Back to Journals » Drug Design, Development and Therapy » Volume 15
Antibacterial and Antimicrobial Effects of Xanthorrhizol in the Prevention of Dental Caries: A Systematic Review
Authors Khalid GS, Hamrah MH, Ghafary ES , Hosseini S, Almasi F
Received 24 November 2020
Accepted for publication 20 February 2021
Published 11 March 2021 Volume 2021:15 Pages 1149—1156
DOI https://doi.org/10.2147/DDDT.S290021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Ghulam Sarwar Khalid,1 Mohammad Hassan Hamrah,2 Elaha Somaya Ghafary,3 Sepideh Hosseini,2 Fateme Almasi2
1Department of Pharmacology, Alberoni University, Kapisa, Afghanistan; 2Department of Pediatric Dentistry, Tehran University of Medical Sciences, Tehran, Iran; 3Department of Periodontics, Kabul University of Medical Sciences, Kabul, Afghanistan
Correspondence: Elaha Somaya Ghafary
Department of Periodontics, Kabul Medical University, Kabul, Afghanistan
Tel/Fax +93776665337
Email [email protected]
Background: Xanthorrhizol is one of the numerous phytochemicals whose pharmacological benefits have been explored for its antibacterial and antimicrobial effects. In light of the role bacteria play for initiating tooth decay, this present systematic review assessed xanthorrhizol’s effect against dental caries.
Methods: The electronic databases including Pubmed, Scopus and Embase were searched up to September 2020, Studies examining the antibacterial and antimicrobial effects of xanthorrhizol in the prevention and treatment of dental caries.
Results: Eleven studies met the criteria for final inclusion. Findings from these studies showed that xanthorrhizol showed significant inhibition of notable caries causing bacteria including Streptococcus mutans, Streptococcus sanguinis, Enterococcus faecalis and Bacillus cereus. Furthermore, there was no reported toxicity. However, it could not selectively target the growth of cariogenic bacteria.
Conclusion: So far, studies exploring the use of xanthorrhizol as a potential drug for the prevention and treatment of dental caries have shown promising outcomes. However, more work needs to be done especially in areas such as optimal dose or concentration, in addition, in vitro, in vivo and clinical studies and selective targeting of cariogenic bacteria has been performed.
Keywords: xanthorrhizol, antibacterial, antimicrobial, dental caries
Introduction
Dental caries (also known as tooth decay) is the most prevalent oral disease as well one of the most common chronic diseases across the globe.1 The formation of caries also describes the carious lesions or cavities resulting from the destruction of the cementum, enamel, and dentin.2 Although dental caries are complex in pathogenesis, the process of decay is initiated by Early life stages of cariogenic bacteria joining the dental biofilm, leading to initiation of the disease at a later stage.3 Some diseases responsible for dental caries in human include diabetes, anemia, oral cancer, eating disorders, etc.
As part of the bacterial flora in the oral cavity, Streptococcus sanguinis (S. sanguinis) and Streptococcus mutans (S. mutans) can be observed. In the pathogenesis of dental caries,4 certain microorganisms are the most dominant. Moreover, as is the case with S. sanguinis, they are concerned with periodontal and systemic diseases such as bacteremia and endocarditis.5
Biofilms are complex microbial communities. In the oral cavity, the number of phylotypes was estimated to exceed 19,000.6 It is composed of proteins, hydrated polysaccharides, glycopeptides, extracellular DNA and lipids.7 A biofilm is composed of several organism species including viruses, fungi and bacteria, existing at a stage or density interface, and embedded in an extracellular matrix that is self-secreted.7 The extracellular matrix surrounding the bacteria limits the penetration of antimicrobial drugs into the deeper sections of the biofilm. Apart from promoting competition for space, cohabitation of several organisms as well enhances the cooperative interactions such as horizontal gene transfer, metabolic cooperation and other synergies. This improves the ability of microorganisms to survive, thus become resistant to antimicrobial drug.8,9
Since biofilms act as a group rather than a separate cell, coordination, communication and signaling from cell to cell are vital.10 Quorum sensing, the secretion and identification by its representatives of diffusible molecules can result in sudden changes in the behavior of microbial species.11 The resistance of biofilms to therapy is controlled by three physiological processes.12,13 Firstly, there is a delayed or reduced penetration of any antimicrobials because of repulsion by the exopolysaccharide matrix. Secondly, the presence of persistent metabolically inactive cells capable of putting up with antimicrobial attacks prevents the colony from being utterly eliminated. Finally, the shut proximity of alternative cells offers rise to associate degree exaggerated chance of sharing resistance-encoding mobile genetic components. They will become 10–1000 times more resistant to the effects of antimicrobial agents when cells live in a biofilm.13 This poses a challenge for therapeutically targeting cariogenic bacteria residing in the biofilm.
Fluoride is the most common therapeutic modality for dental caries.13,14 Fluoride decreases bacteria’s acid resistance and is most effective at acidic pH levels. Fluoride enhances glycolysis in S. mutans under acidic conditions. The anti-caries effect of fluoride not only consists of remineralization but also inhibition of acidogenic bacteria in acidic conditions. Although the most powerful anti-caries agent today is fluoride,14 dental caries remain a public health problem in today’s world. Thus, there is the need to establish new therapeutic alternatives that may complement current treatment modalities.
Dental Caries is mainly a disorder of infection, thus numerous studies have focused mainly on the transmission and presence of certain groups of bacteria as the major determinants of caries. S. mutans have been shown to be one of the key pathogens involved in the development of dental caries, especially in children.15–21 From a microbiological standpoint, preventive approaches might include efforts to prevent or delay transmission of S. mutans to the child, the development of topical antimicrobial agents aimed at preventing key bacteria from reaching pathological levels, vaccination or gene therapy and methods to stimulate salivary flow.2,22 However, the clinical efficacies of these strategies have so far been ineffective.2
Phytochemicals (plant-derived natural products) with abilities to inhibit bacteria growth are an attractive alternative to conventional oral biocides for long-term caries prevention. Phytochemicals have been shown to reduce the development of dental plaque, influence bacterial adhesion, and reduce symptoms of oral diseases.23 For instance, eucalyptus globules and ethyl acetate extracts of acacia nilotica have been observed for their potent antimicrobial effects.24 Natural products have advantage over conventional antimicrobial agents which suppress even health-associated oral microbial communities, and thus disrupting key health benefits of the resident oral microbiome.25
Xanthorrhizol (Figure 1) is one of the numerous phytochemicals whose medical benefits have been explored. This is a bioactive substance isolated from the Curcuma xanthorrhiza rhizome (Java turmeric).26 Xanthorrhizol (1,3,5,10-bisabolatetraen-3-ol) has a molecular weight of 218.33 g/mol and a solubility of 28.90 µg/mL.27 Thus, the surface of a biofilm is supposed to penetrate readily.27 It has been reported to possess numerous pharmacological abilities including antimicrobial, anti-hyperglycemic, antibacterial, anti-inflammatory, antifungal, anticancer and neuroprotective properties.28–31 In light of the role bacteria play for initiating tooth decay, this present systematic review assessed xanthorrhizol’s effect against dental caries.
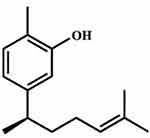 |
Figure 1 Chemical structure of xanthorrhizol. |
Materials and Methods
We adopted the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA)32 guidelines in conducting the present study.
Search Strategy
The Pubmed, Embase and Scopus databases were assessed from the inception of each database till September 2020, to perform a literature search on studies which investigated the antimicrobial and antibacterial effects of xanthorrhizol. These databases were searched for articles published in English language without restriction on publication year. The search keywords were “xanthorrhizol”, “Curcuma xanthorrhiza”, “antimicrobial”, “antibacterial” and “dental caries”. Only original articles (experimental and clinical) were included. However, articles types such as reviews, letters and conference proceedings were excluded. Afterwards, articles from the initial search were screened for duplicates (using Endnote software version 8), followed by screening their titles and abstracts for conformity to the eligibility criteria. Furthermore, references of retained articles were manually screened for possible inclusion of relevant studies. Quality assessment of the relevant studies was conducted using RevMan software version 5.3.
Data Extraction
With the aid of a predesigned data extraction form, two authors were charged with the independent entry of its contents. In the case of disagreements, a third author was consulted and consensuses were reached based on factual evidences. The data extracted from the included studies were as follows: first author name and year, study type, xanthorrhizol concentration, antibacterial activity and major finding(s). The antibacterial and antimicrobial activity of xanthorrhizol against any of the studied microorganism was defined in terms of at least of the following:
- Minimum inhibitory concentration (MIC) (expressed in µg/mL), defined as the lowest concentration of xanthorrhizol required to inhibit the growth of a bacteria compared with the control, in vitro.
- Colony forming unit (CFU) [expressed in log10 (CFU/mL)].
- Cell viability (expressed as a percentage).
Results
Initially, 313 records were identified from the literature search following the removal of duplicates, 295 articles were retained. Afterwards, a further 258 articles were excluded after screening of their titles and abstracts. Thus, the full-texts of 37 articles were screened for eligibility, from which 11 articles met all criteria for final inclusion (Figure 2). Except for one clinical study, all others were in vitro studies. Table 1 presents the major characteristics of the included studies.
 |
Table 1 Summary of Included Studies |
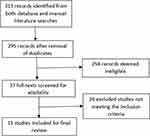 |
Figure 2 PRISMA diagram depicting the process of study inclusion. Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097.32 |
Antibacterial Effect of Xanthorrhizol
Studies by Hwang et al28,33 showed that revealed the fast antibacterial activity of xanthorrhizol against S. mutans. Moreover, in both studies, xanthorrhizol had the highest antibacterial activity against all the five Streptococcus species. Rukayadi and Hwang34 further observed that the antibacterial effect of xanthorrhizol on treated S. mutans biofilms was dependent on the concentration, exposure time and maturation periods (4, 12, 20 and 24 hours) of biofilm.While5 µmol/l xanthorrhizol treated biofilms of S. mutans at the adherent period (4 h) were completely removed, those treated with 50 µmol/l xanthorrhizol at the beginning plateau accumulated phase (20 h) and plateau accumulated phase (24 h) were reduced by 89% and 76%, respectively. These effects of exposure time, concentration and phase growth are particularly important as it has been reported that bacteria on the surfaces of toothbrushes can be viable from 24 hours to 7 days.35
Another study by Rukayadi and Hwang36 showed that the antibacterial activity of xanthorrhizol was similar to that of chlorhexidine. Furthermore, coating the well of a microtiter plate with 5 µg/mlxanthorrhizol resulted in a 60% reduction of adherent cells compared to cells in the uncoated wells. Thus, significantly prevented colonization and biofilm formation of S. mutans. Kim et al37 also reported that 0.1 mg/mL of xanthorrhizol showed a similar antibacterial activity to 2 mg/mL of chlorhexidine on S. mutans biofilms, with a 57% inhibition of cell viability. They attributed the antibacterial mechanism of xanthorrhizol to the disruption of the peptidoglycan layer of S. mutans.37
Lee et al27 explored the photodynamic potential of xanthorrhizol as an antibacterial agent. When irradiated with light, the antimicrobial activity of CXE appeared at 102ng/mL, which is lower than the MIC of xanthorrhizol itself and so indicates that CXE can induce the photodynamic reaction by acting as a photosensitizer. Thus, the viability of S. mutans cells reduced steadily from 85.2% to 0.0% for corresponding xanthorrhizol concentrations of 102 to 104ng/mL. The production of reactive oxygen species (ROS) following irradiation of xanthorrhizol, was attributed to antimicrobial photodynamic activity of xanthorrhizol in the prevention of caries.13
During the treatment of caries, therapeutic resistance could be encountered. It has been shown that the presence of Enterococcus faecalis (E. faecalis) in the root canal following endodontic treatment is an indication of therapeutic failure.38 Thus, it is imperative to identify new drugs with capabilities of fully reaching the canal system. To this aim, Yue et al39 compared the antibacterial effect of xanthorrhizol with chlorhexidine on E. faecalis. Their results showed that although both xanthorrhizol and chlorhexidine had similar antibacterial effect, xanthorrhizol was more effective in alkalization state. Thus, the effective removal of E. faecalis by xanthorrhizol portends a useful strategy for countering resistance during the treatment of caries.
Foodborne pathogens such as Enterobacteriaceae, Staphylococcus aureus and Enterococci have been shown to be contributory factors in the development of dental caries.40–43 Thus, pathogenic bacteria inhibition can be useful to prevent caries and periodontal diseases. Lee et al44 reported effective activity of xanthorrhizol against gram-positive bacteria including Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus and Clostridium perfringens, even at low concentrations. However, among those tested for gram-negative foodborne bacteria, only Vibrio parahaemolyticus and Salmonella typhimurium were observed to be xanthorrhizol sensitive, while xanthorrhizol had no effect on Yersinia enterocolitica, Shigella sonnei, Escherichia coli O157:H7 and Campylobacter jejuni.
Antimicrobial Effect of Xanthorrhizol
Toothbrushes, when manufactured are free of microorganisms. However, when used for the removal of plaque as well as other debris, they become contaminated with bacteria, leading to infection. Moreover, it has been shown that contamination occurs as a fast as 30 seconds to 4 minutes after each use.45 There have also been evidence associating the continued use of these toothbrushes contaminated by several micro organisms including Pseudomonas, Candida, Streptococcus, Klebsiella, Lactobacilli, Escherichia coli and Staphylococcus, to the development of dental caries.46–48 Thus, effective decontamination of toothbrushes is a useful strategy in the prevention of caries, especially as toothbrushes are the most common tool for improving oral hygiene. A clinical trial by Bhat et al35 treated the toothbrushes of 60 children with different antimicrobial agents. Toothbrushes of children in groups 1, 2, 3 and 4 were treated with 3% neem, 5% xanthorrhizol, 0.5% cetylpyridinium chloride and 0.2% chlorhexidine, respectively for 12 hours. Their results showed that the antimicrobial effect of xanthorrhizol on S. mutans was higher than chlorhexidine, but lesser than that of neem and cetylpyridinium chloride. Moreover, there was a 78% reduction in S. mutans following treatment with xanthorrhizol.
In exploring novel drugs that inhibit bacteria that induce dental caries, it is important that the drug does not deplete the “useful” bacteria of plaque microflora. Philip et al49 investigated the efficacy of some antimicrobial agents including xanthorrhizol to ascertain their potentials for selective targeting of S. mutans without upsetting the viability of health-associated S. sanguinis. Results showed that among the tested natural products, xanthorrhizol had the highest antimicrobial activity. Moreover, for all the agents, there was no significant difference between their inhibitions of S. mutans and S. sanguinis. Thus, none of the agents could selectively target the growth of cariogenic bacteria.
It has been shown that biofilm matrix can limit the effectiveness of drugs, thereby making the biofilm resistant to antimicrobial agents.50,51 Thus, Cho et al52 explored the possibility of the use of xanthorrhizol in the form of nanoemulsion, in order to facilitate the ease of penetration of antimicrobial agents into biofilm. Their results showed effective antimicrobial effects of nanoemulsion xanthorrhizol, via inhibition as well as damaging the biofilm formation by S. mutans.
Discussion
To our knowledge, this is the first study to systematically review the antimicrobial and antibacterial capabilities of xanthorrhizol in preventing dental caries. Our findings from the reviewed studies showed that xanthorrhizol has potentials to be an effective drug for the prevention of dental caries. Xanthorrhizol’s mechanism against caries-forming bacteria are determined by the substances that comprise xanthorrhizol: chains of phenols and hydrocarbons. The phenol compounds containing the hydroxyl (-OH) functional groups, interact with bacterial cells through the process of adsorption comprising hydrogen bonds and can change the permeability of cell membranes.53 The high concentration of phenol penetrating into cells could lead to protein coagulation and lysis on cell membranes. Furthermore, the formation of hydrogen bonds between the hydroxyl groups of phenol compounds and cell membrane proteins disrupts the permeability of the membrane, thereby causing the vital cell components to exit the cell leading to the inhibition or death of the bacteria.53 It has also been suggested that the suppression of mitogen-activated protein kinase (MAPK) and nuclear factor kappaB (NF-kB) by xanthorrhizol could be attributed to its antimicrobial effect.54
In recent times, novel antimicrobial and antibacterial agents have been explored.55–60 Chlorhexidine, which is commonly found in mouthwash has faced limitations due to restricted does and possible side effects.61 In contrast, studies involving xanthorrhizol have shown no toxicity in addition to effective antimicrobial and antibacterial activities even at low concentrations. While the MIC of xanthorrhizol is comparable to chlorhexidine, it also suffers from the challenge of not been selective for cariogenic bacterial species.49
According to a recent report by the Global Burden of Disease Study, oral diseases are prevalent in 3.5 billion people.62 Furthermore, it was revealed that untreated dental caries is the most common health burden.63 Dental caries has been shown to have a negative effect on the quality of life of individuals.64 Its economic burden has also been a major concern especially in low and middle-income nations unable to provide services to prevent and treat oral health conditions.65 Thus, exploring the use of natural agents such as xanthorrhizol could go a long way in alleviating these burdens, especially as they are cheap, naturally abundant and less toxic.
The present study had limitations. Most of the reviewed studies were in vitro except for one clinical trial. Moreover, in the clinical study, only a few subjects were enrolled and xanthorrhizol was not administered directly on them, rather it was used to disinfect their toothbrushes. Thus, going forward, future randomized control trials with a large number of subjects, preferably those suffering from dental caries can be enrolled in order to fully examine the clinical efficacy of this drug. Furthermore, while no safety concerns were recorded in any of the reviewed studies, it is imperative that further evaluation of nanoemulsion xanthorrhizol be carried out to ascertain its effects on saliva as well as other soft tissues, before being explored as a potential mouthwash.
Conclusion
Findings from the reviewed studies have shown that xanthorrhizol possesses potent antibacterial and antimicrobial activities, implying a potential drug for the prevention and treatment of dental caries. However, there still remains a lot of work to be done in ensuring its full clinical translation. Further researches are necessary, including in vitro, in vivo and clinical studies. Aspects such as optimal treatment dose or concentration of xanthorrhizol, future randomized control trials and selective inhibition of cariogenic bacterial species would also need to be addressed in order to fulfill its potential to be considered as a standard drug.
Disclosure
The authors report no conflicts on interest in this work.
References
1. Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51–59. doi:10.1016/S0140-6736(07)60031-2
2. Fejerskov O, Kidd E. Dental Caries: The Disease and Its Clinical Management. John Wiley & Sons; 2009.
3. Smith D. Dental caries vaccines: prospects and concerns. Crit Rev Oral Biol Med. 2002;13(4):335–349. doi:10.1177/154411130201300404
4. Kouidhi B, Yma AQ, Chaieb K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb Pathog. 2015;80:39–49. doi:10.1016/j.micpath.2015.02.007
5. Babii C, Bahrin L, Neagu AN, et al. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J Appl Microbiol. 2016;120(3):630–637. doi:10.1111/jam.13048
6. Keijser B, Zaura E, Huse S, et al. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res. 2008;87(11):1016–1020. doi:10.1177/154405910808701104
7. Dong L, Tong Z, Linghu D, et al. Effects of sub-minimum inhibitory concentrations of antimicrobial agents on Streptococcus mutans biofilm formation. Int J Antimicrob Agents. 2012;39(5):390–395. doi:10.1016/j.ijantimicag.2012.01.009
8. Yang L, Liu Y, Wu H, et al. Current understanding of multi‐species biofilms. Int J Oral Sci. 2011;3(2):74–81. doi:10.4248/IJOS11027
9. Wolcott R, Costerton J, Raoult D, et al. The polymicrobial nature of biofilm infection. Clin Microbiol Infect. 2013;19(2):107–112. doi:10.1111/j.1469-0691.2012.04001.x
10. Shrout JD, Tolker-Nielsen T, Givskov M, et al. The contribution of cell-cell signaling and motility to bacterial biofilm formation. MRS Bulletin. 2011;36(5):367–373. doi:10.1557/mrs.2011.67
11. Remis JP, Costerton JW, Auer M. Biofilms: structures that may facilitate cell–cell interactions. ISME J. 2010;4(9):1085–1087. doi:10.1038/ismej.2010.105
12. Høiby N, Bjarnsholt T, Givskov M, et al. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–332. doi:10.1016/j.ijantimicag.2009.12.011
13. Mah T-F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012;7(9):1061–1072. doi:10.2217/fmb.12.76
14. Clarkson JJ, McLoughlin J. Role of fluoride in oral health promotion. Int Dent J. 2000;50(3):119–128. doi:10.1111/j.1875-595X.2000.tb00552.x
15. Fernando S, Tadakamadla S, Bakr M, et al. Indicators of risk for dental caries in children: a holistic approach. JDR Clin Trans Res. 2019;4(4):333–341. doi:10.1177/2380084419834236
16. Parekh MS, Ishnava KB. Antimicrobial and hemolytic activity of seed protein extracts from selected medicinal plants against tooth decaying microorganisms. IJRSMB. 2019. doi:10.20431/2454-9428.0601005
17. Bhattacharya I, Ishnava K, Chauhan J. In vitro anticariogenic activity of some Indian medicinal plants towards human oral pathogen. Asian J Tradit Med. 2016;11(4).
18. Bhoot N, Ishnava KB. Antimicrobial activity of medicinally important essential oils against selected dental microorganisms. Int J Curr Microbiol App Sci. 2017;6(6):1562–1575. doi:10.20546/ijcmas.2017.606.184
19. Ishnava K. Role of herbal medicine in dental health. J Environ Chem Toxicol. 2018;2(1):28–29.
20. Shouche S, Ishanva K. Screening of herbal formulation for anticariogenic activity. J Med Plant. 2018;6(1):243–249.
21. Trivedi P, Chauhan J, Ishnava K. In vitro assessment of inhibition potential of ethanomedicinal plants against cariogenic bacteria. Acta Sci Microbiol. 2018;1(6):43–49.
22. Berkowitz RJ. Causes, treatment and prevention of early childhood caries: a microbiologic perspective. J Can Dent Assoc. 2003;69(5):304–307.
23. Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complementary Altern Med. 2011;2011:1–15. doi:10.1093/ecam/nep067
24. Barad MK, Ishnava KB, Chauhan JB. Anticariogenic activity and phytochemical studies of crude extract from some Indian plant leaves. J Intercult Ethnopharmacol. 2014;3(2):85–90. doi:10.5455/jice.20140228010459
25. Yoo S, Murata R, Duarte S. Antimicrobial traits of tea-and cranberry-derived polyphenols against Streptococcus mutans. Caries Res. 2011;45(4):327–335. doi:10.1159/000329181
26. Rimpler H, Hansel R, Kochendoerfer L. Xanthorrhizol, ein neues Sesquiterpen aus Curcuma xanthorrhiza. Z Naturforsch B J Chem Sci. 1970;25(9):995–998. doi:10.1515/znb-1970-0917
27. Lee H-J, Kang S-M, Jeong S-H, et al. Antibacterial photodynamic therapy with curcumin and Curcuma xanthorrhiza extract against Streptococcus mutans. Photodiagnosis Photodyn Ther. 2017;20:116–119. doi:10.1016/j.pdpdt.2017.09.003
28. Hwang JK, Shim JS, Baek NI, et al. Xanthorrhizol: a potential antibacterial agent from Curcuma xanthorrhiza against Streptococcus mutans. Planta Med. 2000;66(02):196–197. doi:10.1055/s-0029-1243135
29. Choi MA, Kim SH, Chung WY, et al. Xanthorrhizol, a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochem Biophys Res Commun. 2004;326(1):210–217. doi:10.1016/j.bbrc.2004.11.020
30. Lim CS, Jin DQ, Mok H, et al. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia.J. Neurosci Res (N Y). 2005;82(6):831–838. doi:10.1002/jnr.20692
31. Kim MB, Kim C, Song Y, et al. Antihyperglycemic and anti-inflammatory effects of standardized Curcuma xanthorrhiza Roxb. extract and its active compound xanthorrhizol in high-fat diet-induced obese mice. Evid Based Complementary Altern Med. 2014;205915.
32. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
33. Hwang J, Shim J, Pyun Y. Antibacterial activity of xanthorrhizol from Curcuma xanthorrhiza against oral pathogens. Fitoterapia. 2000;71(3):321–323. doi:10.1016/S0367-326X(99)00170-7
34. Rukayadi Y, Hwang JK. In vitro activity of xanthorrhizol against Streptococcus mutans biofilms. Lett Appl Microbiol. 2006;42(4):400–404. doi:10.1111/j.1472-765X.2006.01876.x
35. Bhat PK, Badiyani BK, Sarkar S, et al. Effectiveness of antimicrobial solutions on streptococcus mutans in used toothbrushes. World J Dent. 2012;3(1):6–10. doi:10.5005/jp-journals-10015-1119
36. Rukayadi Y, Hwang JK. Effect of coating the wells of a polystyrene microtiter plate with xanthorrhizol on the biofilm formation of Streptococcus mutans. J Basic Microbiol. 2006;46(5):410–415. doi:10.1002/jobm.200510088
37. Kim JE, Kim HE, Hwang JK, et al. Antibacterial characteristics of Curcuma xanthorrhiza extract on Streptococcus mutans biofilm. J Microbiol. 2008;46(2):228. doi:10.1007/s12275-007-0167-7
38. Rodríguez-Niklitschek C. Clinical implications of Enterococcus faecalis microbial contamination in root canals of devitalized teeth: literature review. Revista Odontológica Mexicana. 2015;19(3):181–186. doi:10.1016/j.rodmex.2015.04.002
39. Yue W, Song M, Kang SM, et al. The antibacterial effect of xanthorrhizol as an endodontic irrigant on Enterococcus faecalis. J Korean Dent Assoc. 2016;54(3):206–216.
40. Mobley C, Marshall TA, Milgrom P, et al. The contribution of dietary factors to dental caries and disparities in caries. Acad Pediatr. 2009;9(6):410–414. doi:10.1016/j.acap.2009.09.008
41. Feglo P, Sakyi K. Bacterial contamination of street vending food in Kumasi, Ghana. J Medical Biomed Sci. 2012;1(1):1–8.
42. Mensah P, Yeboah-Manu D, Owusu-Darko K, et al. Street foods in Accra, Ghana: how safe are they? Bull. World Health Organ. 2002;80:546–554.
43. Ismail SA. Microbiological quality of hawawshy consumed in Ismailia, Egypt. J Food Saf. 2006;26(4):251–263. doi:10.1111/j.1745-4565.2006.00047.x
44. Lee LY, Shim J-S, Rukayadi Y, et al. Antibacterial activity of xanthorrhizol isolated from Curcuma xanthorrhiza Roxb. against foodborne pathogens. J Food Prot. 2008;71(9):1926–1930.
45. Taji S, Rogers A. The microbial contamination of toothbrushes. A pilot study. Aust Dent J. 1998;43(2):128–130. doi:10.1111/j.1834-7819.1998.tb06101.x
46. Wetzel WE, Schaumburg C, Ansari F, et al. Microbial contamination of toothbrushes with different principles of filament anchoring. J Am Dent Assoc. 2005;136(6):758–765. doi:10.14219/jada.archive.2005.0259
47. Karibasappa G, Nagesh L, Sujatha B. Assessment of microbial contamination of toothbrush head: an in vitro study. Indian J Dent Res. 2011;22(1):2. doi:10.4103/0970-9290.79965
48. Vvb S. Contamination of toothbrush at different time intervals and effectiveness of various disinfecting solutions in reducing the contamination of toothbrush. J Indian Sot Pedo Prev Dent. 2002;20(3):81–85.
49. Philip N, Leishman S, Bandara H, et al. Growth inhibitory effects of antimicrobial natural products against cariogenic and health-associated oral bacterial species. Oral Health Prev Dent. 2020;18(3):537–542. doi:10.3290/j.ohpd.a44307
50. Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90(3):294–303. doi:10.1177/0022034510379602
51. Koo H, Allan RN, Howlin RP, et al. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15(12):740. doi:10.1038/nrmicro.2017.99
52. Cho MY, Kang SM, Lee ES, et al. Antimicrobial activity of Curcuma xanthorrhiza nanoemulsions on Streptococcus mutans biofilms. Biofouling. 2020;36(7):825–833. doi:10.1080/08927014.2020.1823376
53. Rukayadi Y, Hwang JK. In vitro activity of xanthorrhizol isolated from the rhizome of Javanese turmeric (Curcuma xanthorrhiza Roxb.) against Candida albicans biofilms. Phytother Res. 2013;27(7):1061–1066. doi:10.1002/ptr.4834
54. Oon SF, Nallappan M, Tee TT, et al. Xanthorrhizol: a review of its pharmacological activities and anticancer properties. Cancer CellInt. 2015;15(1):100.
55. Trong LN, Viet HD, Quoc DT, et al. In vitro antimicrobial activity of essential oil extracted from leaves of Leoheo domatiophorus chaowasku, D.T. Ngo and H.T. Le in Vietnam. Plants. 2020;9(4):453. doi:10.3390/plants9040453
56. Freires IA, Denny C, Benso B, et al. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: a systematic review. Molecules. 2015;20(4):7329–7358. doi:10.3390/molecules20047329
57. Donadu MG, Trong LN, Viet HD, et al. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of Hornstedtia bella Škorničk. Antibiotics. 2020;9(6):334. doi:10.3390/antibiotics9060334
58. Trong LN, Viet HD, Quoc DT, et al. Biological activities of essential oils from leaves of Paramignya trimera (Oliv.) Guillaum and Limnocitrus littoralis (Miq.) swingle. Antibiotics. 2020;9(4):207. doi:10.3390/antibiotics9040207
59. Le NT, Donadu MG, Ho DV, et al. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J Infect Dev Ctries. 2020;14(9):1054–1064. doi:10.3855/jidc.12469
60. Bua A, Usai D, Donadu MG, et al. Antimicrobial activity of Austroeupatorium inulaefolium (H.B.K.) against intracellular and extracellular organisms. Nat Prod Res. 2018;32(23):2869–2871. doi:10.1080/14786419.2017.1385014
61. Brookes ZLS, Bescos R, Belfield LA, et al. Current uses of chlorhexidine for management of oral disease: a narrative review. J Dent. 2020;103:103497. doi:10.1016/j.jdent.2020.103497
62. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858.
63. World Health Organization. Political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases. New York: 66th Session of the Unites Nations General Assembly, WHO; 2011.
64. Åkesson ML, Gerdin EW, Söderström U, Lindahl B, Johansson I. Health-related quality of life and prospective caries development. BMC Oral Health. 2016;16(1):15. doi:10.1186/s12903-016-0166-3
65. Hosseinpoor A, Itani L, Petersen P. Socio-economic inequality in oral healthcare coverage: results from the World Health Survey. J Dent Res. 2012;91(3):275–281. doi:10.1177/0022034511432341
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
