10.1053/j.gastro.2016.01.028
15. Ma J, Yu M, Shao QQ, et al. Both family-based Helicobacter pylori infection control and management strategy and screen-and-treat strategy are cost-effective for gastric cancer prevention. Helicobacter. 2022;27(4):e12911. doi:10.1111/hel.12911
16. Yang L, Zou A, Wu H, et al. World Health Organization: list of bacteria for which new antibiotics are urgently needed. Biomed Res Int. 2017;2021:6150628.
17. Yang Q, Shang Q, Wei GQ, et al. Jinghuaweikang capsules combined with Quadruple therapy in the treatment of Helicobacter pylori infection: a multicenter, randomized, controlled, clinical study. Zhonghua Yi Xue Za Zhi. 2019;99(4):295–300. doi:10.3760/cma.j.issn.0376-2491.2019.04.012
18. Nie SS, Song ZQ, Suo BJ, Xue Y, Meng LM, Zhou LY. The exposure of antibiotics on the eradication of bismuth quadruple therapy in H. pylori infection. Zhonghua Nei Ke Za Zhi. 2021;60(11):977–981. doi:10.3760/cma.j.cn112138-20210101-00001
19. Kasahun GG, Demoz GT, Desta DM. Primary resistance pattern of Helicobacter pylori to antibiotics in adult population: a systematic review. Infect Drug Resist. 2020;13:1567–1573. doi:10.2147/IDR.S250200
20. Miftahussurur M, Waskito LA, Syam AF, et al. Alternative eradication regimens for Helicobacter pylori infection in Indonesian regions with high metronidazole and levofloxacin resistance. Infect Drug Resist. 2019;12:345–358. doi:10.2147/IDR.S187063
21. Song Z, Fu W, Zhou L. Cefuroxime, levofloxacin, esomeprazole, and bismuth as first-line therapy for eradicating Helicobacter pylori in patients allergic to penicillin. BMC Gastroenterol. 2019;19(1):132. doi:10.1186/s12876-019-1056-3
22. Dutta AK, Phull PS. Treatment of Helicobacter pylori infection in the presence of penicillin allergy. World J Gastroenterol. 2021;27(44):7661–7668. doi:10.3748/wjg.v27.i44.7661
23. Luo L, Huang Y, Liang X, Ji Y, Yu L, Lu H. Susceptibility-guided therapy for Helicobacter pylori-infected penicillin-allergic patients: a prospective clinical trial of first-line and rescue therapies. Helicobacter. 2020;25(4):e12699. doi:10.1111/hel.12699
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
