Back to Journals » Infection and Drug Resistance » Volume 14
Anti-Septic Potential of 7-α-Obacunyl Acetate Isolated from the Toona sinensis on Cecal Ligation/Puncture Mice via Suppression of JAK-STAT/NF-κB Signal Pathway
Authors Li D, Weng Y, Wang G, Zhen G
Received 20 January 2021
Accepted for publication 21 March 2021
Published 14 May 2021 Volume 2021:14 Pages 1813—1821
DOI https://doi.org/10.2147/IDR.S302853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Duo Li, Yibing Weng, Guan Wang, Genshen Zhen
Department of Critical Care Medicine, Beijing Luhe Hospital, Capital Medical University, Beijing, 101149, People’s Republic of China
Correspondence: Genshen Zhen
Department of Critical Care Medicine, Beijing Luhe Hospital, Capital Medical University, No. 82, Xinhua South Road, Tongzhou District, Beijing, People’s Republic of China
Tel +86 10-69543901-2121
Email [email protected]
Purpose: Sepsis is a life-threatening clinical syndrome and characterized by an inflammatory and innate immune response to infections. The current study was aimed to evaluate the anti-sepsis effect of 7-α-Obacunyl acetate (7-OBA), the abundant constituent isolated from Toona sinensis (Meliaceae), in cecal ligation and puncture (CLP)-induced mice and to investigate the related molecular mechanisms.
Methods: The CLP operation was performed to establish the sepsis mice model, and the survival rate and temperature were measured after 7-OBA treatment (7.5, 15, and 30 mg/kg; i.p.). Inflammatory cytokines levels of TNF-α, IL-1β, IL-6, and IL-10 were detected by ELISA kits, and the kidney, liver, and heart function were measured using an automatic biochemistry analyzer. Effects of 7-OBA on NF-κB and JAK2-STAT3 signaling pathways were determined by Western blot analysis in a lipopolysaccharide (LPS) stimulated RAW264.7 cells model.
Results: 7-OBA treatment significantly increased the survival rate (p< 0.05 and p< 0.01) and normalized temperature (p< 0.05 and p< 0.01) of sepsis mice. The levels of pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6 in serum were obviously decreased, whereas the anti-inflammatory cytokines of IL-10 were increased. CLP-induced increases of the main markers of kidney, liver, and heart function in mice (p< 0.01) were also obviously reversed by 7-OBA. The anti-sepsis effect of 7-OBA might be associated with regulation of nuclear factor kappa-B (NF-κB) and Janus kinase 2 (JAK2)-signal transducer and activator of transcription 3 (STAT3) signal pathways.
Conclusion: Our investigation indicated that 7-OBA can be developed as an effective agent for treating/curing sepsis in the future.
Keywords: 7-α-obacunyl acetate, Toona sinensis, sepsis, cecal ligation/puncture, JAK-STAT/NF-κB
Introduction
Currently, increasing reports have indicated that sepsis is a leading cause of death in intensive care units, characterized as severe infections caused by a whole-body inflammatory response syndrome (SIRS). It’s considered as the systemic host response to microorganisms of viruses, bacteria, or fungi, and eventually results in associated multiple organs dysfunction syndromes, the heart, lungs, liver, and kidneys in particular.1–3 In a recent extrapolation from high-income country data, there was an estimation of 48.9 million cases and 11 million sepsis-related deaths worldwide in 2017.4 However, specific medicine for treatment of sepsis is heavily lacking, and long-term administration of the antibiotics commonly cause some severe side-effects, such as nausea, vomit, and loss of appetite. Thus, it is urgent for us to find reliable therapeutic methods for curing sepsis or attenuating syndromes of sepsis.
Recently, traditional herbal medicines have been widely used for treating infection and inflammation in folk for thousands of years. Plant-derived compounds/extracts are important source for available drugs discovery, which have been reported to have considerable potential against sepsis in previous studies. Toona sinensis, belonging to the Meliaceae family, is a deciduous plant with edible and medicinal value, distributed throughout the east and southeast of Asia.5,6 The main constituents of T. sinensis are terpenoids, phenylpropanoids, and flavonoids compounds, etc., with extensive pharmacological activities including anti-tumor, anti-diabetic, and anti-inflammatory effects.7–9 Furthermore, Toona sinensis leaf aqueous extract showed strong activity against sepsis in both in vitro and in vivo models.10 Recently, previous studies have revealed that 7-α-Obacunyl acetate (7-OBA; Figure 1) is the abundant constituent in the leaves of T. sinensis, furthermore, which showed a good effect on preventing sepsis in our preliminary experiments. However, whether 7-OBA has reliable sepsis-attenuating effects and its molecular mechanism have never been systematically studied. Thus, the current study was designed to investigate the protective effect of 7-OBA against systemic inflammation in a cecal ligation/puncture (CLP) induced mice model of sepsis and its possible mechanism involved.
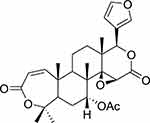 |
Figure 1 Chemical structure of 7-OBA isolated from the Toona sinensis. |
Materials and Methods
Chemicals and Reagents
Mouse TNF-α, IL-1β, IL-6, and IL-10 ELISA kit were purchased from BOSTER biological technology company (Wuhan, China). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin-streptomycin (P/S) were purchased from GIBCO (Grand Island, NY, USA). The primary antibodies of p-JAK2 (ab195055), JAK2 (ab39636), p-STAT3 (ab30647), and STAT3 (ab19352) were purchased from Abcam Biotech (Cambridge, MA); NF-κB p65 (#8242), Histone H1 (#41328), and IκB (#9242) were purchased from Cell Signaling Technology (Beverly, MA). All other chemicals used in this study were of analytical reagent grade.
Plant Materials
Leaves of T. sinensis was purchased from Beijing Tong-ren-tang pharmaceutical Group (Beijing, China), in January 2018. A voucher specimen has been deposited (No. XC–201801011) in the herbarium of the Department of pharmacy, Beijing Luhe Hospital (Beijing, China).
Animals
Male ICR mice (20±2 g, 5–6 weeks) were purchased from the experimental animal center of Peking University (Beijing, China). Mice were kept with standard laboratory chow and distilled water at a relative humidity of 55±3% (25±2°C; 12 hour light/dark cycle).
Cells Culture
The RAW264.7 macrophage cells (American Type Culture Collection, Manassas, VA) were cultured in a humidified atmosphere (37°C, 5% CO2) with DMEM added 10% FBS, 1.5 g/L sodium bicarbonate, and 1% P/S.11 Cells were exposed to 7-OBA (30 μg/mL), and then stimulated with LPS at 100 ng/mL.
Preparation of Cecal Ligation and Puncture Model of Mice and Animal Protocol
Cecal ligation and puncture (CLP) model of mice were carried out according to the method described previously.12 In brief, animals were anesthetized with isoflurane, and cecum ligation was made through an abdominal midline incision. Then, the ligated cecum was punctured three times with a 21-gauge needle. After reposition the cecum into the abdominal cavity, the abdomen was closed through suture method.
A total of 100 mice were randomly divided into five groups (n=20): sham group, control group (0 mg/kg), and three treatment groups (CLP+7.5, 15, and 30 mg/kg 7-OBA). For the control group, the cecal ligation and puncture were performed, and animals in the sham group underwent the same procedure without ligation or puncture of the cecum. Animals were intravenously given 7-OBA or saline at 0, 4, 12, and 24 hours after CLP operation.
Preparation and Identification of the 7-OBA
The powdered leaves (2.5 kg) were extracted with ethyl alcohol three times at room temperature. After the extract was concentrated in vacuum, the residue was suspended in water and extracted with petroleum ether and CHCl3. The CHCl3 fraction (92 g) provided a residue which was subjected to a chromatographic column filled with silica gel using CHCl3-Me2CO mixes and then purified on a RP-C18 column eluted with CH3-OH–water (8:2–7:3) as an eluent to yield 7-OBA (0.86 g). Identification of 7-OBA was confirmed by comparing the NMR spectral data with previous reports.13,14
The 1H-NMR and 13C-NMR peaks of 7-OBA were displayed as follows: HR-EI-MS: [M+H]+ at m/z 499.2328 (cald. 499.2331), C28H35O8; 1H-NMR (600 MHz, CDCl3) δ (ppm): 7.46 (1H, m, α-furans), 6.72 (1H, d, J=11.7 Hz, H-1), 6.42 (1H, d, J=0.8 Hz, β-furans), 5.95 (1H, d, J=11.7 Hz, H-2), 5.54 (1H, s, H-17), 4.46 (1H, s, H-7), 3.56 (1H, s, H-15), 2.14 (3H, s, -COCH3), 1.48, 1.33, 1.32, 1.22, 1.08 (-CH3×5); 13C-NMR (150 MHz, CDCl3) δ (ppm): 169.3 (-CO-), 165.7 (C-3), 162.9 (C-16), 158.8 (C-1), 141.2 (C-23), 138.2 (C-21), 123.1 (C-20), 122.7 (C-2), 111.6 (C-22), 89.3 (C-4), 77.2 (C-17), 74.7 (C-7), 68.4 (C-14), 59.3 (C-15), 48.5 (C-5), 44.2 (C-10), 43.3 (C-8), 41.5 (C-9), 38.6 (C-13), 26.2 (C-12), 25.1 (C-6), 20.6 (-COCH3), 17.1 (C-11), 30.1, 23.7, 18.3, 16.1, 14.7 (-CH3×5).
Survival Analysis and Body Temperatures after CLP Surgery
The survival rate of the mice (n=12) were observed within 7 days; furthermore, the body temperatures in the rectum were measured at 0 (pre-operation), 4, 8, 12, 16, 20, 24, 32, 40, and 48 hours after CLP surgery.
Determination of 7-OBA on the Function Indexes of Kidney, Liver, and Heart in Mice
At 24 hours after surgical operation, eight mice from each group were anesthetized by pentobarbital sodium and the serum were separated by centrifugation at 3,500 r/min for 10 minutes for subsequent assays. An automatic biochemistry analyzer (AU5800, Beckman Coulter, USA) was applied to evaluate the contents of alanine transaminase (ALT), aspartate transaminase (AST), creatinine (Cre), blood urea nitrogen (BUN), heart-type fatty acid-binding protein (H-FABP), N-terminal pronatriuretic peptide (NF-proBNP), and Cardiac troponin I (cTnI) in serum.
Determination of Serum Cytokines of CLP Mice
After the mice were sacrificed under anesthesia with sodium pentobarbital, the blood samples were collected from the heart. After centrifuging, each serum sample was collected and stored at −80°C until used in this study. Serum levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-10 were measured using ELISA kits according to the commercial kits introduction.
Western Blotting Analysis
To elucidate the possible mechanism by which 7-OBA mediates sepsis in mice, we used LPS as the dominant inflammatory response to induce sepsis in the RAW264.7 macrophage cell line. Cells (2.0×106/mL, 2 mL) were cultured in 6-well plates and incubated with or without 7-OBA (30 μg/mL) for 2 hours, and then stimulated with LPS (100 ng/mL) for 4 hours. Total protein concentration was examined using a BCA assay kit, and equal amounts of protein (30 μg) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After the proteins were transferred to PVDF membranes, blots were blocked with buffer (5% non‑fat milk). Then, the proteins were incubated with primary antibodies of NF-κB p65 (dilution 1:700), Histone H1 (dilution 1:500), IκB (dilution), p-JAK2 (dilution 1:1,000), JAK2 (dilution 1:500), p-STAT3 (dilution 1:500), and STAT3 (dilution 1:2,000) overnight at 4°C. Following through washing with TBST buffer for three times, HRP-conjugated secondary antibodies were applied at room temperature and immune complexes were visualized by the chemiluminescence detection system.
Statistical Analysis
Data were expressed as mean±SD and analyzed by SPSS 19.0 software (SPSS Inc., Chicago, IL). The chi-squared value of the exact test was applied to study the differences of mice mortality among different groups. One-way ANOVA test was performed for multiple comparisons. A p-value <0.05 was considered to be statistically significant.
Results
Effect of 7-OBA on Survival of Mice after CLP Surgery
All mice in the control group (0 mg/kg) died within 3 days after CLP surgery. However, in the 7-OBA treatment group (7.5, 15, and 30 mg/kg), the survival of the mice were increased to 20%, 35%, and 60% until the end of the observation, in a dose-dependent manner (p<0.05, p<0.01, and p<0.01, respectively) (Figure 2). The median survival rates of mice in the control group, 7-OBA administration groups (7.5, 15, and 30 mg/kg) were 1, 2, 2, and 7 days after surgery, respectively. In addition, no death of mice was observed in the sham group within 7 days.
Effect of 7-OBA on Body Temperature in CLP-Induced Mice Sepsis
The effects of 7-OBA on body temperature of CLP-induced mice sepsis are shown in Figure 3. As a whole, compared to the sham group, the body temperature was significantly decreased after surgery, and it basically stayed at 33°C in the control group. However, other mice in the 7-OBA treatment group were then progressively returned to normal body temperature from 8 hours to the end of the observation period, especially those with doses of 15 mg/kg and 30 mg/kg (p<0.01).
Effect of 7-OBA on TNF-α, IL-1β, IL-6, and IL-10 in Serum of CLP-Induced Mice Sepsis
The contents of cytokines TNF-α, IL-1β, IL-6, and IL-10 in mice serum are shown in Figure 4. It was found that the proinflammatory cytokines of TNF-α, IL-1β, and IL-6 in serum were significantly increased after CLP surgery, when compared with the sham group (p<0.01). However, treatment with 7-OBA markedly decreased the level of these cytokines, especially at 15 and 30 mg/kg doses (p<0.01). The anti-inflammatory cytokine level of IL-10 in serum was dramatically increased by administration with 7-OBA (7.5, 15, and 30 mg/kg) in a dose-dependent manner (p<0.01).
Effects of 7-OBA on the Function Indexes of Kidney, Liver, and Heart in CLP-Induced Mice Sepsis
As seen in Figure 5, the levels of ALT, AST, Cre, BUN, H-FABP, NF-proBNP, and cTnI in serum were examined for evaluating kidney, liver, and heart function of mice. Compared to the sham group, the ALT, AST, Cre, and BUN levels were significantly increased after CLP surgery, but these increases were markedly inhibited by treatment with 7-OBA (15 and 30 mg/kg) (p<0.01), with a dose-dependent manner. Similarly, the levels of cardiac function markers, H-FABP, NF-proBNP, and cTnI in serum following CLP surgery were also decreased by 7-OBA at 7.5, 15, and 30 mg/kg (p<0.01).
Effects of 7-OBA on NF-κB Signal Pathway in RAW264.7 Cells
As displayed in Figure 6, the results of protein expression of NF-κB p65 and IκB were exhibited by Western blot analysis. Herein, the protein expression of NF-κB p65 (N) in cells incubated with LPS was significantly increased, whereas pretreatment with 30 μg/mL 7-OBA showed a decrease (p<0.01). In addition, 7-OBA strongly reversed LPS-induced reduction of protein expression of NF-κB p65 (C) and IκB (p<0.01). The findings suggested that 7-OBA showed a protective effect in CLP-induced sepsis in mice, maybe through the modulation of NF-κB signaling pathways.
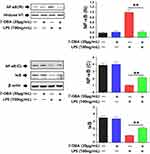 |
Figure 6 Effects of 7-OBA on NF-κB signal pathway in LPS-stimulated RAW264.7 cells. As compared with the control group, **p<0.01 indicates an extremely significant difference. |
Effects of 7-OBA on JAK2-STAT3 Signal Pathway in RAW264.7 Cells
To further investigate the possible molecular mechanism involved in CLP-induced mice sepsis, the protein expression of JAK2 and STAT3 were evaluated. As shown in Figure 7, significant increases of protein expression levels, p-JAK2 and p-STAT3, were observed in RAW264.7 cells stimulated by LPS (100 ng/mL) alone. Interestingly, 7-OBA incubation at 30 μg/mL obviously reversed LPS-induced increases of these two proteins (p<0.01). These results indicated that 7-OBA attenuated CLP-induced sepsis in mice, maybe through the inhibition of JAK2-STAT3 signaling pathways.
 |
Figure 7 Effects of 7-OBA on JAK2-STAT3 signal pathway in LPS-stimulated RAW264.7 cells. As compared with the control group, **p<0.01 indicates an extremely significant difference. |
Discussion
Increasing studies have shown that over 50% of the available agents are related to plant-derived compounds/extracts, and many plant-derived natural constituents have been considered as potential sources of new drugs.15 In the present study, 7-OBA was found to protect CLP sepsis mice, including improved survival of CLP mice, and this protection was closely related to decreased pro-inflammatory cytokines levels and mediated NF-κB and JAK2-STAT3 signaling pathways.
As far as we know, sepsis often leads to an overwhelming response of innate inflammation that has been considered as a protective response initiated by the immune system to defend severe infections and tissue injury, and ultimately resulted in release of pro-inflammatory factors. The Cecal ligation and puncture (CLP) operation, a typical self-infection model, has been commonly used as an effective animal model to discover candidate of anti-sepsis drugs.16 After CLP operation, the levels of TNF-α, IL-1β, and IL-6 in plasma were increased in mice, and additionally cause different levels of mortality.17,18 Liver, heart, and kidney are the main target organs susceptible to be attacked by sepsis, and their functions also reversely affect the sepsis development. AST and ALT are two key markers of liver function, Cre and BUN are two main indices of the kidney function, and H-FABP, NF-proBNP, and cTn are four important markers of heart function.19,20 In this experiment, the markers of these target organ function in serum were significantly increased after CLP surgery. These findings indicated that the animal model of sepsis in this study was effectively established, similar to those symptoms observed in sepsis in the clinic.
An inflammatory reaction plays an important role in the physiological processes of many diseases, such as sepsis. Pro-inflammatory cytokines, such as TNF-α and IL-6, act as an essential role in sepsis development. It has been reported that TNF-α is believed as an upstream regulator of inflammation to regulate numerous inflammatory factors, and IL-6 and IL-8 as the downstream cytokines. Once activated, it can result in tissue injury by increasing several pro-inflammatory cytokines.21,22 The current investigation also showed a regulatory effect on the inflammatory cytokines, such as TNF-α, IL-6, and IL-8.
It is well-known that lipopolysaccharide (LPS) is an effective activator of sepsis to release a good amount of pro-inflammatory cytokines in monocytes. RAW264.7 cells incubated by LPS is a widely accepted in vitro model for discovering anti-sepsis agents.23,24 In this study, the protein expression levels of JAK2 and STAT3 exhibited an obvious reduction in RAW264.7 cells (exposure to LPS) pretreated with 7-OBA. Previous studies have revealed that activation of the JAK/STAT signaling pathway is crucial for the inflammatory responses.25,26 For instance, release of cytokines such as IL-6 and IL‑2 was commonly regulated by activation of JAK/STAT signaling pathway.25,27 The inhibition of this pathway can attenuate dysfunction of target organs in sepsis in mice and, therefore, this signal pathway may be considered as the main molecular mechanism for the anti-sepsis of 7-OBA. However, an extremely limited growing number of reports have shown the role of the JAK/STAT signaling pathway in sepsis.
In addition to the inhibition of the JAK/STAT signaling pathway, other protein expressions such as NF-κB and IκB are also regarded as indicative markers in sepsis. The NF-κB signal pathway is very important in inflammatory responses, which can be stimulated by multiple stimuli agents such as LPS. NF-κB can be activated through phosphorylation of the protein IκB by translocation of NF-κB subunits to the IκB kinases (IKKs) and nucleus. The activation of NF-κB p65 (N) could be detected in CLP-induced sepsis and LPS-cultured RAW 264.7 cells.28,29 In the present study, 7-OBA pretreatment significantly reduced the protein expression of NF-κB (N), whereas the NF-κB (C) and IκB were increased.
Conclusion
Collectively, the current study showed that 7-OBA, the abundant constituent from T. sinensis leaves, has the potential effect on CLP-induced sepsis mice, and the molecular mechanism might be associated with modulation of the NF-κB and JAK/STAT signaling pathway. This investigation provides a new insight into the medicinal role of the bioactive constituents of T. sinensis.
Statement of Ethics
These animal experiments were performed according to the National Institute of Health guidelines of the Care and Use of Laboratory Animals and approved by the Animal Research Ethics Committee of the Animal Care and Use Committee of Beijing Luhe Hospital (No. 201809ky-012).
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest.
References
1. Wang AM, Xiao ZM, Zhou LP, Zhang J, Li XM, He QC. The protective effect of atractylenolide I on systemic inflammation in the mouse model of sepsis created by cecal ligation and puncture. Pharm Biol. 2015;54:146–150. doi:10.3109/13880209.2015.1024330
2. Shi XF, Zhang Y, Wang H, Zeng S. Effect of triggering receptor expressed on Myeloid cells 1 (TREM-1) blockade in rats with cecal ligation and puncture (CLP)-induced sepsis. Med Sci Monit. 2017;23:5049–5055. doi:10.12659/MSM.904386
3. Hagiwara S, Iwasaka H, Hasegawa A, Asai N, Noguchi T. High-dose intravenous immunoglobulin G improves systemic inflammation in a rat model of CLP-induced sepsis. Intensive Care Med. 2008;34(10):1812–1819. doi:10.1007/s00134-008-1161-1
4. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet (London, England). 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
5. Liao JW, Chung YC, Yeh JY, et al. Safety evaluation of water extracts of Toona sinensis Roemor leaf. Food Chem Toxicol. 2007;45:1393–1399. doi:10.1016/j.fct.2007.01.020
6. Peng W, Liu YJ, Hu MB, et al. Toona sinensis: a comprehensive review on its traditional usages, phytochemistry, pharmacology and toxicology. Rev Bras Farmacogn. 2019;29:111–114. doi:10.1016/j.bjp.2018.07.009
7. Cheng KW, Yang RY, Tsou SC, et al. Analysis of antioxidant activity and antioxidant constituents of Chinese toon. J Funct Foods. 2019;1:253–259. doi:10.1016/j.jff.2009.01.013
8. Liao JW, Hsu CK, Wang MF, Hsu W, Chan YC. Beneficial effect of Toona sinensis Roemor on improving cognitive performance and brain degeneration in senescence-accelerated mice. Br J Nutr. 2006;96(2):400–407. doi:10.1079/BJN20061823
9. Su YF, Yang YC, Hsu HK, et al. Toona sinensis leaf extract has antinociceptive effect comparable with non-steroidal anti-inflammatory agents in mouse writhing test. BMC Complement Altern Med. 2015;15:70–78. doi:10.1186/s12906-015-0599-2
10. Yang CJ, Chen YC, Tsai YJ, Huang MS, Wang CC. Toona sinensis leaf aqueous extract displays activity against sepsis in both in vitro and in vivo models. Kaohsiung J Med Sci. 2014;30(6):279–285. doi:10.1016/j.kjms.2014.02.013
11. Wen Q, Mei L, Ye S, et al. Chrysophanol demonstrates anti-inflammatory properties in LPS-primed RAW 264.7 macrophages through activating PPAR-γ. Int Immunopharmacol. 2018;56:90–97. doi:10.1016/j.intimp.2018.01.023
12. Li B, Yu MC, Pan XC, et al. Artesunate reduces serum lipopolysaccharide in cecal ligation/puncture mice via enhanced LPS internalization by macrophages through increased mRNA expression of scavenger receptors. Int J Mol Sci. 2014;15:1143–1161. doi:10.3390/ijms15011143
13. Bennett RD, Hasegawa S. 7α-oxygenated limonoids from the rutaceae. Phytochemistry. 1982;21:2349–2354. doi:10.1016/0031-9422(82)85203-5
14. Luo XD, Wu SH, Ma YB, Wu DG. Limonoids and phytol derivatives from Cedrela sinensis. Fitoterapia. 2000;71:492–496. doi:10.1016/S0367-326X(00)00158-1
15. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi:10.1021/acs.jnatprod.5b01055
16. Yu M, Shao D, Liu J, Zhu J, Zhang Z, Xu J. Effects of ketamine on levels of cytokines, NF-κB and TLRs in rat intestine during CLP-induced sepsis. Int Immunopharmacol. 2007;7(8):1076–1082. doi:10.1016/j.intimp.2007.04.003
17. Davies B, Cohen J. Endotoxin removal devices for the treatment of sepsis and septic shock. Lancet Infect Dis. 2011;11:65–71. doi:10.1016/S1473-3099(10)70220-6
18. Raspéa C, Höcherlb K, Ratha S, Sauvanta C, Bucher M. NF-κB-mediated inverse regulation of fractalkine and CX3CR1 during CLP-induced sepsis. Cytokine. 2013;61:97–103. doi:10.1016/j.cyto.2012.08.034
19. Kang J, Zhang BL. Effect of curcumin on protection of organ function and serum levels of IL-18 in LPS-induced sepsis in rats. J Clin Pediatr. 2012;30:869–873.
20. Wang R, Li YH, Zhang JL, Fu LB. Effects of sodium ferulate on Bcl-2 and Bax expression in septic rats kidney. Chin J Clin Pharmacol. 2011;27:46–49.
21. Zhang YP, Fan HH, Xu J, et al. Network analysis reveals functional cross-links between disease and inflammation genes. Sci Rep. 2013;3:3426. doi:10.1038/srep03426
22. Jayatilaka H, Tyle P, Chen J, et al. Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration. Nat Commun. 2017;8:15584. doi:10.1038/ncomms15584
23. Karki R, Park CH, Kim DW. Extract of buckwheat sprouts scavenges oxidation and inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages (RAW264.7). J Integr Med. 2013;11:246–252. doi:10.3736/jintegrmed2013036
24. Zhang WW, Sun Q, Gao XL, Jiang YM, Li RT, Ye J. Anti-inflammation of spirocyclopiperzazinium salt compound LXM-10 targeting α7 nAChR and M4 mAChR and inhibiting JAK2/STAT3 pathway in rats. PLoS One. 2013;8:e66895. doi:10.1371/journal.pone.0066895
25. O’She JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi:10.1146/annurev-med-051113-024537
26. Zhang Q, Peng W, Wei SJ, et al. Guizhi-Shaoyao-Zhimu decoction possesses anti-arthritic effects on type II collagen-induced arthritis in rats via suppression of inflammatory reactions, inhibition of invasion & migration and induction of apoptosis in synovial fibroblasts. Biomed Pharmacother. 2019;118:109367. doi:10.1016/j.biopha.2019.109367
27. Zhang X, Li J, Qin JJ, et al. Oncostatin M receptor β deficiency attenuates atherogenesis by inhibiting JAK2/STAT3 signaling in macrophages. J Liquid Res. 2017;58(5):895–906. doi:10.1194/jlr.M074112
28. Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi:10.1126/science.1112304
29. Kim SJ. Curcumin suppresses the production of interleukin-6 in Prevotella intermedia lipopolysaccharide-activated RAW 264.7 cells. J Periodontal Implant Sci. 2011;41:157–163. doi:10.5051/jpis.2011.41.3.157
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




