Back to Journals » Drug Design, Development and Therapy » Volume 14
Anti-Tumor Xanthones from Garcinia nujiangensis Suppress Proliferation, and Induce Apoptosis via PARP, PI3K/AKT/mTOR, and MAPK/ERK Signaling Pathways in Human Ovarian Cancers Cells
Received 30 April 2020
Accepted for publication 21 August 2020
Published 25 September 2020 Volume 2020:14 Pages 3965—3976
DOI https://doi.org/10.2147/DDDT.S258811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Zhongyan Tang,1,* Lihua Lu,2,* Zhengxiang Xia3
1Department of Emergency and Critical Care Medicine, Jin Shan Hospital, Fudan University, Shanghai 201508, People’s Republic of China; 2Department of Neonatology, International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200030, People’s Republic of China; 3Department of Pharmacy, School & Hospital of Stomatology, Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Tongji University, Shanghai 200072, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhengxiang Xia Tel +8621-66315500
Email [email protected]
Background: Ovarian cancer (OC) is a serious public health concern in the world. It is important to develop novel drugs to inhibit OC.
Purpose: This study investigated the isolation, elucidation, efficiency, molecular docking, and pharmaceutical mechanisms of xanthones isolated from Garcinia nujiangensis.
Methods: Xanthones were isolated, and purified by different chromatography, including silica gel, reversed-phase silica gel (ODS-C18), and semipreparative HPLC, then identified by analysis of their spectral data. Three xanthones were estimated for their efficiency on the human OC cells HEY and ES-2. 2 was found to be the most potent cytotoxic xanthones of those tested. Further, its mechanisms of action were explored by molecular docking, cell apoptosis, and Western blotting analysis.
Results: Bioassay-guided fractionation of the fruits of Garcinia nujiangensis led to the separation of a new xanthone named nujiangexanthone G ( 1) and two known xanthones. Among these, isojacareubin ( 2) exhibited the most potent cytotoxic compound against the HEY and ES-2 cell lines. The analysis of Western blot suggested that 2 inhibited OC via regulating the PARP, PI3K/AKT/mTOR, and ERK/MAPK signal pathways in the HEY cell lines.
Conclusion: In conclusion, isojacareubin ( 2) might be a potential drug for the treatment of OC.
Keywords: Garcinia nujiangensis, xanthone, ovarian cancer; OC, molecular docking, apoptosis
Introduction
Ovarian cancer (OC) is the deadliest form of gynaecological malignancy in the world, with the increasing number of new cases and deaths in the last year.1 Due to a lack of evident symptoms in the early stage of OC, most women diagnosed with it are at an advanced stage of the disease and this can lead to high mortality rates.2 Cytoreductive surgery and chemotherapy are the first-line treatment for OC, however, the chemotherapy has side and toxic effects, most of the patients diagnosed with advanced OC survive no more than 5 years. Therefore, novel therapeutic strategies are urgently required which can improve survival in OC patients.3
Poly (ADP-ribose) polymerase (PARP), a family of proteins involved in DNA damage repair, genomic stability, and programmed cell death. The development of PARP inhibitors (PARPi) has succeed in the treatment of OC.4 However, resistance to PARPi is one of the major problems. Combination therapy is useful for enhancing therapeutics in the treatment of complex diseases (such as OC).5 The phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathways play an important role in the regulation of cell survival, growth, and proliferation. Irregularities in the major components of those signaling pathways leads to human cancers including OC.6 Natural products regulates multiple signal pathways involved in OC will be a prospect agent.
Natural product xanthones (dibenzo-γ-pyrones) constitutes a significant class of oxygenated heterocycles and occurs as secondary metabolites in microorganisms and plants.7 This kind of metabolites showed numerous pharmacological activities such as anti-cancer,8 anti-oxidant,9 diuresis and saluresis,10 hepatoprotective,11 anti-gout,12 anti-inflammatory,13 anti-diabetes,14 and gastroprotective.15 Recently, these xanthones easily incorporated into micro-molecules attributing to intriguing biological activities had drew attention from phytochemical,16 organic synthesis,17,18 and pharmacological endeavors.19 Xanthones from the genus Garcinia had structural diversity and exhibited significant biological activity.20 Garcinia nujiangensis is a Chinese endemic species, grown in Nujiang, Yunnan province in China. Our previous study on the leaves, twigs, and fruits of this plant led to the discovery of several cytotoxic xanthones.21–23 In our continuing research on new anti-OC compounds from traditional Chinese medicine, the extract of the fruits of G. nujiangensis exhibited inhibitory effects on the HEY cell lines. Bioassay-guided separation of G. nujiangensis led to the isolation of a new xanthone, nujiangexanthone G (1), and two known xanthones. In this article we report the isolation, separation, structure elucidation, and bioassay evaluation of these xanthones, the potential proteins involved in the progress of OC regulated by 2 screened by molecular docking, which was demonstrated by the Western blot analysis.
Materials and Methods
General
HPLC data was performed on an Agilent 1200 instrument loaded with ultraviolet detector. Optical rotations were examined on a JASCO P-1020 polarimeter. NMR data were performed on an Agilent 500M-NMR spectrometer. High-resolution mass spectrometry was carried on a Waters Q-TOF Premier instrument (Micromass MS Technologies, Manchester, UK) loaded with an ESI source. Column chromatography was done with silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Ltd.), reversed-phase C18 silica gel (250 mesh, Merck), and Sephadex LH-20 (Pharmacia). An Agilent 1260 machine was loaded with a C18 column (Zorbax SB, 4.6 × 250 mm, 5 μm) was applied for HPLC analysis. 3-(4, 5-Dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide was obtained from Sigma-Aldrich (St Louis, MO, USA). Dulbecco's Modified Eagle’s Medium (DMEM), penicillin and streptomycin, fetal bovine serum, and 0.05% trypsin was purchased from GibcoBRL (Grand Island, NY, USA). Compounds were dissolved in dimethyl sulphoxide (DMSO). The values of OD were carried on a microplate reader (Bio-Rad, Hercules, CA, USA). Antibodies cleaved Cas-3, cleaved PARP, Bcl-2, Bax, p-PI3K, p-AKT, p-mTOR, p-ERK1, ERK1, p-MEK, p-C-Raf, GAPDH, and Enhanced chemiluminescent (ECL) plus reagent kit were obtained from Beyotime Institute of Biotechnology (Shanghai, China). The cell morphology was visualized by the use of an optical microscope (Olympus, Japan).
Plant Material
The fruits of G. nujiangensis (Clusiaceae), were collected in Nujiang, Yunnan P.R. China, in September 2018, identified by Prof. Yuanchuan Zhou, traditional Chinese medicine of Yunnan university. A voucher specimen (NJ. 028) was deposited in the lab of Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Tongji University.
Extraction and Isolation
The fruits of G. nujiangensis (1200 g) were cut into pieces, and extracted with acetone at the temperature of 25 °C three times. The extract (132 g) was suspended in water (2 L) and extracted with hexane, ethyl acetate, and n-ButOH successively. The ethyl acetate portion (24 g) was subjected to a silica gel column (200–300 mesh, 400 g), using a gradient of dichloromethane–acetone (from 1:0 to 0:1) to afford six fractions (A–F). Fr. C (338 mg) showed moderate cytotoxic activity against the HEY cell lines. It was subjected to ODS-C18 using a gradient of methanol–water (from 65:35 to 95:5) as eluant to obtain three subfractions C1–C3. Fr.C1 (30 mg) was subjected to semi-preparative HPLC using methanol–water (55:45 including 0.1% TFA, 3 mL/min) to afford compounds 2 (12 mg) and 3 (8 mg). Fr.B2 (80 mg) was subject to semi-preparative HPLC using methanol–water (65:35, including 0.1% TFA, 3 mL/min) to obtain compound 1 (5 mg).
Cell Culture
Human OC cell lines HEY and ES2, and human bronchial epithelioid cell lines 16HBE were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Cells were cultured in DMEM medium including 10% fetal bovine serum. Cells were preserved at 37 °C in a humidified environment under 5% CO2.
Cytotoxicity Bioassay
Three xanthones were dissolved in DMSO to make stock solutions and further diluted in culture medium for experiments. To evaluate the effects of these xanthones on cell viability, cell number was counted using a standard colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test. Cells were plated in a 96-well plate (5 × 103 cells/well) and allowed to attach overnight. Cells were added with 1, 5, 10, 20, 40, and 80 μM of each compound in culture medium for 24 and 48 h, respectively, then added with 10 μL of MTT (5 mg/mL stock in PBS) per well and hatched for 4 h at 37 °C. In the end, the culture medium was given up and 100 μL of DMSO was mixed per well to dissolve the purple formazan crystals. Absorbance of the mixed solution was determined on a microplate reader spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA), at a wavelength of 472 nm. Triplicate wells were used for each sample and the experiments were repeated at least three times to get means and standard deviations.
Xanthones Inhibited the Proliferation of Ovarian Cancer Cells
The HEY and ES-2 cell lines were plated in a 6-well plate (1 × 105 cells/well) and attached overnight, then added with 5 and 10 μM of 2 in every culture medium for 24 h, respectively, the cell morphology was observed using an optical microscope.
Apoptosis Assay
To analyze cell apoptosis, the HEY cells were added with 2 at the concentration of 5, and 10 µM, respectively, then stained with Annexin V-fluorescein isothiocyanate (Annexin V-FITC) and propidium iodide (PI, Biolegend) according to the manufacturer’s instruction. Stained cells (1 × 106 per reaction) were tested on a flow cytometer (FACSCalibur, Becton Dickinson). Flow cytometry assay was repeated 3 times.
Molecular Docking
The 2D chemical structure of the xanthones were drawn and the 2D coordinates were converted into the 3D format in Chemdraw 10. The Gasteiger-Huckel charges were computed using the FF12SB method of Chimera 1.10.1. The molecular targets were obtained from the Protein Data Bank (PDB) according to the best resolution value. The co-crystalized ligands were used to analyze the active site on the molecular targets. The molecular docking assay was carried on the AutoDock Vina software with the default settings. The molecular docking was done with the GridScore scoring function. A redocking process was carried out to confirm the accuracy of the docking method to illustrate the crystallographic orientation of the complexed ligands to the selected targets. The results were evaluated by Root-Mean-Square Deviation (RMSD) computed on the DOCK6.8 software. Next, 2 were docked with all the candidate proteins.
Western Blot
The HEY cells were treated with 2 at the concentration of 5 and 10 μM, respectively, 24 h later, they were splitted in RIPA lysis buffer (Beyotime, Jiangsu, China) including 1% PMSF (Thermo, Waltham, MA) at 4 °C for 30 min, then centrifuged at 10,000 rpm for 30 minutes. The concentration of protein was measured using a BCA Protein Assay Kit (Beyotime, Shanghai, China), then boiled for 10 minutes at 100 °C, the collected protein was carried out at 30 μg/per lane and separated on SDS-PAGE gel, electrophoretically transferred to a polyvinylidene difluoride membrane (Beyotime, Shanghai, China). The primary antibody incubated overnight and the secondary antibody incubated at 25 °C for 2 h, the bands were detected by super ELC detection reagent (Beyotime, Shanghai, China). Images were determined by Image-Pro Plus software (version 7.0, Olympus, Tokyo, Japan). All experiments were repeated 3 times.
Statistical Analysis
Graphs were produced from GraphPad Prism 7 and ggplot 2. Statistical significance was depended on the Statistical Package for the Social Sciences (SPSS) 12.0. All experimental results were determined according to one-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons, and there was no remarkable difference in the variance homogeneity measurement (P > 0.05).
Results and Discussion
Isolation and Identification of Xanthones from Garcinia nujiangensis
The fruits of G. nujiangensis were broken into pieces, then extracted with acetone at 20 °C three times. The extract was solved in water and extracted with hexane, EtOAc, and n-ButOH, successively. The EtOAc portion was subjected to silica gel, reversed-phase silica gel (ODS-C18), and semipreparative HPLC, to obtain a new xanthone named nujiangexanthone G (1), together with two other known xanthones: isojacareubin (2), and 1,5,6-trihydroxy-2-prenyl-6′,6′-dimethyl-2H-pyrano(2′,3′:3,4) xanthone (3) (Figure 1). The identified of the compound was analyzed by 1D-NMR data (1H, 13C) (Figure 1) and 2D-NMR data (1H-1H-COSY [Figure S1], HSQC [Figure S2], and HMBC [Figure S3]), and HRESIMS.
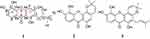 |
Figure 1 Compounds from the fruits of Garcinia nujiangensis. (A) The left ring of xanthone. (B) The middle ring of xanthone. (C) The right ring of xanthone. |
Compound 1 was obtained as a yellow power. The molecular formula deduced as C18H16O7 according to HRESIMS at m/z 343.0816 [M–1]– with a degree of unsaturation of 11. The UV absorptions (MeOH) at λmax 264 and 332 nm suggested 1 to be a xanthone derivative modified by the hydroxyl groups. The 1H NMR spectrum of 1 (Table 1) showed the proton signals of a chelated hydroxy proton signal at δH 13.43 (1H, s), two adjacent aromatic protons signal at δH 6.89 (1H, J = 8.65 Hz, H-7), and δH 7.50 (1H, J = 8.65 Hz, H-8), a single aromatic proton signal at δH 6.45 (1H, s, H-4), a methylene signal at δH 2.72 (2H, m, H-4); a 2-hydroxy-3-methylbut-3-enyl moiety with two olefinic protons at 4.61 (1H, s, H-15) and 4.65 (1H, s, H-15), a methyl proton at δH 1.74 (3H, s, H-14), and a methylene proton modified an oxygenated group at δH 4.27 (1H, s, H-12). The 1H and 13C NMR data (Figure 2B and C, Table 1), aided by a HSQC experiment, which suggested the presence of a carbonyl, ten sp2 quaternary carbons (six of which are oxidized), three sp2 methines, one sp3 methylene, one oxygen sp2 methine, and one sp3 methyl groups. Based on the NMR spectroscopic data of 1, which suggested that the molecule consisted of a xanthone skeleton decorated with a 2-hydroxy-3-methylbut-3-enyl moiety. The location of the 2-hydroxy-3-methylbut-3-enyl moiety was located at C-2 (δC 107.7) based on the correlations observed in the HMBC spectrum (Figure 2A). On the other hand, a chelated hydroxy proton signal at δH 13.43 (1H, s, OH-1) suggested a cross peak to the carbon signals at C-1 (δC160.5) and C-2 (δC 107.7) (Figure 2A). Analysis of the 1H and 13C NMR data of 1 with those of known compounds indicated that they possessed similar skeletons, the substituted pattern of ring A was similar to that of isojacareubin,24 and the substituted pattern of ring C was similar to that of caledol.25 The absolute configuration of C-12 was determined to be S based on its specific rotation, [α] −16 (c 0.25, MeOH) with that of nujiangxanthone E, [α] −17 (c 0.30, MeOH),22 and Garcihombronone B, [α] −16.2 (c 0.29, MeOH).26 Thus, 1 was established as 1,3,5,6-tetrahydroxy-2-(2-hydroxy-3-methylbut-3-enyl) xanthone. Therefore, the structure of nujiangxanthone G (1) was deduced as shown.
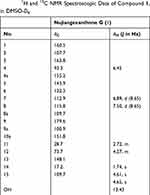 |
Table 1 1H and 13C NMR Spectroscopic Data of Compound 1, in DMSO-D6 |
 |
Figure 2 (A) The key HMBC of 1. NMR spectra of 1. (B) 13C NMR spectrum. (C) 13C NMR spectrum. |
The two known compounds, named isojacareubin (2)24 and 1,5,6-trihydroxy-2-prenyl-6′,6′-dimethyl-2H-pyrano(2′,3′:3,4)xanthone (3)27 were identified by comparison of spectroscopic data with reported values. Isojacareubin was isolated from Garcinia livingstonei,28 Garcinia Fusca,29 and Hypericum stellatum,30 in the family Clusiaceae; and showed inhibitory effects on hepatocellular carcinoma via protein kinase C in vitro and in vivo.31
Cell Viability
Three xanthones were tested for their effects on human OC cell lines. The HEY and ES-2 cells were treated with three xanthones for 24 and 48 h, respectively, the cell viability was determined according to the MTT assay. To calculated the IC50 values, the cells were incubated with a series of concentrations of three xanthones for 24 and 48 h, respectively. Comparing to the control, the results showed that three xanthones were positive (p < 0.05) to the HEY and ES2 cell lines. Those xanthones inhibited proliferation of the HEY and ES-2 cells in a concentration and time-dependent manner (Figure 3). Among them, 2 showed the strongest inhibitory effect on the HEY and ES-2 cells with IC50 value of 6.5 ± 0.4 μM, 9.5 ± 0.3 μM for 24 h, respectively. In the meantime, the selective cytotoxicity of three xanthones on tumor cells was evaluated on an immortalized normal human bronchial epithelial cell line 16HBE. The cell viability of the 16HBE cells was almost unaffected after the treatment of three xanthones for 48 h, indicating a selective effect of three xanthones against the HEY and ES-2 cells. From the above analysis, 2 was found to be the most potent cytotoxic compound of those tested, so we focused on the mechanism of action of 2 against OC cells.
2 Induced the Morphological Changes on Ovarian Cancer Cells
The HEY and ES-2 cell lines were treated with 5 and 10 μM of 2 for 24 h, respectively, the cell morphology was exhibited in (Figure 4), the cell morphology was obviously changed after 24 h treatment of 2 at the concentration of 5 μM, as exhibited by the cell shrinkage, cell size reduction, and loose arrangement, and there were less cells attached to the plate compared with the control group. Moreover, those two cells treated with 2 at the higher concentration of 10 μM, there were death cells in the plate. The results suggested that 2 could inhibit proliferation and might induce apoptosis of the HEY and ES-2 cell lines in a concentration-dependent manner.
 |
Figure 4 2 induced the morphological changes of human OC cells HEY and ES-2. (100×, scale bar=50 μm). |
2 Induced Apoptosis in Ovarian Cancer Cells HEY
To detect cell apoptosis triggered by 2, the HEY cells added with 5 and 10 μM of 2 for 24 h, respectively, the results were exhibited (Figure 5). 5 µM of 2 induced moderate cell apoptosis compared with the control, moreover, 10 µM of 2 striking stimulated cell apoptosis compared with the control, these results showed 2 prompted OC cells HEY apoptosis in a concentration-dependent manner. The underlying molecular mechanism would predicted by molecular docking, then the potential targets regulated were explored by Western blot experiments.
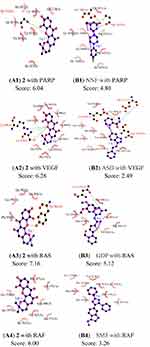
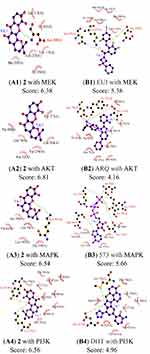
Molecular Docking Analysis
Computational docking experiments were subjected to mimic the characteristics of constituent-target, which is a effective method to screen the active compounds and predict the mechanism of action of compounds.32 The crystal structure of the key target proteins were obtained from the Protein Data Bank, their information was listed in Table S1. The docking exercise was conducted using Autodock (Figures S4–S11). The results indicated that 2 formed stronger interactions with the key target proteins (PARP, VEGF, RAS, RAF, MEK, PI3K, AKT, and MAPK) compared to the native ligands (Figures 6 and 7). The interactions between the xanthones and proteins including hydrophobic, hydrogen, and Pi-Pi bonds, which were listed in (Figure 6). PARP had docked with 2 was analyzed in detail, the dock score was 6.89, 2 formed hydrophobic interactions with the side chain of Glu1138, Tyr1060, His1031, Tyr 1071, Tyr 1050, and Ile 1075. On the other hand, the oxygen atom in the ether of 2 formed a hydrogen bond with the hydroxyl group of Ser1033.
A molecular docking approach was widely used to explain the interactions between the ligand molecules and target proteins, which offered the exploration of the molecular mechanisms of action of ligand molecules binding to the amino acid residues of the target proteins. These key target proteins participated in the progression of human OC were screened by molecular docking. The results showed that nine targets had excellent interactions with 2, such as, PARP, Vascular endothelial growth Factor (VEGF), Rapidly accelerated fibrosarcoma (Raf), Ras, mitogentic effector kinase (MEK), mitogen-activated protein kinases (MAPKs), PI3K/AKT (phosphatidylinositol-3-kinase/serine/threonine kinase). Owing to they played pivotal roles in OC progression through proliferation, survival, invasion, and immune evasion. PARP had an essential part in the repair of DNA single-strand breaks (SSBs). The SSBs would not be repaired if PARP was inhibited. PARPi had been widely used in OC patients.33 Angiogenesis had a significant role in the growth, metastasis, and ascites formation of cancer. VEGF was confirmed to be related to malignant progression and poor prognosis of OC patients, the activation of VEGF/VEGFR signal pathway presented as a dominant mechanism.34 RAS/RAF/MEK/ERK signaling pathway was participated in the development, maintenance, and progression of cancer, RAS/RAF/MEK/ERK signaling pathway had been showed to be one of the most frequently activated oncogenic signaling pathways in the progress of human cancer. Additionally, new studies had suggested that RAS/RAF/MEK/ERK cascade was a key downstream intracellular signal of EGFR.35 Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) played a key role in regulating the proliferation, the cycle, and apoptosis of cells. Many studies showed that the PI3K/AKT signaling pathway was relevant to certain gynecological tumors. Deregulation of the PI3K/AKT pathway is common in OC and is associated with poor prognosis.36 Thus, some relevant drug molecules were used to block or inhibit the PI3K/AKT signaling pathway to identify the anti-tumor targets. The Ras/MAPK pathway has an important role in the major cellular processes, such as cell survival and proliferation, and its deregulation was involved in malignant transformation and progression of OC.37
The Potential Target of Xanthones Validated by Western Blot Analysis
Based on these xanthones potency for the treatment of the HEY and ES-2 cell lines, although their potential molecular mechanism of action had been predicted by molecular docking, their direct evidence was still margin. To solve the problem, 8 target proteins (PARP, VEGF, RAS, RAF, MEK, PI3K, AKT, and MAPK) had excellent interactions with 2 screened by molecular docking. Owing to 2 showed high sensibility to the HEY cells, the expression levels of potential targets regulated by 2 were detected by Western blot analysis. Fortunately, the results were exhibited in (Figures 8 and 9), which indicated that 2 could regulate the expression levels of the key target proteins in the PARP, PI3K/AKT/mTOR, and ERK/MAPK signal pathways against the HEY cells in a concentration-depend manner.
Natural products have gotten great interest is gradually increasing in the globe. Bioactive compounds from herbal medicine have a long history of use as a good source of ingredients for valuable medical usages.38 Natural xanthone mangiferin from traditional Chinese medicine exhibited suppressed activities on human epithelial OC.39 A number of xanthones from Garcinia nujiangensis showed potent cytotoxicity against selective cancer cells,21–23,40 among them, Nujiangexathone A inhibited cervical cancer via hnRNPK,41 and induced caspase-dependent apoptosis via ROS/JNK pathway,42 in addition, it had anti-inflammatory and anti-allergic effects.43 2 featured with a xanthone skeleton showed inhibitory effects on the proliferation and induced apoptosis on human OC.
Studying on the mechanisms of action of potential drugs were challenged, costly, and time-consuming, the molecular docking was an excellent strategy to screen the active compounds and predict the possible pharmaceutical mechanisms. After high screening, some key targets showed excellent interactions with 2, which belonged to the signal pathways (PARP, PI3K/AKT/mTOR, and ERK/MAPK) involved in the progress of OC.44 Apoptosis was considered as a form of programmed cellular suicide played a crucial role in various human diseases, and evasion of apoptosis, had been recognized as a hallmark of cancer cells.45 2 induced apoptosis at the concentration 5 and 10 μM tested on a flow cytometer stained with Annexin V-FITC and PI. Further, the Western blot showed 2 upregulated the expression levels of the pro-apoptotic protein Bax, and downregulated the expression levels of the anti-apoptotic protein Bcl-2, increased the expression levels of the cysteine proteases cleaved caspase-3 and the apoptosis marker cleaved PARP. And all these changes exhibited in a concentration-depend manner. The PI3K/AKT/mTOR pathway was involved in many human cancers, including OC, which induced the apoptotic response through its ability to interplay with a great quantity of major genes in the apoptosis.46 Once this pathway was activated, it would initiate phosphorylation of transcription factors, then upregulate the expression levels of anti-apoptotic genes and downregulate levels of pro-apoptotic proteins. Thus, an inhibitor targeted on the PI3K/AKT/mTOR pathway might also induced apoptosis. Moreover, the AKT pathway could enhance PARP inhibition under the microenvironment of chemotherapy.47 In all, compounds targeted on both the PARP and PI3K/AKT pathways, would be a greater clinical benefit to the cancer. Generally, PI3K/AKT/mTOR and MAPK/ERK signal pathways were participated in the proliferation, differentiation, and apoptosis of many cancer cells. Thus, an inhibitor, one stone two birds, one kind of combined therapy, simultaneously regulated the PI3K/AKT/mTOR and MAPK/ERK pathways could increase efficacy and reduce resistance. Consequently, these xanthones, especially 2 was a potential inhibitor for the treatment of the human OC.
Conclusion
In this study, bioassay-directed fractionation of the fruit of G. nujiangensis, led to a new xanthone named nujiangexanthone G (1) together with two known analogues: isojacareubin (2), and 1,5,6-trihydroxy-2-prenyl-6′,6′-dimethyl-2H-pyrano(2′,3′:3,4) xanthone (3). The activity test suggested that 2 had moderate cytotoxic activity against human OC cell lines HEY and ES-2 with the IC50 values of 6.5 ± 0.4 μM, 9.5 ± 0.3 μM, respectively, respectively. Moreover, those xanthones exhibit no cytotoxic activity toward normal cell 16HBE. Molecular docking succeed in screening the potential targets involved in the progression of human OC., the results suggested that PARP, VEGF, RAS, RAF, MEK, MAPK, PI3K, and AKT had excellent interactions with 2. Finally, the Western blot analysis indicated that 2 could inhibit proliferation, and induced apoptosis on the HEY cell lines via PARP, PI3K/AKT/mTOR, and ERK/MAPK signal pathways. Therefore, these xanthones especially 2 was a potential inhibitor against OC via PARP, PI3K/AKT/mTOR, and ERK/MAPK signal pathways.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Special subject for scientific research of Chinese Medicine from Shanghai Municipal Commission of Health and Family Planning (No. 2018JP007).
Disclosure
The authors have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: gLOBOCANestimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Volpe J, Filipi JG, Cooper OR, Penson RT. Frontline therapy of ovarian cancer: trials and tribulations. Curr Opin Obstet Gynecol. 2018;30:1–6. doi:10.1097/GCO.0000000000000434
3. Kang K, Wang Y. Sevoflurane inhibits proliferation and invasion of human ovarian cancer cells by regulating JNK and p38 MAPK signaling pathway. Drug Des Devel Ther. 2019;13:4451–4460. doi:10.2147/DDDT.S223581
4. Xie HY, Wang WJ, Xia BR, Jin WL, Lou G. Therapeutic applications of PARP inhibitors in ovarian cancer. Biomed Pharmacother. 2020;127:110204–110216. doi:10.1016/j.biopha.2020.110204
5. Deshaies RJ. Multispecific drugs herald a new era of biopharmaceutical innovation. Nature. 2020;580:329–338. doi:10.1038/s41586-020-2168-1
6. Caumanns JJ, van Wijngaarden A, Kol A, et al. Low-dose triple drug combination targeting the PI3K/AKT/mTOR pathway and the MAPK pathway is an effective approach in ovarian clear cell carcinoma. Cancer Lett. 2019;461:102–111. doi:10.1016/j.canlet.2019.07.004
7. Loureiro DRP, Soares JX, Costa JC, et al. Structures, activities and drug-likeness of anti-infective xanthone derivatives isolated from the marine environment: a review. Molecules. 2019;24:E243–E354. doi:10.3390/molecules24020243
8. Wang SP, Wang L, Chen MW, Wang YT. Gambogic acid sensitizes resistant breast cancer cells to doxorubicin through inhibiting P-glycoprotein and suppressing survivin expression. Chem Biol Interact. 2015;235:76–84. doi:10.1016/j.cbi.2015.03.017
9. Herrera-Aco DR, Medina-Campos ON, Pedraza-Chaverri J, Sciutto-Conde E, Rosas-Salgado G, Fragoso-González G. Alpha-mangostin: anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem Toxicol. 2019;124:300–315. doi:10.1016/j.fct.2018.12.018
10. Mariano LNB, Boeing T, Silva RCMVAF, Cechinel-Filho V, Andrade SF. 1,3,5,6-Tetrahydroxyxanthone, a natural xanthone, induces diuresis and saluresis in normotensive and hypertensive rats. Chem Biol Interact. 2019;311:108778–108786. doi:10.1016/j.cbi.2019.108778
11. Rodniem S, Tiyao V, Nilbu-Nga C, Poonkhum R, Pongmayteegul S, Pradidarcheep W. Protective effect of alpha-mangostin on thioacetamide-induced liver fibrosis in rats as revealed by morpho-functional analysis. Histol Histopathol. 2019;34:419–430. doi:10.14670/HH-18-052
12. Zhou LY, Peng JL, Wang JM, Geng YY, Zuo ZL, Hua Y. Structure-activity relationship of xanthones as inhibitors of xanthine oxidase. Molecules. 2018;23:E365–E374. doi:10.3390/molecules23020365
13. Jang JH, Lee KH, Jung HK, Sim MO, Cho HW. Anti-inflammatory effects of 6′-O-acetyl mangiferin from Iris rossii Baker via NF-κb signal blocking in lipopolysaccharide-stimulated RAW 264.7 cells. Chem Biol Interact. 2016;257:54–60. doi:10.1016/j.cbi.2016.07.029
14. Santos CMM, Freitas M, Fernandes E. A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur J Med Chem. 2018;157:1460–1479. doi:10.1016/j.ejmech.2018.07.073
15. Nathália L, Luisa BM, Silva M, et al. Gastroprotective xanthones isolated from Garcinia achachairu: study on mucosal defensive factors and H+, K+-ATPase activity. Chem Biol Interact. 2016;258:30–39. doi:10.1016/j.cbi.2016.08.009
16. Ibrahim MY, Mohd Hashim N, Mohan S, et al. Involvement of NF-κB and HSP70 signaling pathways in the apoptosis of MDA-MB-231 cells induced by a prenylated xanthone compound, α-mangostin, from Cratoxylum arborescens. Drug Des Devel Ther. 2018;12:533–534. doi:10.2147/DDDT.S167650
17. Szwalbe AJ, Williams K, Song Z, et al. Characterisation of the biosynthetic pathway to agnestins A and B reveals the reductive route to chrysophanol in fungi. Chem Sci. 2018;10:233–238. doi:10.1039/c8sc03778g
18. Smith MJ, Reichl KD, Escobar RA, et al. Asymmetric synthesis of griffipavixanthone employing a chiral phosphoric acid-catalyzed cycloaddition. J Am Chem Soc. 2019;141:148–153. doi:10.1021/jacs.8b12520
19. Wu JQ, Dai JW, Zhang YY, et al. Synthesis of novel xanthone analogues and their growth inhibitory activity against human lung cancer A549 cells. Drug Des Devel Ther. 2019;13:4239–4246. doi:10.2147/DDDT.S217827
20. Winter DK, Sloman DL, Porco JA. Polycyclic xanthone natural products: structure, biological activity and chemical synthesis. Nat Prod Rep. 2013;30:382–391. doi:10.1039/c3np20122h
21. Xia ZX, Zhang DD, Liang S, et al. Bioassay-guided isolation of prenylated xanthones and polycyclic acylphloroglucinols from the leaves of Garcinia nujiangensis. J Nat Prod. 2012;75:1459–1464. doi:10.1021/np3003639
22. Tang ZY, Xia ZX, Qiao SP, et al. Four new cytotoxic xanthones from Garcinia nujiangensis. Fitoterapia. 2015;102:109–114. doi:10.1016/j.fitote.2015.02.011
23. Tang ZY, Lu LH, Zhou XY, et al. A new cytotoxic polycyclic polyprenylated acylphloroglucinol from Garcinia nujiangensis screened by the LC-PDA and LC-MS. Nat Prod Res. 2020;34:2448–2455. doi:10.1080/14786419.2018
24. Helboe P, Arends P. Xanthone studies. VI. Synthesis of jacareubin, isojacareubin, and some hydroxyxanthones with allylic substituents. Arch Pharm Chemi Sci Ed. 1973;1:549–555.
25. Jean-Michel O, Cécile M, Jean-Jacques H, et al. First 2-hydroxy-3-methylbut-3-enyl substituted xanthones isolated from plants: structure elucidation, synthesis and antifungal activity. Nat Prod Res. 2003;17:195–199. doi:10.1080/1057563021000040808
26. Klaiklay S, Sukpondma Y, Rukachaisirikul V, Phongpaichit HS. Friedolanostanes and xanthones from the twigs of Garcinia hombroniana. Phytochemistry. 2013;85:161–166. doi:10.1016/j.phytochem.2012.08.020
27. Yang NY, Han QB, Cao XW, et al. Two new xanthones isolated from the stem bark of Garcinia lancilimba. Chem Pharm Bull. 2007;55:950–952. doi:10.1248/cpb.55.950
28. Mulholland DA, Mwangi EM, Dlova NC, Plant N, Crouch NR, Coombes PH. Non-toxic melanin production inhibitors from Garcinia livingstonei (Clusiaceae). J Ethnopharmacol. 2013;149:570–575. doi:10.1016/j.jep.2013.07.023
29. Nontakham J, Charoenram N, Upamai W, Taweechotipatr M, Suksamrarn S. Anti-helicobacter pylori xanthones of Garcinia fusca. Arch Pharm Res. 2014;37:972–977. doi:10.1007/s12272-013-0266-4
30. Ji Y, Zhang R, Zhang C, et al. Cytotoxic xanthones from hypericum stellatum, an ethnomedicine in Southwest China. Molecules. 2019;24:3568–3579. doi:10.3390/molecules24193568
31. Yuan X, Chen H, Li X, et al. Inhibition of protein kinase C by isojacareubin suppresses hepatocellular carcinoma metastasis and induces apoptosis in vitro and in vivo. Sci Rep. 2015;5:12889–12902. doi:10.1038/srep12889
32. Ul Bari W, Zahoor M, Zeb A, et al. Isolation, pharmacological evaluation and molecular docking studies of bioactive compounds from Grewia optiva. Drug Des Devel Ther. 2019;13:3029–3036. doi:10.2147/DDDT.S220510
33. Ledermann JA. Extending the scope of PARP inhibitors in ovarian cancer. Lancet Oncol. 2019;20:470–472. doi:10.1016/S1470-2045(19)30019-1
34. Dhani NC, Oza AM. Targeting angiogenesis: taming the medusa of ovarian cancer. Hematol Oncol Clin North Am. 2018;32:1041–1055. doi:10.1016/j.hoc.2018.07.008
35. Wu D, Liu Z, Li J, et al. Epigallocatechin-3-gallate inhibits the growth and increases the apoptosis of human thyroid carcinoma cells through suppression of EGFR/RAS/RAF/MEK/ERK signaling pathway. Cancer Cell Int. 2019;19:43–51. doi:10.1186/s12935-019-0762-9
36. Liu L, Wu N, Wang Y, et al. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. J Exp Clin Cancer Res. 2019;38:106–119. doi:10.1186/s13046-019-1061-y
37. Chen S, Cavazza E, Barlier C, et al. Beside P53 and PTEN: identification of molecular alterations of the RAS/MAPK and PI3K/AKT signaling pathways in high-grade serous ovarian carcinomas to determine potential novel therapeutic targets. Oncol Lett. 2016;12:3264–3272. doi:10.3892/ol.2016.5083
38. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. doi:10.1021/acs.jnatprod.9b01285
39. Zeng Z, Lin C, Wang S, et al. Suppressive activities of mangiferin on human epithelial ovarian cancer. Phytomedicine. 2020;76:153267. doi:10.1016/j.phymed.2020.153267
40. Liu XJ, Hu X, Peng XH, et al. Polyprenylated xanthones from the twigs and leaves of Garcinia nujiangensisand their cytotoxic evaluation. Bioorg Chem. 2020;94:103370–103382. doi:10.1016/j.bioorg.2019.103370
41. Zhang L, Feng J, Kong S, et al. A novel compound from Garcinia nujiangensis, suppresses cervical cancer growth by targeting hnRNPK. Cancer Lett. 2016;380:447–456. doi:10.1016/j.canlet.2016.07.005
42. Zhang L, Kong SY, Zheng ZQ, et al. A novel compound derived from garcinia nujiangensis, induces caspase-dependent apoptosis in cervical cancer through the ROS/JNK pathway. Molecules. 2016;21:1360–1372. doi:10.3390/molecules21101360
43. Lu Y, Cai S, Nie J, et al. The natural compound nujiangexanthone A suppresses mast cell activation and allergic asthma. Biochem Pharmacol. 2016;100:61–72. doi:10.1016/j.bcp.2015.11.004
44. Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16:329–344. doi:10.1038/nrm3999
45. Guan LY, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med. 2018;26:219–229.
46. Huang TT, Lampert EJ, Coots C, Lee JM. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin Cancer Biol. 2019;59:147–160. doi:10.1016/j.ctrv.2020.102021
47. Franzese E, Centonze S, Diana A, et al. PARP inhibitors in ovarian cancer. Cancer Treat Rev. 2019;73:1–9. doi:10.1016/j.ctrv.2018.12.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.