Back to Journals » Journal of Inflammation Research » Volume 14
Antibiotic Combined with Epitope-Specific Monoclonal Antibody Cocktail Protects Mice Against Bacteremia and Acute Pneumonia from Methicillin-Resistant Staphylococcus aureus Infection
Authors Duan L, Zhang J , Chen Z, Gou Q, Xiong Q, Yuan Y, Jing H, Zhu J, Ni L, Zheng Y, Liu Z , Zhang X, Zeng H, Zou Q, Zhao Z
Received 25 June 2021
Accepted for publication 20 August 2021
Published 30 August 2021 Volume 2021:14 Pages 4267—4282
DOI https://doi.org/10.2147/JIR.S325286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
LianLi Duan,1,* Jinyong Zhang,1,* Zhifu Chen,1 Qiang Gou,1 Qingshan Xiong,1 Yue Yuan,1 Haiming Jing,1 Jiang Zhu,2 Li Ni,3 Yuling Zheng,4 Zhiyong Liu,5 Xiaokai Zhang,1 Hao Zeng,1 Quanming Zou,1 Zhuo Zhao1
1National Engineering Research Center of Immunological Products, Department of Microbiology and Biochemical Pharmacy, College of Pharmacy, Army Medical University, Chongqing, 400038, People’s Republic of China; 2Department of Pathology, Southwest Hospital, Army Medical University, Chongqing, 400038, People’s Republic of China; 3Obstetrics and Gynecology, The First People’s Hospital of Jiulongpo District, Chongqing, 400050, People’s Republic of China; 4State Key Laboratory of Pathogens and Biosecurity, Institute of Microbiology and Epidemiology, Beijing, 100071, People’s Republic of China; 5Department of Laboratory Medicine, Southwest Hospital, Army Medical University, Chongqing, 400038, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhuo Zhao; Quanming Zou Email [email protected]; [email protected]
Purpose: We previously reported that monoclonal antibody (mAb) cocktail improves survival in Staphylococcus aureus infection. In this study, we used acute pneumonia model and lethal sepsis model to investigate the efficacy of antibiotic combined with epitope-specific mAb cocktail in treating MRSA252 infection.
Methods: MRSA252 was challenged by tail vein injection or tracheal intubation to establish sepsis model or pneumonia model. One hour after infection, the mice received a single intravenous injection of normal saline, vancomycin, and vancomycin combined monoclonal antibody, linezolid alone or linezolid combined monoclonal antibody. Daily record survival rate (total 7 days), bacterial load, histology, cytokine analysis of serum and alveolar lavage fluid, and in vitro determination of the neutralizing ability of antibodies to SEB toxin and Hla toxin explained the mechanism of antibody action.
Results: The mAb cocktail combined with low doses of vancomycin or linezolid improved survival rates in acute pneumonia model (70%, 80%) and lethal sepsis model (80%, 80%). Epitope-specific monoclonal antibodies reduced bacterial colonization in the kidneys and lungs of mice and inhibited the biological functions of the toxins Hla and SEB in vitro. Compared to the antibiotic alone or PBS groups, the combination group had higher levels of IL-1α, IL-1β and IFN-γ and lower levels of IL-6, IL-10, TNF-α. Further, the combination of antibiotic and mAb cocktail improved infection survival against the clinical MRSA isolates in a lethal sepsis model.
Conclusion: This study demonstrates a novel method to treat people with low immunity against drug-resistant S. aureus infections.
Keywords: methicillin-resistant Staphylococcus aureus, immunodominant epitope, monoclonal antibody, lethal sepsis, pneumonia
Plain Language Summary
We previously reported that an immunodominant epitope-specific monoclonal antibody (mAb) cocktail improves survival in a mouse model of MRSA (Methicillin-resistant Staphylococcus aureus) bacteremia. The mAb cocktail includes four B-cell immunodominant epitope-specific mAbs, including Hla48-65-mAb, IsdB432-449-mAb, SEB78-95-mAb, and SEB222-239-mAb. This study confirmed that the combination treatment of the mAb cocktail and low-dose vancomycin or linezolid was effective against MRSA252 infection in lethal sepsis and acute pneumonia mouse models. Among these mAbs, SEB222-239-mAb inhibited the ability of native SEB to induce T cell mitogenesis and cytokine production in splenocytes, and Hla48-65-mAb inhibited the hemolytic activity of native Hla. Compared to the antibiotic alone or PBS groups, the combination group had higher levels of IL-1α, IL-1β and IFN-γ and lower levels of IL-6, IL-10, TNF-α. Further, the combination of antibiotic and mAb cocktail improved infection survival against the clinical MRSA isolates in a lethal sepsis model. This study demonstrates a novel method to treat people with low immunity against drug-resistant S. aureus infections.
Introduction
Staphylococcus aureus (S. aureus) is a very common human pathogenic microorganism that causes a variety of clinical infections. Methicillin-resistant S. aureus (MRSA) is a serious threat, and the spread of its drug resistance has posed a challenge to the healthcare system worldwide.1 Clinically, it causes sepsis, pneumonia, skin infections, fractures, and trauma-associated infections.2 MRSA infections are associated with an enormous burden of morbidity and mortality in children and adults.3 Current treatments for MRSA include vancomycin, daptomycin, teicoplanin, linezolid, and other antibiotics, among which the current standard therapy for MRSA bacteremia is vancomycin or daptomycin.4–6 In developed countries such as the United States, the resistance of MRSA to β-lactam antibiotics has posed a major challenge in hospitals and other medical institutions.7 However, antibiotic resistance is an unavoidable problem, and the massive use of these antibiotics causes side-effects such as ototoxicity, nephrotoxicity, and neurotoxicity,8–10 which cannot be ignored. Clinical treatment of multidrug-resistant (MDR) bacterial infections is usually carried out with new antibiotics, but this may not solve the fundamental problem of drug resistance.11 Therefore, it is urgent to find new treatment methods.
Antibodies were produced by B cells, they have multiple functions in pathophysiology, including infection. Antibodies are versatile therapeutic tools. They can neutralize pathogens and their toxins, which reduce host damage associated with infection. Antibody-based approaches have been proven to be effective against S. aureus infections.12 Monoclonal antibodies will recruit the host’s immune system to perform effector functions such as ADCC (antibody-dependent cytotoxicity), complement fixation, and opsonization. Although both antibiotics and phagocytic antibodies kill bacteria, but they do not prevent tissue damage caused by bacterial toxins. Therefore, monoclonal antibody targeting bacterial toxins combined with antibiotic therapy may be a more effective method to treat bacterial infections, such as drug-resistant S. aureus infections.
S. aureus has various virulence factors which mainly include some surface antigens and secreted toxins, it is unlikely that focusing on a single antigen will cripple the bacterium. There is a general consensus that the protective immunity must target several virulence factors with different roles, which might mean that the protection should focus on the combination of antibodies targeting different antigens in the strains. In a previous study, we developed a five-antigen S. aureus vaccine (rFSAV) comprising different antigens that is currently in clinical trials (Phase I of China Clinical Trial: CTR20160004, http://www.chinadrugtrials.org.cn/). The rFSAV vaccine contains five conserved antigens, namely, the secreted factors α-hemolysin (Hla), staphylococcal enterotoxin B (SEB), and three surface proteins staphylococcal protein A (SpA), iron surface determinant B N2 domain (IsdB-N2), and manganese transport protein C (MntC). Among these antigens, SEB is one of the superantigens of S. aureus, Hla, also known as α-Toxin, targets cell membranes resulting in cell lysis, and IsdB is one of the surface proteins that promote heme-iron scavenging from host hemoglobin. As the complete antigen is not as potent as the immunodominant epitope,13 only a few immunodominant epitopes are sufficient to induce a protective response.14 We identified B-cell immunodominant epitopes of the four antigens of rFSAV using antisera from rFSAV-immunized volunteers and developed a mAb cocktail targeting B-cell immunodominant epitopes (Hla48–65, IsdB432–449, SEB78–95, and SEB222–239). As clinical treatment of staphylococcus aureus infection often use antibiotics such as vancomycin or linezolid, and the use of large doses of antibiotics against bacterial infection has side effects.8–10 To determine whether S. aureus immunodominant epitope-specific mAbs combined with antibiotics can treat severe S. aureus infections, we used an mAb cocktail combined with vancomycin or linezolid to treat against MRSA252 infection. The results from our study suggest that the combination of the mAb cocktail and vancomycin or linezolid could reduce mortality in a lethal sepsis model and an acute pneumonia model.
Materials and Methods
Ethics Statement
All animal experiments were approved by the Animal Ethical and Experimental Committee of the Army Medical University (Chongqing, Permit No. 2011–04). The experiments were carried out in accordance with the approved guidelines of the Animal Ethical and Experimental Committee of the Army Medical University (Chongqing, permit number 2011-04). The clinical strain samples were not specifically isolated for this research, and we had obtained informed consents from all the subjects.
Animal, Bacterial Strains, Serum and Epitope-Specific mAbs
Six to eight-week-old specific pathogen-free female BALB/c mice weighing about 20 g were purchased from Beijing HFK Bioscience Co., Ltd (Beijing, China). The MRSA252 strain was purchased from the American Type Culture Collection (Manassas, Virginia). The bacterial strains were first cultivated in MHA plates, then take a single colony and culture it in MHB medium for about 6 hours. After centrifugation to remove the supernatant, dilute with PBS to the desired colony concentration. The mAb cocktail (Hla48-65-mAb, IsdB432-449-mAb, SEB78-95-mAb, and SEB222-239-mAb) were generated by China Peptides Co., Ltd as previously described.15 The isotypes of each mAb in the epitope-specific mAb cocktail and its affinity with the specific epitope have been reported in our previous study.16 Serum of MRSA infected patients and healthy people was collected from Southwest Hospital of Army Medical University (Chongqing, China). The samples with MRSA infection were confirmed after bacterial culture and clinical drug susceptibility tests.
Mouse Infection Models
To evaluate the inhibitory effect of different concentrations of antibiotics on bacteria, mice were intravenously injected with 100 μL saline containing 7×108 colony-forming units (CFU) of MRSA252. Before this study, we had chosen 1 h or 2 h and 4 h post infection to detect the treatment effect of vancomycin or linezolid. The survival rate results show that the treatment effect is best when given to mice 1h after infection (data not shown). One hour after infection, mice received an intravenous injection of saline alone, vancomycin (0.4, 0.2, 0.1, 0.05, or 0.025 mg), or linezolid (0.4, 0.2, 0.1, 0.05, or 0.025 mg). After 48 h of infection, the mice were euthanized by inhalation of CO2. To perform bacterial count, the lung and kidney tissues were dissected and homogenized. Bacterial counts were determined by the serial dilution and plating method described in our previous research.17 The inhibitory effect of different concentrations of antibiotics on bacterial growth was evaluated according to the bacterial numbers in the organs.
To generate an acute pneumonia model, mice were anesthetized with an intraperitoneal injection of 100 μL of 1% pentobarbital sodium before infection. Twenty microliters of saline containing 1×109 CFU of MRSA252 were intranasally inoculated into each mouse. One hour after infection, mice were intranasally administrated with saline alone, vancomycin alone (0.05 mg), vancomycin (0.05 mg) combined with mAbs (0.5 mg), linezolid alone (0.2 mg), or linezolid (0.2 mg) combined with mAbs (0.5 mg). In our study, mAb cocktail contain Hla48-65-mAb 0.125mg, IsdB432-449-mAb 0.125mg, SEB78-95-mAb 0.125mg, and SEB222-239-mAb 0.125mg. The mice were monitored for 7 days for illness and survival.
To establish a lethal sepsis model, each mouse was intravenously injected with 100 μL saline containing 8.7×108 CFU of MRSA252. One hour after infection, mice received an intravenous injection of saline alone, vancomycin alone (0.05 mg), vancomycin (0.05 mg) combined with mAbs (0.5 mg), linezolid alone (0.1 mg), or linezolid (0.1 mg) combined with mAbs (0.5 mg). The mice were monitored for 7 days for illness and survival.
Analysis of Bacterial Burden, Serum Collection, and Tissue Histology
After 48 h of intranasal infection with 100 μL saline containing 7×108 CFU of MRSA252, the mice were euthanized by inhalation of CO2 and their eyeball blood collected. The eyeball blood samples were placed in a refrigerator for overnight at 4°C and centrifuged at 4000 rpm for 10 min to collect the serum, which was stored at −80°C for later use.
At 48 h post therapy with antibiotic combined with mAb cocktail, the lungs and kidneys were harvested. The bacterial numbers in the organs were enumerated by preparing the organ homogenates in PBS and plating 10-fold serial dilutions on an MHA plate. The colonies were counted after 24 h of incubation at 37 °C. The number of CFUs per gram of tissue (CFUs/g) was calculated from each plate. For histopathology, the organs were fixed with 4% paraformaldehyde and embedded in paraffin. Four-micrometer thick sections were prepared and stained with hematoxylin and eosin for microscopic examination.
Cytokine Analysis
The bronchoalveolar lavage (BAL) was harvested as per the previously described protocol.18 Briefly, 48 h after infection, the BAL fluid was harvested using a 1-mL syringe. The samples were centrifuged at 8000 rpm for 5 min, and the supernatants were harvested and stored at −80°C until analysis. Chemokine levels in the BAL and serum were determined using the Cytometric Bead Array-based FlowCytomix assay according to manufacturer’s instructions (LEGENDplexTM Panel, Biolegend, Cat No.740446) for the following proinflammatory cytokines: interleukin IL-1α, IL-1β, IL-10, IL-6 tumor necrosis factor TNF-α, and interferon IFN-γ. Samples were allowed to thaw at 37°C only once before testing. All assays were performed in duplicates.
Analysis of the Ability of the mAb to Neutralize Native SEB Toxins
The abilities of the mAbs to neutralize native SEB toxins were determined by ELISA. A microtiter plate was coated with the native SEB (purchased from the Academy of Military Science of China) at a concentration of 2 μg/well; any non-specific binding was prevented by blocking the microtiter plates with PBS containing 2% bovine serum albumin (BSA). The wells were then treated with SEB78–95 mAb, SEB222–239 mAb, SEB78–95 mAb + SEB222–239 mAb, and normal serum for 1 h at 37°C. After washing, the wells were sequentially treated with a horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (Sigma) and 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. The reaction was terminated by adding 100 μL of 2 M sulfuric acid. The optical density was read at 450 nm using a microplate reader (Bio-Rad).
Mononuclear leukocytes isolated from the spleen tissue of a mouse were counted and plated at 1×106 cells/well (100 μL) before incubation in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Beijing, China) supplemented with 15% fetal calf serum (Gibco, USA). Each well was then treated with 100 μL SEB (0.1 mg/mL) that had been incubated 30 min prior to the start of the study with 0.5 or 1 mg of the mAb cocktail. After 24 h, culture supernatants were harvested and the levels of IL-2, TNF-α, and IFN-γ were quantified using ELISA. Normal serum was used as negative control. The remaining cells were cultured in 90 μL RPMI-1640 medium mixed with 10 μL of Cell Counting Kit (CCK)-8 solution (Dojindo) and incubated at 37°C for 2 h. The absorbance value of each well was measured at 450 nm wavelength using microplate reader.
Detection of Hla Monoclonal Antibody Inhibiting Hla Hemolytic Activity
Hemolytic activity assay was carried out based on a previously established method.19 Briefly, in 96-well, U-bottom plates, Hla had been incubated at 37°C with different concentrations of Hla48-56-mAb 30 min prior to the start of the study. Equal volumes of 1% Triton X-100 and Hla were used as positive controls, while PBS and mHla were used as negative controls. mHla, a Hla mutant without hemolytic activity, was produced in our lab according to the previous study about Hla.20 Then, 100 μL of 4% rabbit erythrocyte suspension was added to each well and incubated at 37°C for 60 min. The mixtures were centrifuged at 400 ×g for 10 min, and the hemolytic activity was determined from the release of hemoglobin, which was spectrophotometrically measured at 540 nm and presented as percent hemolysis of the positive control (Triton X-100).
Challenge with Clinical MSRA Isolates
The strains for challenge were selected from distinct clades based on evolutionary analyses according to the previous report.21 Based on the phylogeny of the human clinical MRSA isolates and SEB sequence-known MRSA strains, four clinical MRSA isolates were selected. The human clinical isolates CQ19, BJ2 and GZ9 are sequenced MRSA strains from different regions of China. For challenge experiments, mice were challenged by tail-vein injection of 100ul solutions containing 1*108 CFU one of these strains, and then were injected with mAb cocktail or PBS control. Survival analysis was then monitored as described above.
Clinical Human Serum Cross-Reactivity Test
To determine the reactivity of serum samples from clinical MRSA infected patients against theses four dominant epitope peptides (SEB78–95, SEB222–239, Hla48–65 and IsdB432–449), wells of microtiter plates were coated with 10μg of each peptide dissolved in 50mM carbonate buffer (pH9.6). Phosphate buffered saline (PBS, pH7.4) containing 2% BSA was used to prevent nonspecific binding. Serum of MRSA infected patients and healthy people was collected from Southwest Hospital of Third Military Medical University (Chongqing, China). The samples with MRSA infection were confirmed after bacterial culture and clinical drug susceptibility tests. Serum of both patients and healthy people came were gradient diluted starting from 1:50, 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200 to 1:6400 with PBS and were used as the primary antibodies, and peroxidase-conjugated rabbit anti-human antibodies (Bioss, Beijing, China) were used as secondary antibodies at a dilution of 1:5000. The results shown as absorbance values observed at 450 nm. The values that were 2.1-fold higher than the mean absorbance value of blank wells were defined as positive.
Statistical Analysis
All data were analyzed using GraphPad Prism (version 8.0) and presented as the mean ± standard deviation (SD). The Fisher’s exact test was used to analyze the statistical significance of protective immunity data generated by the single mAb. One-tailed Student’s t-test was used to analyze the statistical significance of renal abscesses and pulmonary data. Mantel-Cox log-rank analysis was utilized to determine differences in survival times. P < 0.05 was considered statistically significant.
Result
Different Concentrations of Vancomycin or Linezolid Were Used for the Treatment of MRSA252 Infection in a Lethal Sepsis Model
To study the effects of different concentrations of antibiotics on MRSA252 infection, we injected different doses of linezolid and vancomycin into mice intravenously challenged with MRSA252. We observed bacterial colonization in the lungs and kidneys of mice. As the colonization of bacteria in the lungs was unstable, we used the kidney samples to evaluate the effect of antibiotics. In comparison with the PBS treatment group, the groups treated with 0.4, 0.2, 0.1, 0.05 and 0.025 mg linezolid (Figure 1A) and 0.4, 0.2, 0.1 and 0.05 mg vancomycin (Figure 1B) showed an obvious reduction in MRSA252 counts in the kidney tissue (P < 0.05). The treatment with 0.05 mg linezolid had no obvious protective effect. There was a concentration-dependent effect of antibiotics on the number of bacteria. As shown in Figure 1, the smallest effective dose of linezolid against bacteria both in the kidney and lung tissues was 0.1 mg, while that of vancomycin was 0.05 mg. These doses were then used for the subsequent experiments.
Antibiotic Combined with the Immunodominant Epitope-Specific mAb Cocktail Reduced MRSA252 Infection in Lethal Sepsis Model
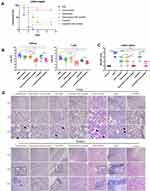
Survival analysis showed that all mice from the PBS treatment group died within 4 days from MRSA252 infection, which was consistent with previous studies and indicated that the bacteremia model was successful.16 The mice in the PBS group mice died within 4 days. The mice treated with vancomycin combined with mAb cocktail or linezolid combined with mAb cocktail had higher survival rates after MRSA252 infection (80%) than the vancomycin alone (20%), vancomycin alone (20%) or mAb cocktail alone-treated mice (30%). The significance of therapeutic effect by the mAb cocktail combined with the low-dose of antibiotic was measured using the log-rank (Mantel-Cox) test compared to the low doses of antibiotic alone or mAb cocktail alone groups. Compared with PBS group, vancomycin plus mAb cocktail (p<0.0001) and linezolid plus mAb cocktail (p=0.0004). Compared with antibiotic alone group, vancomycin plus mAb cocktail (P=0.005) and linezolid plus mAb cocktail (p=0.0111). Compared with mAb cocktail alone, vancomycin plus mAb cocktail (p=0.0251) and linezolid plus mAb cocktail (p=0.0497) (Figure 2A).
After 48 h of MRSA252 challenge, the bacterial load was evaluated in the organs of the mice treated with the mAb cocktail and antibiotic mixture. The results showed that the bacterial burden in the kidneys and lungs was significantly lower in the mice treated with the mAb cocktail and antibiotics than in those treated with PBS. As shown in Figure 2B, in the lung, compared with mAb cocktail alone, mAb cocktail plus vancomycin (p=0.0086) and mAb cocktail plus linezolid (p=0.0094). Compared with vancomycin alone, mAb cocktail plus vancomycin (p=0.0273). Compared with the linezolid alone, mAb cocktail plus linezolid (p= 0.0056). In the kidney, compared with mAb cocktail alone, mAb cocktail plus vancomycin (p= 0.001) and mAb cocktail plus linezolid (p=0.0012). Compared with vancomycin alone, mAb cocktail plus vancomycin (p =0.0048). Compared with the linezolid alone, mAb cocktail plus linezolid (p=0.008). Therefore, the effects of antibiotics combined with the mAb cocktail treatment was better than those of the antibiotics alone and also better than mAb cocktail alone. The severity scores of kidney and lung for semi-quantitative analyses of kidney and lung injury were shown in Figure 2C.
Histological analysis showed that the structures of renal tubules and alveoli in MRSA252-challenged mice treated with mAb cocktail and antibiotics were normal. In contrast, abscesses and scattered bacterial colonies were readily observed in the kidney or lung of the PBS-control mice, the mice treated with antibiotics alone or mAb cocktail alone (Figure 2D). Therefore, the combination of mAb cocktail and antibiotic treatment could provide better protection against MRSA252 challenge than low doses of antibiotics alone. These results demonstrated that the therapeutic effect of the mAb cocktail combined with antibiotics is better than that of using low doses of vancomycin or linezolid alone or that of using mAb cocktail alone.
The Combination of Antibiotic and mAb Cocktail Reduced MRSA252 Infection in an Acute Pneumonia Model
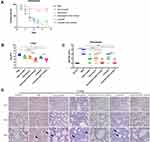
To evaluate the therapeutic effect of the combination of antibiotic and mAb cocktail therapy in an acute pneumonia model of MRSA252 infection, mice were infected with MRSA252 through tracheal intubation and then treated with different regimens. The significance of therapeutic effect by the mAb cocktail combined with the low-dose of antibiotic was measured using the log-rank (Mantel-Cox) test compared to the low doses of antibiotic alone or mAb cocktail alone groups. As shown in Figure 3A, compared with PBS group, vancomycin plus mAb cocktail (p=0.0015) and linezolid plus mAb cocktail (p=0.0008). Compared with antibiotic alone group, vancomycin plus mAb cocktail (p=0.006) and linezolid plus mAb cocktail (p=0.0116). Compared with mAb cocktail alone, vancomycin plus mAb cocktail (p=0.0482) and linezolid plus mAb cocktail (p=0.0281).
As shown in Figure 3B, the amount of bacteria colonizing the lung of the mice treated with mAb cocktail was significantly reduced, while that in the lungs of the mice treated with antibiotics and mAb cocktails was lower than that in the group treated with antibiotics alone. Compared with mAb cocktail alone, mAb cocktail plus vancomycin (p=0.0347) and mAb cocktail plus linezolid (p=0.0322). Compared with vancomycin alone, mAb cocktail plus vancomycin (p=0.0067). Compared with the linezolid alone, mAb cocktail plus linezolid (p= 0.0053). The severity scores of kidney and lung for semi-quantitative analyses of kidney and lung injury were shown in Figure 3C. As shown in Figure 3D, histological analysis showed that no normal alveolar structure was observed in the lung of the PBS-control mice. The lungs of the mice from the linezolid and vancomycin treatment groups showed scattered bacterial colonies. However, the alveolar structures of the mice from the combination treatment groups were intact. Together, these results indicate that the combination of antibiotic and mAb cocktail therapy can mediate significant therapeutic benefits in acute pneumonia model of MRSA252 infection. Therefore, the efficacy of the combination therapy on infections caused by MRSA252 is promising.
Analysis of Cytokines in the Serum and BAL
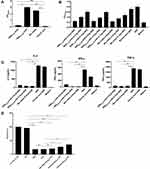
The levels of the cytokines IL-1α, IL-1β, IL-6, IL-10, IFN-γ, and TNF-α were analyzed to evaluate the inflammatory response in mice after MRSA infection. As shown in Figure 4A and B, the groups treated with vancomycin combined with mAb cocktail and linezolid combined with mAb cocktail had higher levels of IL-1α, IL-1β and IFN-γ and lower levels of IL-6, IL-10, TNF-α than the PBS and antibiotic alone groups in both of the lethal sepsis and the acute pneumonia models.
Epitope-Specific Monoclonal Antibodies Inhibited the Biological Functions of Hla and SEB in vitro
Our mAb cocktail comprised three toxin antigens (SEB78–95, SEB222–239, and Hla48–56). Therefore, we analyzed the toxin-neutralizing ability of these two antigens to explain the underlying mechanism of action. SEB is a super-antigen that can strongly increase the proliferation of mouse mononuclear leukocytes and increase the production of IFN-γ, IL-2, and TNF-α.22,23 We evaluated the binding ability of the mAb and SEB. As shown in Figure 5A, SEB78-95-mAb could weakly bind to SEB, but SEB222-239-mAb showed a strong binding with SEB. The mix-mAb of SEB78-95-mAb and SEB222-239-mAb also showed a strong affinity toward SEB. We also studied the toxin-neutralizing ability of SEB78-95-mAb, SEB222-239-mAb, and mix-mAb on the native. As shown in Figure 5B, SEB exposure obviously increased the proliferation of mouse mononuclear leukocytes as compared with the negative control. SEB78-95-mAb, SEB222-239-mAb, or mix-mAb incubated with SEB could reduce the proliferation of mouse mononuclear leukocytes. As the concentration of the antibody decreased, the proliferation of mouse monocytes gradually increased. In comparison with the negative control, SEB treatment could significantly increase the secretion of IFN-γ, IL-2, and TNF-α (Figure 5C). In addition, the incubation of SEB with SEB78-95-mAb, SEB222-239-mAb, or mix-mAb resulted in a significant decrease in the production of IFN-γ, IL-2, and TNF-α. However, this effect was not observed following incubation of SEB with normal serum. These observations confirmed the inhibitory effect of SEB78-95-mAb, SEB222-239-mAb, and mix-mAb on the superantigen activity of SEB.
We evaluated the inhibitory effect of Hla48-56-mAb on the hemolysis of rabbit erythrocytes by native Hla. We had used different concentrations of Hla, the neutralizing toxin-neutralizing effect of Hla48-56-mAb is was the most obvious at an Hla concentration of 0.625 μg/mL (data not shown). Therefore, we chose 0.625 μg/mL Hla to evaluate the inhibitory effect of Hla48-56-mAb on the Hla-mediated hemolysis of rabbit erythrocytes. As shown in Figure 5D, Hla showed hemolytic activity similar to that of the positive control (1% Triton X-100). In contrast, the incubation of Hla48-56-mAb with Hla resulted in a dose-dependent inhibition of the hemolytic activity. There was no significant difference in the hemolytic activity when Hla was incubated with Hla48-56-mAb and the negative controls (PBS, mHla), indicating that these antibodies was sufficient to completely inhibit the hemolytic activity of Hla.
The Combination of Antibiotic and mAb Cocktail Improved Infection Survival Against the Clinical MRSA Isolates in a Lethal Sepsis Model
Protection of the mAb cocktail combined with antibiotics must be achieved against a wide variety of different strains in order to be useful for treating MRSA infections more generally in the future. Thus, we selected the distantly related MRSA strains which were previous reported. Survival analysis was performed after mice challenged by different clinical MRSA isolates. As shown in Figure 6A, the results revealed that mAb cocktail combined with antibiotics could significantly treat the infection of the four clinical MRSA isolates. For BJ2 clinical isolates, compared with PBS group, vancomycin plus mAb cocktail (p<0.0001) and linezolid plus mAb cocktail (p=0.0131). For GZ9 clinical isolates, compared with PBS group, vancomycin plus mAb cocktail (p<0.0001) and linezolid plus mAb cocktail (p=0.0075). For CQ19 clinical isolates, compared with PBS group, vancomycin plus mAb cocktail (p=0.012) and linezolid plus mAb cocktail (p=0.12). As shown in Figure 6B, all of these four immunodominant epitopes had cross reactivity with the clinical serum from the patients recovering from MRSA infection.
Discussion
Studies have shown that antibody-based passive immunity plays an important role in protecting humans against pathogens such as Streptococcus pneumoniae24 and hepatitis C virus infections.25 At the same time, mAbs can exhibit good therapeutic effects in immunosuppressed patients.26 Using mAbs in combination with antibiotics can mediate significant protective effects even at subinhibitory concentrations and alleviate the emergence of bacterial drug resistance. With robust specific antibodies, antibiotics can work even at very low concentrations. The remarkable specificity of mAbs toward pathogens can limit the possibility of drug resistance and provide better curative effects by acting synergistically with antibiotics.27,28
SEB is an exotoxin of S. aureus, which can stimulate T cells to release IL-2 and IFNs, inhibit humoral and cellular immunity, and mediate bacterial proliferation.29 Previous study showed that SEB mAb 20B1 worked as an immunotherapeutic agent for severe diverse S. aureus infections.30 Hla is believed to play a significant role in S. aureus colonization and pathogenesis, which form circumscribed transmembrane pores in target cells.31 Previous study reported that the humanized Hla mAb AR-301 can effectively eradicate S. aureus infection in pneumonia patients in the ICU.32 IsdB is a hemoglobin receptor necessary in regulating iron utilization by S. aureus, and is associated with increased mortality rates in patients with S. aureus infections, in which IsdB which functions to capture heme from hemoglobin for import into the bacteria.33 In a murine lethal sepsis model, a fully human IsdB mAb CS-D7 conferred protection from death when dosed prior to challenge, but not when dosed after challenge.34 Given their conserved sequences and structures, these antigens can serve as candidate antigens for MRSA vaccines.35 In our previous studies, protective epitopes were screened from the serum and epitope-specific mAbs were prepared. These epitope-specific mAbs (Hla48-65-mAb, IsdB432-449-mAb, SEB78-95-mAb, and SEB222-239-mAb) exhibited protective effects in a mouse model of lethal sepsis.16 rFSAV was proven to be safe for inducing robust antigen-specific antibodies in volunteers in phase I clinical trial. In a previous study, we developed a mAb cocktail targeting the human B-cell immunodominant epitopes of Hla, IsdB, and SEB.16 In this study, we suggest that the combination of the mAb cocktail of anti-Hla48–65, anti-IsdB432–449, anti-SEB78–95, and anti-SEB222–239 with vancomycin or linezolid could reduce mortality in bacteremia and acute pneumonia models of MRSA infection.
To confirm the robust effects of the combination of low-dose antibiotics and mAb immunization, we first analyzed the effects of vancomycin or linezolid at different concentrations on MRSA252 colonization in the kidneys and lungs. Vancomycin and linezolid showed a dose-dependent negative correlation with the number of bacteria. The smallest dose that exerted bactericidal effects was selected in the subsequent experiment to determine the effects of the combination of either of these antibiotics and the antibody cocktail to treat infections. The combination group not only showed higher survival rate but had histopathology similar to that of normal mice. MRSA252 is a hospital-acquired epidemic human MRSA strain associated with septicemia.36 Vancomycin is usually used to treat septicemia caused by gram-positive bacteria, including MRSA.37 Linezolid has better efficacy and safety than vancomycin in the treatment of nosocomial pneumonia caused by MRSA.38 In this study, the combination of mAb and either of these antibiotics could significantly reduce the bacterial colonization and pathological changes in organs and improve the survival rate of mice both in the septicemia and the pneumonia model.
The changes in the levels of inflammatory factors are important indicators of the prognosis of S. aureus infection. Studies have shown that the mortality from S. aureus infection is related to an imbalance in the immune system. Mortality or more serious diseases are associated with low IL-1, IL-1α, IL-1β, IFN-γ, and TNF-α and high levels of IL-6 and IL-10.39,40 For example, IL-10 is related to the prognosis of infection. High levels of IL-10 are associated with poor outcomes and low levels of IL-10 are related to high survival.41 IL-6 is important for stimulating B cells to produce antibodies and could mediate the transformation of naive CD4+T cells to Th17 cells.42 The activation of Th17 cells is critical for the control of S. aureus infections.43 Low levels of IL-6 and IL-8 are related to decreased respiratory failure in S. aureus bacteremia, while low levels of IFN-γ correlate with death.44 In our study, the changes in cytokine levels were consistent with those reported in the literature, highlighting that the combination of antibiotics and mAbs can indeed protect against sepsis and pneumonia caused by MRSA252. We used low-dose antibiotics in this study to alleviate the side-effects of antibiotics and prevent antibiotic resistance.
Researchers have reported that toxin antibodies are good companions of antibiotics in MRSA infection.45 Some studies using an animal model of antigen-induced conditioned phagocytic antibodies, but in clinical trials in the crowd did not play an effective role in protection.46 Although antibiotic or phagocytic antibody can kill bacteria, they cannot prevent the tissue damage caused by the secreted bacterial toxins of S. aureus. Therefore, toxin specific antibodies which can neutralize the toxins are necessary for prevention and treatment of S. aureus infection. Among these mAbs, SEB222-239-mAb inhibited the ability of native toxin SEB to induce T cell mitogenesis and cytokine production, while Hla48-65-mAb inhibited the hemolytic activity of native toxin Hla. According to previous study, IsdB contains two functional domains N1 and N2, and only N1 region exhibits hemoglobin binding activity.47 However, in our study IsdB432-449-mAb targets its N2 region. We thought that in this study, IsdB432-449-mAb might cooperate with other antibodies (SEB222-239-mAb and Hla48-65-mAb) to play a synergistic protective role. Therefore, the above experiments indicated that the therapeutic effect of the cocktail is attributed to the ability of antibodies to neutralize Hla and SEB as well as from the synergy of the four antibodies.
Protection against diverse clinical pathogenic isolates from diverse forms of disease is of crucial importance for broad-spectrum treatment efforts. Thus, we aimed at the combination of mAb cocktail and the antibiotics would be effective against a wide range of MRSA isolates. Three clinical MRSA strains were selected according to our previous study, in which we sequenced several genes including SEA, SEB, SEC, SED, SEE, and SpA for the clinical MRSA isolates and these clinical strains BJ2, GZ9, and CQ19 were selected from distinct clades based on phylogenic SEB gene sequences.21 The resistance analysis showed that MRSA252 and the 3 clinical isolates BJ2, GZ9 and CQ19 were all susceptible to vancomycin and linezolid (Supplementary Table S). Therefore, there are no potential differences in natural resistance against the two antibiotics used in this study. For the three clinical MRSA strains, the combination of mAb cocktail and the antibiotics also afforded robust protection. Since the sequence homology between SEB and SEC varies from 42% to 67%,48 the mAb cocktail might be cross-reactive to non-SEB-secreting strains and SEC-secreting strains. The mAb cocktail in our study is a mixture of four mAbs. In our previous study, the homology analysis indicated that among the four epitopes for the mAb cocktail targets, except SEB222–239, other epitopes Hla48–65, IsdB432–449 and SEB78–95 were highly conserved among different S. aureus strains.21 In this study, besides SEB, we also sequenced genes including Hla and IsdB for the three clinical MRSA strains (data not shown). Therefore, we held the opinion that the differences in protection rates might result from differences in the virulence of the clinical strains themselves, rather than from differences in the binding of the mAbs targeting the antigens.
In this study, we tested the therapeutic efficacy of low dose of antibiotics combined with the mAb cocktail in murine pneumonia model and lethal sepsis model, in which the mAb cocktail targeted the immunodominant epitopes that were screened using human antisera in the clinical trial (Phase I of China Clinical Trial: CTR20160004, http://www.chinadrugtrials.org.cn/). The results showed that immunodominant epitope-specific toxin antibodies could be good companions of antibiotics to treat MRSA infections. These results pave the way for new therapies based on the low-dose of antibiotics and for treating patients with low immunity against MRSA infections.
Author Contributions
Zhuo Zhao and Quanming Zou designed the experiments, LianLi Duan wrote the manuscript. LianLi Duan, Zhuo Zhao, Jinyong Zhang, Jiang Zhu, Li Ni, Yuling Zheng, Zhiyong Liu, Xiaokai Zhang and Hao Zeng analyzed experimental results. LianLi Duan, Jinyong Zhang, Zhifu Chen, Qiang Gou, Qingshan Xiong, Yue Yuan and Haiming Jing carried out the experiments. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 31970869, 31970138 and 32070941) and the Natural Science Foundation Project of Chongqing (cstc2019jcyj-msxmX0320).
Disclosure
The authors report no conflicts of interest for this work and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Senobar Tahaei SA, Stájer A, Barrak I, et al. Correlation between biofilm-formation and the antibiotic resistant phenotype in staphylococcus aureus isolates: a Laboratory-Based Study in Hungary and a review of the literature. Infect Drug Resist. 2021;14:1155–1168. doi:10.2147/IDR.S303992
2. Gajdács M, Urbán E. Epidemiology and resistance trends of Staphylococcus aureus isolated from vaginal samples: a 10-year retrospective study in Hungary. Acta Dermatovenerol Alp Pannonica Adriat. 2019;28(4):143–147.
3. Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet. 2015;386(9998):1097–1108. doi:10.1016/S0140-6736(15)60733-4
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55.
5. Davis JS, Van Hal S, Tong SY. Combination antibiotic treatment of serious methicillin-resistant Staphylococcus aureus infections. Semin Respir Crit Care Med. 2015;36(1):3–16. doi:10.1055/s-0034-1396906
6. Gajdács M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics. 2019;8(2):52. doi:10.3390/antibiotics8020052
7. Bush K, Courvalin P, Dantas G, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894–896. doi:10.1038/nrmicro2693
8. Beibei L, Yun C, Mengli C, et al. Linezolid versus vancomycin for the treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2010;35(1):3–12. doi:10.1016/j.ijantimicag.2009.09.013
9. Conaughty JM, Chen J, Martinez OV, et al. Efficacy of linezolid versus vancomycin in the treatment of methicillin-resistant Staphylococcus aureus discitis: a controlled animal model. Spine. 2006;31(22):E830–E832. doi:10.1097/01.brs.0000241065.19723.13
10. Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi:10.3390/molecules24050892
11. Aslam A, Gajdács M, Zin CS, et al. Evidence of the practice of self-medication with antibiotics among the lay public in low- and middle-income countries: a scoping review. Antibiotics. 2020;9(9):597. doi:10.3390/antibiotics9090597
12. Shopsin B, Kaveri S, Bayry J. Tackling difficult Staphylococcus aureus infections: antibodies show the way. Cell Host Microbe. 2016;20(5):555–557. doi:10.1016/j.chom.2016.10.018
13. Correia B, Bates JT, Loomis RJ, et al. Proof of principle for epitope-focused vaccine design. Nature. 2014;507(7491):201–206. doi:10.1038/nature12966
14. Akram A, Inman R. Immunodominance: a pivotal principle in host response to viral infections. Clin Immunol. 2012;143(2):99–115. doi:10.1016/j.clim.2012.01.015
15. Kilpatrick K, Wring SA, Walker DH, et al. Rapid development of affinity matured monoclonal antibodies using RIMMS. Hybridoma. 1997;16(4):381–389. doi:10.1089/hyb.1997.16.381
16. Zhao Z, Zhang J, Song X, et al. An immunodominant epitope-specific monoclonal antibody cocktail improves survival in a mouse model of Staphylococcus aureus bacteremia. J Infect Dis. 2021;223(10):1743–1752.
17. Chen Z, Gou Q, Xiong Q, et al. Immunodominance of epitopes and protective efficacy of HI antigen are differentially altered using different adjuvants in a mouse model of Staphylococcus aureus bacteremia. Front Immunol. 2021;12:684823. doi:10.3389/fimmu.2021.684823
18. Hua L, Hilliard JJ, Shi Y, et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother. 2014;58(2):1108–1117. doi:10.1128/AAC.02190-13
19. Zhang J, Yang F, Zhang X, et al. Protective efficacy and mechanism of passive immunization with polyclonal antibodies in a sepsis model of Staphylococcus aureus infection. Sci Rep. 2015;5(1):15553. doi:10.1038/srep15553
20. Bhakdi S, Jursch R, Bröker M, et al. Functionally inactive S. aureus alpha-toxin containing a single amino acid substitution: potential usefulness as a vaccine. Behring Inst Mitt. 1994;95:80–84.
21. Zhao Z, Sun H-Q, Wei -S-S, et al. Multiple B-cell epitope vaccine induces a Staphylococcus enterotoxin B-specific IgG1 protective response against MRSA infection. Sci Rep. 2015;5(1):12371. doi:10.1038/srep12371
22. Rieder S, Nagarkatti P, Nagarkatti M, Bliska JB. CD1d-independent activation of invariant natural killer T cells by staphylococcal enterotoxin B through major histocompatibility complex class II/T cell receptor interaction results in acute lung injury. Infect Immun. 2011;79(8):3141–3148. doi:10.1128/IAI.00177-11
23. Krakauer T, Buckley M, Fisher D. Murine models of staphylococcal enterotoxin B-induced toxic shock. Mil Med. 2010;175(11):917–922. doi:10.7205/MILMED-D-10-00148
24. Sanders ME, Taylor S, Tullos N, et al. Passive immunization with Pneumovax®23 and pneumolysin in combination with vancomycin for pneumococcal endophthalmitis. BMC Ophthalmol. 2013;13(1):8. doi:10.1186/1471-2415-13-8
25. Salinas E, Boisvert M, Upadhyay AA, et al. Early T follicular helper cell activity accelerates hepatitis C virus-specific B cell expansion. J Clin Invest. 2021;131(2):e140590. doi:10.1172/JCI140590
26. Domenech M, Sempere J, de Miguel S, et al. Combination of antibodies and antibiotics as a promising strategy against multidrug-resistant pathogens of the respiratory tract. Front Immunol. 2018;9:2700. doi:10.3389/fimmu.2018.02700
27. Ramos-Sevillano E, Rodríguez-Sosa C, Díez-Martínez R, et al. Macrolides and β-lactam antibiotics enhance C3b deposition on the surface of multidrug-resistant Streptococcus pneumoniae strains by a LytA autolysin-dependent mechanism. Antimicrob Agents Chemother. 2012;56(11):5534–5540. doi:10.1128/AAC.01470-12
28. Cafini F, Yuste J, Giménez M-J, et al. Enhanced in vivo activity of cefditoren in pre-immunized mice against penicillin-resistant S. pneumoniae (serotypes 6B, 19F and 23F) in a sepsis model. PLoS One. 2010;5(8):e12041. doi:10.1371/journal.pone.0012041
29. Krakauer T, Pradhan K, Stiles B. Staphylococcal superantigens spark host-mediated danger signals. Front Immunol. 2016;7:23. doi:10.3389/fimmu.2016.00023
30. Varshney AK, Wang X, Scharff MD, et al. Staphylococcal enterotoxin B–specific monoclonal antibody 20b1 successfully treats diverse Staphylococcus aureus Infections. J Infect Dis. 2013;208(12):2058–2066. doi:10.1093/infdis/jit421
31. von Hoven G, Qin Q, Neukirch C, Husmann M, Hellmann N. Staphylococcus aureus α-toxin: small pore, large consequences. Biol Chem. 2019;400(10):1261–1276.
32. Francois B, Mercier E, Gonzalez C, et al. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med. 2018;44(11):1787–1796. doi:10.1007/s00134-018-5229-2
33. Mazmanian SK, Skaar EP, Gaspar AH, et al. Passage of heme iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–909. doi:10.1126/science.1081147
34. Ebert T, Smith S, Pancari G, et al. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) with functional activity in vitro and in vivo. Hum Antibodies. 2010;19(4):113–128. doi:10.3233/HAB-2010-0235
35. Miller L, Fowler VG
36. Holden MT, Feil EJ, Lindsay JA, et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A. 2004;101(26):9786–9791. doi:10.1073/pnas.0402521101
37. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American society of health-system pharmacists, the infectious diseases society of America, and the society of infectious diseases pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi:10.2146/ajhp080434
38. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–629. doi:10.1093/cid/cir895
39. Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis. 2012;206(10):1604–1611. doi:10.1093/infdis/jis552
40. Chantratita N, Tandhavanant S, Seal S, et al. TLR4 genetic variation is associated with inflammatory responses in gram-positive sepsis. Clin Microbiol Infect. 2017;23(1):
41. Rose WE, Shukla SK, Berti AD, et al. Increased endovascular Staphylococcus aureus inoculum is the link between elevated serum interleukin 10 concentrations and mortality in patients with bacteremia. Clin Infect Dis. 2017;64(10):1406–1412. doi:10.1093/cid/cix157
42. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi:10.1101/cshperspect.a016295
43. Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5(12):e1000703. doi:10.1371/journal.ppat.1000703
44. Minejima E, Bensman J, She RC, et al. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med. 2016;44(4):671–679. doi:10.1097/CCM.0000000000001465
45. Bagnoli F. Staphylococcus aureus toxin antibodies: good companions of antibiotics and vaccines. Virulence. 2017;8(7):1037–1042. doi:10.1080/21505594.2017.1295205
46. Proctor RA. Immunity to Staphylococcus aureus: implications for vaccine development. Microbiol Spectr. 2019;7(4):4–7.
47. Gaudin CFM, Grigg JC, Arrieta AL, Murphy ME. Unique heme-iron coordination by the hemoglobin receptor IsdB of Staphylococcus aureus. Biochemistry. 2011;50(24):5443–5452. doi:10.1021/bi200369p
48. Krakauer T, Stiles BG. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence. 2013;4(8):759–773. doi:10.4161/viru.23905
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.