Back to Journals » OncoTargets and Therapy » Volume 12
Anti-tumor effect of chitin oligosaccharide plus cisplatin in vitro and in vivo
Authors Liu X, Zhang Y, Liu Z, Xie X
Received 24 June 2019
Accepted for publication 30 August 2019
Published 16 September 2019 Volume 2019:12 Pages 7581—7590
DOI https://doi.org/10.2147/OTT.S220619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Xing Liu,1,* Yan Zhang,2,* Zhaozhe Liu,1 Xiaodong Xie1
1Oncology Department, General Hospital of Northern Theater Command, Shenyang, Liaoning Province, People’s Republic of China; 2Medical Examination Center, General Hospital of Northern Theater Command, Shenyang, Liaoning Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaodong Xie
Oncology Department, General Hospital of Northern Theater Command, Wenhua Road, Shenhe District, Shenyang, Liaoning Province, People’s Republic of China
Tel +86 242 885 1781
Email [email protected]
Background: Lung cancer is one of the most common malignant tumors in human beings, and cisplatin is a widely used chemotherapy drug, but its clinical application is limited because of its dose-dependent toxicity and drug resistance. Chitin is known to have various biological activities including anti-tumor, but the insoluble feature in common solvents greatly restricts its application. Chitin oligosaccharide is a small water-soluble molecule degraded from chitin without any toxic effect.
Methods: Chitin oligosaccharide was adopted to investigate the effects on lung adenocarcinoma A549 cells and tumor xenografts of nude mice. The experiments were divided into control group, chitin oligosaccharide group, cisplatin group and combination group. MTS assay, cell scratch test and migration assay were used to observe the proliferation and migration of A549 cells, and Western blot was used to detect the expression levels of caspase8, caspase3 and BAK. Ki67 and P53 expressions of tumor xenografts were detected to explore the effects of drugs on tumor prognosis.
Results: The results in vitro showed that chitin oligosaccharides could inhibit the proliferation and migration of A549 cells, and the effect was superior to chitin oligosaccharide or cisplatin when combined with cisplatin. Chitin oligosaccharide plus cisplatin up-regulated the expression level of caspase8 and caspase3, while had minor influence on the expression level of BAK. In vivo experiments showed that chitin oligosaccharide plus cisplatin could down-regulate the expression level of Ki67, while had minor influence on the expression level of P53.
Conclusion: The study demonstrated that chitin oligosaccharide plus cisplatin had positive synergistic effects, and it is possible to improve the prognosis of lung adenocarcinoma patients by up-regulating the expression level of caspase8, caspase3 and down-regulating the expression level of Ki67.
Keywords: chitin oligosaccharide, cisplatin, lung adenocarcinoma cell line A549
Introduction
Lung cancer is one of the most common malignant tumors in human beings;1,2,3 its 5-year survival rate is less than 15%4,5 and the fatality rate accounts for about 22.7% of all tumor deaths.6 About 85% of lung cancer is non-small cell lung cancer (NSCLC),7 mainly including squamous cell carcinoma, adenocarcinoma and large cell carcinoma. Adenocarcinoma has become a major type of lung cancer in many countries at present.8 The chemotherapy regimens on the basis of the platinum have been widely recognized as the main treatment of lung cancer, but its clinical application is limited due to dose-dependent toxicity and drug resistance.9 Finding out a kind of anti-tumor substance with tolerable adverse reactions will provide important theoretical basis for the future development of anti-tumor drugs for pharmaceutical industry.
Chitin is an alkaline polysaccharide which widely exists as the major structural component in the exoskeleton of crustacean arthropod (such as crab and shrimp), the epidermis of insects, organs of mollusks, cell wall of plants and fungal and green algae.10–12 The insoluble feature in common solvents greatly restricts in the applications of different fields.13 Chitin oligosaccharide is a small molecule degraded from chitin and readily soluble in water because of its shorter chain lengths.10 It has good affinity to human body and less possibility to cause drug resistance without any toxic effects.14 Chitin is known to have various biological activities including anti-oxidation, anti-inflammation, immunity-enhancing, anti-tumor, etc.15–18 However, in view of the few studies on the anti-tumor effect, the soluble chitin oligosaccharide was adopted alone and combined with cisplatin to investigate the anti-tumor activity on lung adenocarcinoma A549 cells and tumor xenografts.
Materials and methods
Materials
Soluble chitin oligosaccharide was provided by Shenyang Institute of Metal Research. Lung adenocarcinoma A549 cell line (ATCC®CCL-185TM) was selected for the experiment, which was preserved at the General Hospital of Northern Theater Command. Twenty BALB/C male nude mice, 6–8 weeks old and 22–24 g, were purchased from Beijing Weitong Lihua Experimental Animal Technology Co. Ltd. China.
Cell experiments
MTS assay
The density of A549 cells was adjusted to 2×103/mL; 100μL cell suspension was seeded in triplicate in 96 well plates. Each experimental group was given the drug of chitin oligosaccharide. At 24 hrs, the cells of each well were stained with 20 μL MTS solution (Promega, China). The absorbance of 492 nm was measured and IC50 was calculated. Cell density was readjusted to 5×103/mL, MTS assay was repeated and the experimental groups were divided into Chitin oligosaccharide group, cisplatin group and chitin oligosaccharide plus cisplatin group. The final concentration of chitin oligosaccharide and cisplatin were 7mg/mL and 3μg/mL. The absorbance of 492 nm at 24 hrs, 48 hrs, 72 hrs and 96 hrs was measured and the inhibition rate was calculated.
Cell scratch test
5×105 A549 cells were suspended in the RPMI-1640 medium (BioInd, Israel) with 10% fetal bovine serum (FBS, Zhejiang Tianhang Biotechnology Co., Ltd, China) and placed in 6 well plates. Cells were scratched to create a transverse wound when 90% confluent was formed overnight. Cellular debris was removed and the cells were exposed to serum-free medium before it was photographed. Chitin oligosaccharide was given in each well and the same point was observed and photographed after 24 hrs. RPMI-1640 containing 10% FBS was replaced and the medicine was readded; the same point was observed and photographed after 48 hrs and the relative scratch width was calculated. Cell scratch test was repeated and the experimental groups were divided into control group, chitin oligosaccharide group, cisplatin group and chitin oligosaccharide plus cisplatin group; the same point was observed and photographed at 24 hrs, 48 hrs and the relative scratch width was calculated.
Transwell migration experiment
A549 cells were starved for 24 hrs, and readjusted to 5×105/mL. 500 μL RPMI-1640 containing 10% FBS was added into the lower chamber of transwell (Costar, USA). The upper chamber was divided into control group, chitin oligosaccharide group, cisplatin group and chitin oligosaccharide plus cisplatin group, and the final volume and cell numbers were identical after the administration of drugs in each upper chamber. The upper chamber was placed into the lower one for 24 hrs, formaldehyde fixed for 20 mins and stained with 0.1% crystal violet (Solarbio, China) for 20 mins. It was photographed and the mean and standard deviation was calculated.
Western blot
The experimental groups were divided into control group, chitin oligosaccharide group, cisplatin group and chitin oligosaccharide plus cisplatin group. A549 cells were collected after incubated for 72 hrs and lysed with RIPA buffer. The concentrations of proteins were measured by BCA method and samples were separated by SDS-PAGE. After transfer, the PVDF membrane was blocked with 5% fat-free milk for 2 hrs and incubated with primary antibodies at 4°C overnight. Primary antibodies were rabbit anti-human caspase3 antibody (1:500; cell signaling technology, USA), mouse anti-human caspase8 antibody (1:500; cell signaling technology, USA), rabbit anti-human BAK antibody (1:500; cell signaling technology, USA) and mouse anti-human β-actin antibody (1:500; santa cruz biotechnology, USA). The membrane was then incubated with a horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody (1:40,000; cell signaling technology, USA) after it was rinsed by TBS-T for three times. ECL method was used for immunoblot analysis.
Animal experiment
The logarithmic growth of A549 cells was used to make the cell suspension with a density of 1×108/mL. 100 μL cell suspension was inoculated in the right gluteal subcutaneous tissue of nude mice, and they were randomly divided into control group, chitin oligosaccharide group, cisplatin group and chitin oligosaccharide plus cisplatin group. The regimen of chitin oligosaccharide was intraperitoneal injected by 80 mg/kg·d once a day, and the injection was conducted for 10 days successively. The regimen of cisplatin was intraperitoneal injected by 3 mg/kg·d, and was given on d1 and d8. The nude mice were killed by the spinal disconnection after 10 days administration, and the tumor was completely stripped and placed in formalin solution. The morphological changes and the expression level of Ki67 and P53 of tumor tissues were observed by HE staining and immunohistochemical methods. The scoring was modified slightly on the basis of Pu’s criterion.19
Statistical analysis
The measurement data were expressed as mean ± standard deviation (.x±s). GraphPad Prism 5.0 software was used to analyze the results of MTS assay and ImageJ statistical software to analyze the cell count of transwell experiment. Statistical analysis of the data was made by SPSS 17.0. One-way ANOVA was adopted to compare the difference among the groups of immunohistochemical staining. P<0.05 indicates the difference has statistical significance.
Ethical statement
Ethical approval for this investigation was obtained from the medical ethics committee of General Hospital of Northern Theater Command, and all animal experiments were performed following the GB/T 35892–2018 Laboratory animal guideline for ethical review of animal welfare, which is the National Standards of the People’s Republic of China.
Results
24 hrs IC50 determination of chitin oligosaccharide
MTS assay was used to investigate the effects on the proliferation of A549 cells with different concentration of chitin oligosaccharide, and we found that when the concentration was more than 5mg/mL, it could inhibit the proliferation of A549 cells obviously, and the effect was increased with the increase of drug’s concentration and calculated the 24 hrs IC50 was 6.840 mg/mL.
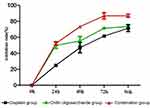
The inhibition rates of chitin oligosaccharide plus cisplatin after 48 hrs were persistently increased compared with chitin oligosaccharide or cisplatin group
Chitin oligosaccharide, cisplatin, chitin oligosaccharide plus cisplatin were used to investigate the effects on the proliferation of A549 cells by MTS assay. It was shown that chitin oligosaccharide’s inhibition rates on cell proliferation of 24 hrs, 48 hrs, 72 hrs and 96 hrs were 49.80%, 55.90%, 71.30% and 73.50%, respectively. The inhibition rates of Cisplatin were 25%, 47.4%, 61.7% and 71.2%, respectively, and the inhibition rates of the combination group were 52.4%, 73.7%, 86.8% and 87.4%, respectively. After drugs acted for 24 hrs, the inhibition rate had no significant difference between the combination group and chitin oligosaccharide group (P>0.05), but compared with cisplatin group, the difference was statistically significant (P<0.05). After drugs acted for 48 hrs, 72 hrs and 96 hrs, the inhibition rates of the combination group were significantly increased compared with other two groups; the difference was statistically significant (P<0.05) (Figure 1).
The relative scratch width for 48 hrs in 3 mg/mL chitin oligosaccharide group was significantly wider compared with 1 mg/mL chitin oligosaccharide group or control group
Cell scratch test was used to observe the migration of A549 cells with different concentration of chitin oligosaccharide. The experiment showed that when the concentration of chitin oligosaccharide was 1 mg/mL, the relative scratch width of 24 hrs and 48 hrs were 82.33% and 42.68%, respectively. When the concentration of chitin oligosaccharide was 3 mg/mL, the relative scratch width of 24 hrs and 48 hrs were 83.46% and 60.52%, respectively, while in the control group they were 84.78% and 21.79%, respectively. There was no significant difference after drug acted for 24 hrs (P>0.05), but the difference of 48 hrs was statistically significant (P<0.05) (Figure 2). Chitin oligosaccharide could inhibit the migration of A549 cells and the effect was increased with the increase of drug’s concentration.
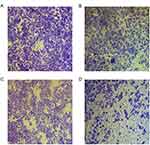
Chitin oligosaccharide plus cisplatin could inhibit the migration of A549 cells obviously
We have preliminarily confirmed that chitin oligosaccharide could inhibit the migration of A549 cells. To further verify the effect of chitin oligosaccharide plus cisplatin on A549 cells, chitin oligosaccharide, cisplatin, chitin oligosaccharide plus cisplatin were used by cell scratch test and transwell migration experiment. Cell scratch test showed that when the concentration of chitin oligosaccharide was 7 mg/mL, the relative scratch width of 24 hrs and 48 hrs were 82.49%, 64.07%, respectively. When the concentration of cisplatin was 3 μg/mL, the relative scratch width of 24 hrs and 48 hrs were 83.23%, 55.91%, respectively. The relative scratch width of the combination group was 83.91%, 69.67%, and the control group was 84.31%, 23.09%, respectively. The differences were not statistically significant after drugs acted for 24 hrs (P>0.05); however, the differences of 48 hrs were statistically significant (P<0.05) (Figure 3). Transwell migration experiment showed that the 24 hrs calculated number of A549 cells were control group 578±26, chitin oligosaccharide group 472±21, cisplatin group 423±28 and combination group 236±23. The number of cells passing through the basement membrane in the combination group decreased significantly compared with other groups (P<0.05) (Figure 4). The effect of combination group acted on the migration of lung cancer cells for 24 hrs was reduced by 59.2% compared with the control group. It indicated that chitin oligosaccharide plus cisplatin could inhibit the migration of A549 cells obviously.
Chitin oligosaccharide plus cisplatin could up-regulate the expression level of caspase8 and caspase3, while had minor influence on the expression level of BAK
To determine the potential mechanism of chitin oligosaccharide in A549 cells, we measured protein expression of caspase8, caspase3 and BAK by Western blot. The results showed that caspase8 and caspase3 expressions were both up-regulated in the combination group, while BAK expression had minor change in all groups. These results indicated that chitin oligosaccharide plus cisplatin promoted the apoptosis of A549 cells by increasing the expression of caspase8 and caspase3 (Figure 5).
 |
Figure 5 Western blot analysis. The protein level of caspase8 and caspase3 were markedly up-regulated in the combination group, while the protein level of BAK had minor change. |
Chitin oligosaccharide plus cisplatin may promote tumor cell necrosis
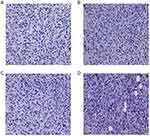
The HE staining of tumor tissue of nude mice showed that cancer cells had the same morphological features in the control group, chitin oligosaccharide group and cisplatin group. They exhibited diffuse distribution, obviously heteromorphism, pathological mitosis, and there were punctiform necrosis in tumor tissue. In addition to the above performance, the combination group also exhibited focal necrosis, nuclear fragmentation and pyknosis, chromatin margination (Figure 6).
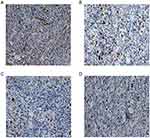
Chitin oligosaccharide plus cisplatin could down-regulating the expression level of ki67, while the expression level of P53 almost had no change
Immunohistochemistry staining showed that the integral median of Ki67 among the control group, chitin oligosaccharide group, cisplatin group and combination group were 9, 5, 5, 1, expression levels were strongly positive (+ + +), positive (+ +), positive (+ +) and weakly positive (+), respectively. The expression of Ki67 in each medication group was significantly lower than that in the control group (P<0.05); especially, the treatment group decreased more significantly compared with that of other groups and the difference was statistically significant (P<0.05) (Figure 7). The integral medians of P53 were 2, 1, 1, 1, expression levels all weakly positive (+). There was no significant difference between the combination group and other groups (P>0.05) (Figure 8).
Discussion
NSCLC is a serious threat to human health. The survival rate and quality of life of patients with NSCLC have gradually become a common concern in clinic, and drug treatment plays an increasingly important role in individualized treatment. Chitin oligosaccharide is a small water-soluble molecule degraded from chitin without any toxic effect. Its biological activity has attracted much attention because of its widespread existence and availability in nature.
This study investigated the anti-tumor activity of chitin oligosaccharide and combined with cisplatin acted on lung adenocarcinoma A549 cells and tumor xenografts. Here, we first investigated the anti-tumor activity of chitin oligosaccharide alone and combined with cisplatin in vitro, and then we studied the effects of chitin oligosaccharide plus cisplatin in vivo by observing the expression level of Ki67 and P53 of tumor xenografts.
The proliferation of tumor cells determines the growth rate of tumor tissue. MTS assay showed that chitin oligosaccharide had the effect of inhibiting the proliferation of A549 cells, and this effect was increased with the increase of drug concentration. When combined with cisplatin, the inhibition rate in 24 hrs had no significant difference compared with chitin oligosaccharide group, but it showed high efficacy than cisplatin. After drugs acted for 48 hrs, 72 hrs and 96 hrs, the inhibitory effects of proliferation of three groups on A549 cells were increased with time prolonging. However, the combination group showed obvious advantages (P<0.05). It indicated that chitin oligosaccharide plus cisplatin could significantly inhibit the proliferation of lung adenocarcinoma cells.
The migrate ability of tumor cells is a key factor affecting tumor metastasis. Both cell scratch test and transwell migration experiment showed that chitin oligosaccharides could inhibit the migration of A549 cells, and combined with cisplatin, its effect was superior to chitin oligosaccharide or cisplatin. But both of them are the experiments to measure the migration of cells, why did scratch test had no significant change in relative scratch width between the experimental group and the control group in the initial 24 hrs and the effect of tumor cell migration was significantly inhibited after 48 hrs? The transwell experiment showed that tumor cell migration was significantly inhibited at 24 hrs. Considering this situation, it might be related to the high dependence of A549 cells on serum. Both inhibitory effects on the migration ability of tumor cells occurred obviously after the cell starvation. Comprehensive experiment results indicate that chitin oligosaccharide plus cisplatin showed a good inhibitory effect on migration of A549 cells. It indirectly reflected that chitin oligosaccharides plus cisplatin could inhibit the distant metastasis of tumor cells.
In the nude mice experiment, HE staining of tumor tissue showed that chitin oligosaccharide plus cisplatin could induce focal necrosis, nuclear fragmentation and pyknosis, chromatin margination, explain that chitin oligosaccharide plus cisplatin could promote tumor cell necrosis. Immunohistochemistry staining showed that the combination of chitin oligosaccharide and cisplatin could significantly reduce the expression level of Ki67 in tumor tissues. Ki67 obviously correlated with the proliferation activity of malignant tumor cells,20 indicating that chitin oligosaccharide plus cisplatin may be possible to inhibit the growth of tumor cells and improve the prognosis by down-regulating the expression level of Ki67. P53 gene is closely related to the tumor apoptosis and drug resistance.21 The experimental results showed that the chitin oligosaccharide plus cisplatin had no significant effect on the expression of P53 in tumor tissue. It is suggested that the combination of chitin oligosaccharide and cisplatin inhibits the proliferation; migration of tumor cells may not up-regulate the expression level of P53. All the results indicated that chitin oligosaccharide and cisplatin could not increase the expression level of P53 in tumor tissues of A549 cells, while it could reduce the expression level of Ki67, especially in the combination group of the two drugs. Chitin oligosaccharide plus cisplatin may be possible to improve the prognosis by down-regulating the expression level of Ki67. It was also showed that chitin oligosaccharide plus cisplatin had positive synergistic effects on the inhibition of proliferation of A549 cells.
It was found that chemotherapies inhibited the growth of tumors by inducing apoptosis of tumor cells. For a long time, apoptosis has been widely regarded as a non-immunogenicity process. However, more and more evidences showed that some chemotherapeutic drugs could stimulate the body to produce anti-tumor immune response and induce immunogenic cell death (ICD), accompanied by inducing apoptosis of tumor cells. ICD relied on the ability of specific stimuli to kill cells and stimulation of the coordinated emission of immunogenic signals. The signals were conveyed by damage-associated molecular patterns (DAMPs), such as the endoplasmic reticulum (ER), chaperone calreticulin (CALR), adenosine-triphosphate (ATP) and high mobility group protein B1 (HMGB1). The DAMPs played a key role in the process of inducing immunogenic response. CALR was expressed on the cell surface of early ICD, and its mechanism was related to the activation of Caspase8 and BAK1. The complex mechanism of ATP included four caspase-dependent processes. Permanent loss of mitochondrial transmembrane potential caused by mitochondrial extra-membrane permeability (MOMP), accompanied by activation of caspase9 and caspase3, was one of the main hallmarks of mitochondrial apoptosis.22 In our study, we found that both caspase8 and caspase3 expressions were increased in the combination group, while BAK expression had minor change in all groups, which indicated that chitin oligosaccharide plus cisplatin promoted the apoptosis of A549 cells via caspase cascade pathway induced by activated initiator caspase8 and effector caspase3. Although we did not detect the expression of CALR, ATP and HMGB1, we could observe the promising effect of chitin oligosaccharide plus cisplatin in triggering ICD process by our present findings.
This study preliminarily confirms that the chitin oligosaccharide could inhibit the proliferation, migration and has positive synergistic effects with cisplatin. The efficacy is higher than chitin oligosaccharide or cisplatin. However, the potential molecular mechanism remains to be further studied. Chitin oligosaccharide is a promising anti-tumor drug, and whether it can be widely used in the clinic is still needed to be verified by large sample studies.
Acknowledgment
We thank Prof. Dongchu Ma, Huiying Yu, Yun’en Liu and Tao Han for their technical help in Medical Laboratory, Severe and War-related Trauma Laboratory and Oncology Department. We also thank Prof Yan Zhao of Shenyang Institute of Metal Research for providing drug and related literatures.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE–5 – a population based study. Lancet Oncol. 2014;15(1):23–34. doi:10.1016/S1470-2045(13)70546-1
2. Gansler T, Ganz PA, Grant M, et al. Sixty years of CA: a cancer journal for clinicians. CA Cancer J Clin. 2010;60(6):345–350. doi:10.3322/caac.20088
3. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208
4. Ge X, Jiang L. Research progress of thyroid transcription factor-1 as molecular marker in lung carcinoma. Chin J Lung Cancer. 2014;17(6):491–495. doi:10.3779/j.issn.1009-3419.2014.06.10
5. Mazières J, Pujol JL, Kalampalikis N, et al. Perception of lung cancer among the general population and comparison with other cancers. J Thorac Oncol. 2015;10(3):420–425. doi:10.1097/JTO.0000000000000433
6. Evans M. Lung cancer: needs assessment, treatment and therapies. Br J Nurs. 2013;22(17):
7. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi:10.3322/caac.21149
8. Chen H, Yang C, Yan B, et al. Occurrence and survival condition of lung cancer with different histologies among residents in Pudong new area. Chin J Lung Cancer. 2014;17(3):203–208.
9. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
10. Azuma K, Osaki T, Minami S, Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J Funct Biomater. 2015;6(1):33–49. doi:10.3390/jfb6010033
11. Muanprasat C, Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther. 2017;170:80–97. doi:10.1016/j.pharmthera.2016.10.013
12. Winkler AJ, Dominguez-Nuñez JA, Aranaz I, et al. Short-chain chitin oligomers: promoters of plant growth. Mar Drugs. 2017;15(2):
13. Lodhi G, Kim YS
14. Phil L, Naveed M, Mohammad IS, et al. Chitooligosaccharide: an evaluation of physicochemical and biological properties with the proposition for determination of thermal degradation products. Biomed Pharmacother. 2018;102:438–451. doi:10.1016/j.biopha.2018.03.108
15. Shen KT, Chen MH, Chan HY, et al. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem Toxicol. 2009;47(8):1864–1871. doi:10.1016/j.fct.2009.04.044
16. Kerch G. The potential of chitosan and its derivatives in prevention and treatment of age-related diseases. Mar Drug. 2015;13(4):2158–2182. doi:10.3390/md13042158
17. Zou P, Yang X, Wang J, et al. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016;190:1174–1181. doi:10.1016/j.foodchem.2015.06.076
18. Thadathil N. VelappanSP. Recent developments in chitosanase research and its biotechnological applications. Food Chem. 2014;150:392–399. doi:10.1016/j.foodchem.2013.10.083
19. Bu J The Influence of Combining Rapamycin or 3-MA with Cisplatin in A549 and Orthotopically Implanted Tumors in Nude Mice. [Dissertation]. Hebei Medical University; 2015.
20. Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer. Mol Med Rep. 2015;11(3):1566–1572. doi:10.3892/mmr.2014.2914
21. Liu J, Zhang C, Feng Z. Tumor suppressor p53 and its gain-of-function mutants in cancer. Acta Biochim Biophys Sin. 2014;46(3):170–179. doi:10.1093/abbs/gmu092
22. Oliver K, Laura S, Ilio V, et al. Consensus guidelines for the detection of immunogenic cell death. OncoImmunology. 2014;3:e955691. doi:10.4161/21624011.2014.955691
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.