Back to Journals » International Journal of Nanomedicine » Volume 12
Antibacterial properties of PEKK for orthopedic applications
Authors Wang M , Bhardwaj G, Webster TJ
Received 17 February 2017
Accepted for publication 6 August 2017
Published 5 September 2017 Volume 2017:12 Pages 6471—6476
DOI https://doi.org/10.2147/IJN.S134983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Carlos Rinaldi
Mian Wang, 1 Garima Bhardwaj, 1 Thomas J Webster 1,2
1Department of Chemical Engineering, Northeastern University, Boston, MA, USA; 2Wenzhou Institute of Biomaterials and Engineering, Wenzhou Medical University, Wenzhou, People’s Republic of China
Abstract: Orthopedic implant infections have been steadily increasing while, at the same time, antibiotics developed to kill such bacteria have proven less and less effective with every passing day. It is clear that new approaches that do not rely on the use of antibiotics are needed to decrease medical device infections. Inspired by cicada wing surface topographical features, nanostructured surfaces represent a new approach for imposing antibacterial properties to biomaterials without using drugs. Moreover, new chemistries with altered surface energetics may decrease bacterial attachment and growth. In this study, a nanostructured surface was fabricated on poly-ether-ketone-ketone (PEKK), a new orthopedic implant chemistry, comprised of nanopillars with random interpillar spacing. Specifically, after 5 days, when compared to the orthopedic industry standard poly-ether-ether-ketone (PEEK), more than 37% less Staphylococcus epidermidis were found on the PEKK surface. Pseudomonas aeruginosa attachment and growth also decreased 28% after one day of culture, with around a 50% decrease after 5 days of culture when compared to PEEK. Such decreases in bacteria function were achieved without using antibiotics. In this manner, this study demonstrated for the first time, the promise that nanostructured PEKK has for numerous anti-infection orthopedic implant applications.
Keywords: PEKK, PEEK, orthopedic, bacteria, infection
Corrigendum for this paper has been published
Erratum for this paper has been published
Introduction
Infection has been widely reported on numerous implantable devices, such as orthopedic implants, heart valves, peritoneal dialysis catheters, and endotracheal tubes.1 Especially for orthopedic implants, infection reports are on the rise.2,3 Due to a failure in treating infection, which can progress to septic failure, revision surgeries have become all too common for numerous orthopedic devices including total knee arthroplasties (25%), the third most common cause of failure in all total hip arthroplasties (15%), and the most common reason for the removal of all total knee arthroplasties and total hip arthroplasties (79% and 74%, respectively) while costing about $70,000 per episode.4,5 More specifically for spinal implants, the total adult infection rate was 2% (0.8% superficial and 1.2% deep infection).4,5 In addition, bacterial infections are a serious complication that can innervate into deep tissue and that can usually only be cured by removing the implant, since the biofilm formed on the implant surface protects the bacteria from host immune system clearance.6
Efforts had been made to prevent orthopedic implant infections through the use of antibiotics, as a conventional treatment method.7 For instance, incorporating antibiotics into bone cements decreases the risk of orthopedic device-related infections after initial arthroplasty.8 However, conventional antibiotic approaches do not always work since the long-term usage of antibiotics has led to the development of antibiotic-resistant bacteria with genetic changes and altered growth rates, leading to the generation of bacteria that we can no longer kill.9
Another approach that has been developed to reduce orthopedic device infection centers on fabricating antimicrobial coatings on implant surfaces.10 However, the loss of such antimicrobial coatings (such as nanoparticles and functionalized peptides) can create additional biological concerns, such as micromotion of the implant due to increased fretting and production of debris particles that cause bone necrosis. The loss of surface coatings can also lead to an aggressive inflammatory response, further decreasing chances of implant efficacy.11 It is clear that we need novel, nonpharmaceutical, and/or noncoating approaches to decrease orthopedic implant infections.
Along these lines, there has been significant promise in the use of nanomaterials or nanostructured implant surfaces to decrease bacteria attachment and prevent biofilm formation.12,13 Inspired by cicada wings, which are covered with nanopillar-shaped structures that are capable of killing bacteria through physical means rather than by using drug, scientists have discovered a way to reduce bacteria growth.14–22 Specifically, studies have shown that when bacteria land on such nanopillar surfaces with features much smaller than the bacteria themselves, bacterial membranes are ruptured.14,15 Therefore, an approach which relies on creating nanostructured surface features on the same material to be implanted may represent a novel nonpharmaceutical or non coating approach to reduce orthopedic implant infections.
For the above reasons, the objective of the present in vitro study was to determine bacteria functions on a new orthopedic implant chemistry, poly-ether-ketone-ketone (PEKK), manufactured to possess novel nanostructured surface features. Since, based on clinical statistics, Staphylococcus epidermidis and Pseudomonas aeruginosa are the most common pathogens responsible for medical device infections, they were the focus of the present study. Due to its popularity in the orthopedic industry, bacteria functions on PEKK were compared to the orthopedic industry standard poly-ether-ether-ketone (PEEK).
Methods
Materials and surface characterization
PEKK samples (TETRAFUSE™; RTI Surgical Inc., Alachua, FL, USA) are a proprietary form of 3D printed PEKK, manufactured via OsteoFab Technology (Oxford Performance Materials, South Windsor, CT, USA). PEEK samples (RTI Surgical) were manufactured to represent commercially available spinal implants.
Contact angle measurement
Contact angle measurements using water was carried out for the PEEK and PEKK samples in order to identify possible differences between their wetting abilities. The contact angles were measured using a video contact angle instrument (Samsung FA-CED camera, Samsung, Seoul, Korea) immediately after deionized water was allowed to fall freely onto the surfaces of the flat scaffolds. The contact angle in each case was taken as the average of three measurements carried out at different locations on the surface of scaffolds.
Scanning electron microscopy
A Hitachi S-4800 high resolution field emission scanning electron microscope was used to visualize the topographical features of the PEKK and PEEK samples.
Bacterial culture
Bacterial cell lines used in this study were S. epidermidis and P. aeruginosa obtained in freeze-dried form from the American Type Culture Collection (35984 and 25668, respectively). The dry pellet was rehydrated in 6 mL of Luria broth (LB) consisting of 10 g tryptone, 5 g yeast extract, and 5 g NaCl per liter of double distilled water with the pH adjusted to 7.4 (all chemicals were obtained from Sigma Aldrich, St Louis, MO, USA). The bacterial solution was agitated under standard cell conditions (5% CO2/95% humidified air at 37°C) for 24 h until the stationary phase was reached. For the second passage, the bacteria were diluted at a ratio of 1:200 into fresh LB supplemented with 10% fetal bovine serum (FBS) and incubated until it reached stationary phase. The second passage was then frozen in one part LB supplemented with 10% FBS and one part glycerol (Sigma Aldrich) and stored at 18°C. All experiments were conducted from this frozen stock. One day before bacterial seeding, a sterile 10 mL loop was used to withdraw bacteria from the frozen stock and to inoculate a centrifuge tube with 3 mL of fresh LB supplemented with 10% FBS.
Bacterial adhesion and growth
All samples were sterilized with UV light for 20 min on each side before use in experiments. Two species bacteria (S. epidermidis and P. aeruginosa) were used to evaluate the antibacterial properties of PEKK samples, with untreated PEEK samples used as a control. The two strains of bacteria were diluted in fresh LB supplemented with 10% FBS to a concentration of 105 CFU per mL. To track colonization by bacteria, each sample was incubated (5% CO2/95% humidified air at 37°C) in 2 mL of bacterial suspension and the medium was changed every 24 hours. At the appropriate time points (1, 3, and 5 days), the samples were gently rinsed three times with PBS (pH =7.4) to remove the nonadherent bacteria. The adherent bacteria on each sample were detached into 1 mL of PBS by ultrasonic vibration done for 5 min two times. Then, solutions with detached bacteria were serially diluted 10-fold with sterile PBS and three droplets (10 μL) were dropped per experimental condition onto LB-agar plates and incubated for 14 h at 37°C, then the bacterial colonies were again counted.
Bacteria live/dead assay
Fluorescence confocal microscopy (Olympus FluoView FV1000; Olympus, Hamburg, Germany) was used to visualize the colonization of bacteria on the samples of interest. Again, two kinds of bacteria (one Gram-positive [S. epidermidis] and one Gram-negative [P. aeruginosa]) were inoculated onto PEKK and PEEK samples at 105 CFU/mL. After 24 h of incubation, the medium was removed and cells were stained using the Live/Dead® BacLight™ kit (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. Living cells were stained by SYTO 9 (green), while dead cells were stained with propidium iodide (red).
Statistical analysis
All bacterial experiments were performed in triplicate and repeated three times per substrate. Data are presented as the mean value ± standard error of the mean and were analyzed with Student t-tests and 2-way analysis of variance. Statistical significance was considered at p<0.05.
Results and discussion
Surface characterization
Figure 1 highlights the surface morphology changes between the PEEK and PEKK samples. The surface of PEEK was relatively flat and smooth. In contrast, PEKK possessed a high degree of nanometer surface features, thus, creating a more nanometer-rough surface topography and significantly increasing surface area and exposure of the novel PEKK chemistry. PEKK showed an uneven surface with random nano-pin like patterns. This nanopattern on the surface had estimated sizes of 50–100 nm (diameter of feature), as estimated from the scanning electrin microscopy images. Contact angle measurements showed hydrophobic surface for nanostructured PEKK sample with a contact angle of 108°±0.79° compared with reference with a contact angle of 64°±0.82° (Figure 2).
  | Figure 2 Contact angle results of PEEK and PEKK samples. |
Bacterial responses
Figures 3 and 4 showed, for the first time, that both S. epidermidis and P. aeruginosa attachment and growth after 5 days were altered on the substrates of interest. Impressively, without resorting to the use of antibiotics, the results of this study revealed that both the bacterial strains adhered and grew less on the nanopatterned substrates (Figure 5). After 5 days, when compared to PEEK, more than 37% less S. epidermidis was found on the PEKK surface. P. aeruginosa attachment and growth also decreased 28% after one-day of culture, with around a 50% decreased after 5 days culture when compared to PEEK.
  | Figure 5 Difference of resistance to nanostructured PEKK surface between Gram-positive and Gram-negative bacteria. |
In addition, an interesting finding of this study was that Gram-negative bacteria (P. aeruginosa) attached less on the PEKK surface when compared to the Gram-positive bacteria (S. epidermidis). PEKK had more than 55% of an antibacterial effect for P. aeruginosa and a 40% effect on S. epidermidis after 5 days of culture. A reasonable explanation for this could be the difference in the mechanism of resistance of two bacterial species due to their membrane structure. It has been speculated that physical forces resulting in the disruption of bacterial membranes may play an important role in the mechanism of killing bacteria by nanostructured surfaces. The structure of a bacterial cell wall contains a cross-linked peptide, peptidoglycan, which builds a continuous macromolecular sacculus that provides cell wall rigidity. Gram-negative bacteria have a single layer of peptidoglycan, while Gram-positive bacteria contain numerous peptidoglycan layers, thereby giving Gram-positive bacteria a rigid membrane. Thus, it can be suggested that the rigidity of the bacteria cell wall is the most significant factor to determine the susceptibility of bacteria to nanostructured surfaces. While Gram-positive bacteria with rigid membranes appear to be more resistant to the lethal effects of the nanostructured surface, Gram-negative bacteria that have more elastic membranes may be more susceptible. In fact, when scientists microwaved Gram-positive bacteria to make their membranes more elastic, these bacteria also became susceptible to the lethal effects of the nanostructured surface.
In addition, whereas the nanocolumns on cicada wings form a regular and hexagonally arranged pattern with around 200 nm spacing between each nanocolumn, the nanocolumns on the presently designed PEKK were randomly orientated with a nonuniform spacing but were, on average, 50–100 nm in diameter.15–22 More importantly, the tips of cicada wing nanocolumns were round and capped, which was in contrast to the tips of PEKK which were sharp-edged. Future studies will investigate the influence of the geometry of these PEKK nanoscale surface features and the resultant antibacterial response.
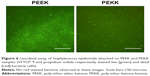
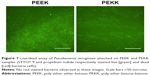
Finally, Figures 6 and 7 show live/dead bacteria images for the PEEK and PEKK substrates. The live/dead assay supported the aforementioned experimental results and indicated that both Gram-positive and Gram-negative bacterial adhesion and growth were significantly decreased after one-day of culture on the PEKK samples compared to PEEK. Representative fluorescence micrographs for S. epidermidis (Figure 5) also indicate that Gram-positive bacterial adhesion and growth was less affected (albeit still significantly affected compared to PEEK) after one-day of culture on PEKK.
In addition, the influence of this novel nanostructured PEKK surface on mammalian cells (such as osteoblasts) will also be investigated. There is a significant amount of evidence showing that, compared to conventional nanosmooth surfaces, nanorough surfaces not only possess antibacterial properties but also promote mammalian cell functions.23 The adhesion of bacteria may be inhibited by nanostructured features since bacteria are small (average diameter of 1 μm) and have relatively stiff membranes, thus inhibiting their attachment to such sharp, close nanostructures. However, osteoblasts are much larger (average diameter of 50 μm) with a more flexible membrane allowing them to contort and attach more to the nanostructures. Moreover, researchers have previously reported that nanoroughness alone altered surface energetics, which influenced select enhanced protein adsorption and bioactivity, thereby improving osteoblast adhesion and tissue growth.21,24,25 Elucidating an exact mechanism of action for the currently observed antibacterial properties on this novel nanostructured PEKK will also be the focus of a future study.
Conclusion
A simple method for the reduction of bacteria on and subsequent in vitro infection of PEKK with nanorough surface features was explored here for promising orthopedic applications. Results of this in vitro study indicated decreased adhesion and growth of P. aeruginosa and S. epidermidis on nanorough PEKK surface compared with conventional PEEK surfaces. This study provides convincing results that one may reduce bacteria functions by creating nanorough surfaces on PEKK, and thus nanostructured PEKK should be further studied for a wide range of antibacterial orthopedic applications.
Acknowledgments
The authors would like to thank RTI Surgical for funding this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Stanton T, Haas J, Phillips M, Immerman I. Study points to savings with infection-screening program before TJR. AAOS Now, 2011. | ||
Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52–56. | ||
Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128–133. | ||
Ercan B, Taylor E, Alpaslan E, Webster TJ. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology. 2011;22(29):295102. | ||
Smith JS, Shaffrey IC, Sansur CA, et al. Rates of infection following spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee: Paper #71. Spine: Affiliated Society Meeting Abstracts. 2009;10:104. | ||
Josef G, Ojan A, Michael B, Axel K. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials – catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdiszip. 2011;6(1):18. | ||
Zhijun S, Lotte B, Niels H, Hong W, Torben S, Arne B. Prosthesis infections after orthopedic joint replacement: The possible role of bacterial biofilms. Orthop Rev (Pavia). 2013;5(2):e14. | ||
Carlo L, Sara S, Enrico G, Delia R, Lorenzo D. Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama. J Orthop Surg Res. 2015;10:157. | ||
Julian D, Dorothy D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–433. | ||
Jiri G, Martin H, Calin M. Antibacterial surface treatment for orthopaedic implants. Int J Mol Sci. 2014;15(8):13849–13880. | ||
Bill Z, Damian M, Gordon GW, Milan B, Peter C. Bioactive coatings for orthopaedic implants – Recent trends in development of implant coatings. Int J Mol Sci. 2014;15(7):11878–11921. | ||
Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31(4):706–713. | ||
Webster TJ, Patel AA, Rahaman MN, Sonny Bal B. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012;8(12):4447–4454. | ||
Kelleher SM, Habimana O, Lawler J, et al. Cicada wing surface topography: An investigation into the bactericidal properties of nanostructural features. ACS Appl Mater Interfaces. 2016;8(24):14966–14974. | ||
Diu T, Faruqui N, Sjöström T, et al. Cicada-inspired cell-instructive nanopatterned arrays. Sci Rep. 2014;20(4):7122. | ||
Bazaka K, Jacob M, Chrzanowski W, Ostrikov K. Anti-bacterial surfaces: natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015;5:48739–48759. | ||
Wang M, Favi P, Cheng X, et al. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomaterialia. 2016;12(46):256–265. | ||
Jaroslaw D, Emil C, Dennis M, Konrad T. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter. 2011;7(21):9804–9828. | ||
Hoshian S, Jokinen V, Somerkivi V, Lokanathan AR, Franssila S. Robust superhydrophobic silicon without a low surface-energy hydrophobic coating. ACS Appl Mater Interfaces. 2015;7(1):941–949. | ||
Quéré D, Reyssat M. Non-adhesive lotus and other hydrophobic materials. Philos Trans A Math Phys Eng Sci. 2008;366(1870):1539–1556. | ||
Hong SH, Hwang J, Lee H. Replication of cicada wing’s nano-patterns by hot embossing and UV nanoimprinting. Nanotechnology. 2009;20(38):385303. | ||
Bagherifard S, Hickey DJ, de Luca C, et al. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials. 2015;73:185–197. | ||
Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25:e4731–e4739. | ||
Izquierdo-Barba I, García-Martín JM, Álvarez R, et al. Nanocolumnar coatings with selective behavior towards osteoblast and Staphylococcus aureus proliferation. Acta Biomater. 2015;15:20–28. | ||
Colon G, Ward BC, Webster TJ. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res A. 2006;78(3):595–604. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.