Back to Journals » Infection and Drug Resistance » Volume 15
Antibiograms of Gut Flora of Poultry Farms Workers Reveal Higher Resistance Levels as Compared to Non-Workers
Authors Saeed MQ , Aziz M, Afzal RK , Hussain A, Manzoor H, Rasul S, Ruby T, Akrem A, Masud S, Arshad A, Latif A, Yousif M, Aali H, Fatima M, Bhutta MM, Khan AA
Received 9 May 2022
Accepted for publication 13 September 2022
Published 29 December 2022 Volume 2022:15 Pages 7699—7705
DOI https://doi.org/10.2147/IDR.S371930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Muhammad Qamar Saeed,1 Mubashar Aziz,1 Raja Kamran Afzal,2 Aamir Hussain,3 Hamid Manzoor,4 Sumaira Rasul,4 Tahira Ruby,5 Ahmed Akrem,5 Samrah Masud,5 Adnan Arshad,1 Ayesha Latif,1 Muhammad Yousif,1 Hamdan Aali,1 Menahil Fatima,1 Muhammad Mujahid Bhutta,1 Aleem Ahmed Khan5
1Dr. Ghulam Nabi Chaudhary Laboratory of Microbial Technologies, Department of Microbiology and Molecular Genetics, Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan; 2Department of Microbiology, CMH Institute of Medical Sciences, Multan, Pakistan; 3Institute of Basic Medical Sciences, Khyber Medical University, Peshawar, Pakistan; 4Institute of Molecular Biology and Biotechnology, Bahauddin Zakariya University Multan, Multan, Pakistan; 5Institute of Pure and Applied Biology, Bahauddin Zakariya University Multan, Multan, Pakistan
Correspondence: Muhammad Qamar Saeed, Dr. Ghulam Nabi Chaudhary Laboratory of Microbial Technologies, Department of Microbiology, Institute of Pure and Applied Biology, Bahauddin Zakariya University Multan, Pakistan, Tel +92-333-0231222, Email [email protected]
Introduction: Antibiotics are being used in humans and animals for treatment and control of bacterial infections. Excessive use of antibiotics in the production of poultry is a popular practice, but it poses serious health issues by transferring resistance from farm to humans via food or direct exposure.
Study Objective: The objective of this study was to carry out a comparison of the resistance and sensitivity profile of isolated isolates from sewage of toilets that were in use of workers inside the farm and from sewage of household toilets.
Methodology: In this study, a total of 320 sewage samples were collected. The antibiotic susceptibility profile was checked by Kirby-Bauer disc diffusion method, and the statistical analysis was carried out by MS excel. Chi-square test was performed to determine whether the antibiograms from two sample types were statistically different from each other or not.
Results: From 320 sewage samples, a total of 296 bacterial isolates were isolated among which the leading bacterium was E. coli. The proportion of resistance, ESBL production and MDR was significantly higher in bacteria isolated from sewage of toilets under use of poultry farm workers as compared to the sewage from domestic use toilets.
Conclusion: Resistance significantly increased in the bacteria isolated from toilets under use of poultry farm workers as compared to the ones isolated from control sewage samples.
Keywords: E. coli, antibiotic resistance, MDR, sewage, human microbiota
Introduction
Poultry industry is one the leading food providers in the world.1 Intensive farming, however, necessitates use of antibiotics as prophylactics and growth promoters. This use is contributing to the development and spread of antibiotic resistance in the environment. Environmental dynamics, ultimately, lead to transfer of resistance determinants into bugs of public health importance. Although, antibiotic resistance is an inevitable outcome of natural selection; excessive use of antibiotics has exacerbated the problem.2 Zoonotic infections caused by MDR bugs are now challenging the health care establishments as increased morbidity and mortality is being reported worldwide.3
Poultry farms are hotspots of highest concern where resistance emerges, persists and spreads into the environment.4,5 Antibiotics are being used in poultry not only for therapeutic purposes but also to protect them from environmental stresses, such as stocking density, and as growth promoters.6 Therefore, the use of antibiotics in poultry farm has increased in order to cope with the challenges of intensive farming and thus increasing the production of poultry.7 The incorporation of antibiotics in farms has developed antibiotic resistant strains through genetic and/or environmental processes.8 The efficacy of currently available antimicrobial agents is diminishing because of these resistant strains causing infections.9
Studies showed that in Egypt, excessive use of antibiotics to increase poultry production has increased drug resistance in enteric pathogens including E. coli and has made the treatment of these infections difficult.10 Researchers believe that most of the antibiotics that animals consume are excreted out of the animal body in the form of feces without any metabolism. In this way it becomes part of soil and by rinsing off water from such surfaces, these residues come into the water sources. Thus, veterinary antimicrobials threaten the environment as well as human health.11
Most commonly used antibiotics in agricultural field and veterinary are lincosamides, sulphonamides, aminoglycosides, macrolides, quinolones and β-lactams.12,13 It has been observed that E. coli isolated from poultry has increased resistance to ampicillin and tetracyclines, and lower resistance to gentamicin and co-trimoxazole.14 Similarly, salmonella exhibit higher resistance to sulfisoxazole and tetracyclines while lower resistance to cefoxitin.15 In Jamaica, E. coli was found to be resistant against kanamycin and nalidixic acid and was sensitive to gentamicin.16 Frequent interaction between animals and humans is a potent cause of resistance transfer from poultry to poultry attendants. As there is an interaction between animals and humans, so resistance can transfer from poultry to poultry farm attendants.17
Our study aims to investigate the resistance profile in gut flora of poultry farm workers in an attempt to see whether their working environment has an effect on normal commensal microbes. For this, we took sewage samples from toilets present within the farm area and under exclusive use of workers. We compared resistance profiles of the isolates with those from toilets in the households. Statistically significant differences in resistance profiles were noted, indicating that their work environment has an influence on the fecal microbiota.
Methodology
Sampling
A total of 320 sewage samples were taken from toilets that were in use of poultry farm workers and from the sewage of toilets that were for domestic use (control sewage). Sampling sites were randomly selected. We collected 160 samples from toilets within poultry farm establishments and 160 from domestic toilets. In rural areas, toilets are usually linked to “gutters” to store and decompose fecal material as it is flushed from toilets. Samples were collected those gutters in sterile containers. The sampling was basically carried out to collect the human fecal material from sewage. Containers were kept in ice until further processing. All the samples were processed within 8 hours of collection.
Culturing Characteristics and Identification
The samples were directly spread on MacConkey and blood agar plates in parallel followed by incubation at 37°C for 24 h. The colonies were identified by different biochemical tests and further confirmed by commercial kits such as rapID (Oxoid and Remel).18
Antimicrobial Susceptibility Testing
Kirby-Bauer disc diffusion method was used to check the susceptibility of bacterial isolates to different antibiotic classes. The inhibition zones were observed and classified according to Clinical Laboratory Standards Institute (CLSI) standards.
ESBL Production
Combined disc test was used to check the production of ESBL. Cefepime, cefotaxime, ceftazidime and cefpodoxime discs were. The inhibition zones around the discs were measured and results were predicted by use of CARBA plus calculator (Mast-Group UK, Cat No. D73C).
Statistical Analysis
Microsoft Excel was used to carry out the statistical analysis. Mean values and percentages were calculated. Chi-square test was performed and p-value was calculated accordingly.19 It was hypothesized that resistance patterns of the samples collected from household toilets represented normal baseline resistance values. Therefore, if working environment had no influence on fecal microbiota of workers; Chi-square test would give us insignificant value. P-value was calculated and P-value <0.05 was considered statistically significant, which meant that work environment had a role in shaping resistance profiles of fecal microbes.
Results
A total of 320 sewage samples were taken from toilets that were in use of poultry farm workers and from the control sewage.
Isolation of Bacteria and Antibiotic Sensitivity and Resistance
Among total 320 sewage samples, 296 bacterial isolates were recovered. In these 296 isolates, 146 bacterial isolates were obtained from toilets that were in use of poultry farm workers and 150 bacterial isolates were recovered from control sewage (toilets for domestic use). In both sample types, E. coli was the leading species. Toilets under workers’ use yielded 102 (69.8%) E. coli isolates, while 105 (70%) E. coli were recovered from domestic toilets. We also recovered Salmonella spp.,Enterococcus spp., Shigella spp. and Enterobacter spp. from sewage samples but isolate counts were very low (Table 1).
 |
Table 1 Resistance Profile of Isolated Isolates to Different Classes of Antibiotics |
We applied 14 different antibiotics belonging to various classes on these isolates. For all antibiotics applied, we found higher number and percentage of resistant bugs recovered from workers’ toilets (Table 1).
Comparison of Antimicrobial Sensitivity and Resistance of E. coli
The leading strain isolated from sewage samples was E. coli. Data of Kirby-Bauer disc diffusion suggests that the sensitivity of this bacterium to antibiotics isolated from sewage of toilets for domestic use was higher than the isolates isolated from sewage of toilets that were under use of poultry farm workers (Table 1). Therefore, we decided to run chi-square test to see whether the difference between resistance profiles was statistically significant or not (Table 2). We found that the differences were statistically significant in all cases except two (amoxiclav and cefepime).
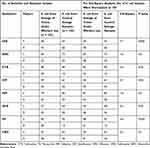 |
Table 2 Comparison of Sensitive and Resistant Profile of Isolated Isolates |
Production of ESBL and MDR Isolates
The total number of isolates isolated from sewage of toilets under workers use that were producing ESBL were 20 (13.6%) and among 102 isolates of E. coli 11 (10.7%) were producers of ESBL. While isolates from control sewage samples, 11 (7.3%) were producing ESBL and among 105 E. coli isolates, 5 (4.7%) were producers of ESBL. The MDR isolates isolated from sewage samples of toilets under workers use were 37 (25.3%) and MDR isolates isolated from control sewage samples were 24 (16%) (Table 3).
 |
Table 3 Association of Antibiotic Exposure to Production of ESBL and MDR |
Discussion
The use of antibiotics is primarily aimed at improving animal health and increasing meat production. But it has serious consequences for human health specifically in the situations where same type of antibiotics are being used for humans.20 Usually hospitals are considered hotspots of resistance and sewage management is advocated for them. We show that farm environment carries risks not only for the workers but also for extended environment, as resistant bugs are carried through sewage into ecological chain. Use of antibiotics develops resistance not only in the bacteria in the farm but also in the endogenous microflora of the individuals that are exposed to that environment.21 At the time of slaughtering, the resistant isolates from the gut of poultry animals may contaminate the meat.22 Also, the eggs become contaminated during laying with the resistant isolates. In this way the humans are affected with the resistant isolates either directly via exposure or indirectly via food.23 The resistant isolates may colonize the gut of humans and they can also transfer the resistance genes to the normal microbiota of the gut.
In this study, we compared the bacterial isolates isolated from the sewage lines of toilets that were under use of poultry farm workers and from the sewage lines of toilets from the households. This study was carried out to check the effects of antibiotic usage in the poultry farm on the gut bacteria of workers and farm handlers. Results indicated the higher proportion of resistant bacteria isolated from sewage of toilets that were under use of poultry farm workers as compared to the bacteria isolated from control sewage. The possibility of this acquired resistance is through the excessive usage of antibiotics inside the poultry farm.
Leading species isolated from all the sewage samples was E. coli. The proportion of resistant E. coli was higher in samples isolated from sewage of toilets that were under use of poultry farm workers as compared to those isolated from control sewage. MDR E. coli were found in both type of samples, but results showed significant difference in overall MDR isolates isolated from sewage of toilets under farm workers use and household toilets. MDR isolates isolated from sewage of toilets that were under use of poultry farm workers were higher as compared to the sewage of household toilets. This indicate that direct exposure of workers to the inside environment of poultry farm may develop multidrug resistance in the gut microflora of the workers.
The type of antibiotics being used inside the poultry farm had a significant effect on the resistance profile of isolated bacteria. This indicates that the resistance is being transferred directly or indirectly between the isolates.
The matter of serious concern regarding antibiotic resistance is the production of ESBLs. The isolates that were isolated from sewage of toilets used by poultry farm workers and handlers had a higher production of ESBLs due to which they were posing higher level of resistance to different classes of antibiotics. The incidents of increasing ESBL production in the bacteria may limit therapeutic options and are a serious threat for environment, humans, and the health care workers.
Conclusion
This study focuses on the fact that the use of antimicrobial agents inside the poultry farm has effects on the individuals who are exposed to this environment. The resistance profile of the bacteria isolated from control sewage is lower as compared to the isolates isolated from sewage of toilets that were under use of poultry farm workers. This indicates that the resistance is being transferred from farms to humans either directly or indirectly. This practice is reducing the efficacy of therapeutics to treat life threatening infections. Thus, it is necessary to develop some other ways to increase production of poultry rather than use of antibiotics and to minimize direct exposure of workers to the inside environment of poultry farm. Additionally, protocols must be developed to keep farm sewage from entering into normal sewerage lines without treatment.
Ethical Approval
Ethical approval was not required as samples were not collected from individual persons but from toilet sewages under use of workers and non-workers.
Acknowledgments
We acknowledge Bahauddin Zakariya University for their annual research grant.
Funding
Annual research grant of BZU.
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Erian I, Phillips CJC. Public understanding and attitudes towards meat chicken production and relations to consumption. Animals. 2017;7(3):20. doi:10.3390/ani7030020
2. Fey PD, Safranek TJ, Rupp ME, et al. Ceftriaxone-resistant salmonella infection acquired by a child from cattle. N Engl J Med. 2000;342(17):1242–1249. doi:10.1056/NEJM200004273421703
3. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya | BMC Microbiology | full Text. Available from: https://bmcmicrobiol.biomedcentral.com/articles/10.1186/1471-2180-6-101.
4. Kousar S, Rehman N, Javed A, et al. Intensive Poultry Farming Practices Influence Antibiotic Resistance Profiles in Pseudomonas aeruginosa Inhabiting Nearby Soils. Infect Drug Resist. 2021;14:4511–4516. doi:10.2147/IDR.S324055
5. Gohar M, Mustafa A, Aziz J, et al. Controlled-shed poultry farming practices in Pakistan select for antibiotic resistant Escherichia coli. J Glob Antimicrob Resist. 2018;12:24–25. doi:10.1016/j.jgar.2017.11.015
6. Akinyemi F, Adewole D. Environmental stress in chickens and the potential effectiveness of dietary vitamin supplementation. Front Anim Sci. 2021;2:775311.
7. Fasure AK, Deji-Agboola AM, Akinyemi KO. Antimicrobial resistance patterns and emerging fluoroquinolone resistant Salmonella isolates from poultry and asymptomatic poultry workers. Afr J Microbiol Res. 2012;6(11):2610–2615. doi:10.5897/AJMR11.950
8. Agada GOA, Abdullahi IO, Aminu M, et al. Prevalence and Antibiotic Resistance Profile of Salmonella Isolates from Commercial Poultry and Poultry Farm-handlers in Jos, Plateau State, Nigeria. Microbiol Res J Int. 2014:462–479. doi:10.9734/BMRJ/2014/5872
9. Nawaz SK, Riaz S, Riaz S, Hasnain S. Screening for anti-methicillin resistant Staphylococcus aureus (MRSA) bacteriocin producing bacteria. Afr J Biotechnol. 2009;8:3. doi:10.4314/ajb.v8i3.59814
10. Andersson DI. Persistence of antibiotic resistant bacteria. Curr Opin Microbiol. 2003;6(5):452–456. doi:10.1016/j.mib.2003.09.001
11. Boxall ABA, Kolpin DW, Halling-Sørensen B, Tolls J. Peer Reviewed: are Veterinary Medicines Causing Environmental Risks? Environ Sci Technol. 2003;37(15):286A–294A. doi:10.1021/es032519b
12. Finley RL, Collignon P, Larsson DGJ, et al. The scourge of antibiotic resistance: the important role of the environment. Clin Infect Dis. 2013;57(5):704–710. doi:10.1093/cid/cit355
13. De Briyne N, Atkinson J, Pokludová L, Borriello SP. Antibiotics used most commonly to treat animals in Europe. Vet Rec. 2014;175(13):325. doi:10.1136/vr.102462
14. Mendonça N, Figueiredo R, Mendes C, Card RM, Anjum MF, Da Silva GJ. Microarray evaluation of antimicrobial resistance and virulence of Escherichia coli isolates from Portuguese poultry. Antibiotics. 2016;5(1):4. doi:10.3390/antibiotics5010004
15. Yang B, Cui Y, Shi C, et al. Counts, serotypes, and antimicrobial resistance of Salmonella isolates on retail raw poultry in the People’s Republic of China. J Food Prot. 2014;77(6):894–902. doi:10.4315/0362-028X.JFP-13-439
16. Miles TD, McLaughlin W, Brown PD. Antimicrobial resistance of Escherichia coli isolates from broiler chickens and humans. BMC Vet Res. 2006;2:7. doi:10.1186/1746-6148-2-7
17. Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: potential Public Health Implications. Mol J Synth Chem Nat Prod Chem. 2018;23(4):795. doi:10.3390/molecules23040795
18. Mikkili I, Peele A, Dulla J, Nath S, Vidya Prabhakar K. Isolation, Screening and Extraction of Polyhydroxybutyrate (PHB) producing bacteria from Sewage sample. Int J Pharm Tech Res. 2014;6:974–4304.
19. Perla R, Carifio J. Use of the Chi-square Test to Determine Significance of Cumulative Antibiogram Data. Am J Infect Dis. 2005;1:775311. doi:10.3844/ajidsp.2005.162.167
20. Wegener HC. Antibiotics in animal feed and their role in resistance development. Curr Opin Microbiol. 2003;6(5):439–445. doi:10.1016/j.mib.2003.09.009
21. Bogaard A, Mertens P, London N, Stobberingh E. High prevalence of colonization with vancomycin- and pristinamycin-resistant enterococci in healthy humans and pigs in The Netherlands: is the addition of antibiotics to animal feeds to blame? J Antimicrob Chemother. 1997;40:454–456. doi:10.1093/jac/40.3.454
22. Turtura GC, Massa S, Ghazvinizadeh H. Antibiotic resistance among coliform bacteria isolated from carcasses of commercially slaughtered chickens. Int J Food Microbiol. 1990;11(3):351–354. doi:10.1016/0168-1605(90)
23. Lakhotia RL, Stephens JF. Drug Resistance and R Factors Among Enterobacteria Isolated from Eggs. Poult Sci. 1973;52(5):1955–1962. doi:10.3382/ps.0521955
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
