Back to Journals » Journal of Experimental Pharmacology » Volume 12
Anti-Oxidant Potential and Antimalarial Effects of Acanthus polystachyus Delile (Acanthaceae) Against Plasmodium berghei: Evidence for in vivo Antimalarial Activity
Authors Kifle ZD , Atnafie SA
Received 16 September 2020
Accepted for publication 26 November 2020
Published 11 December 2020 Volume 2020:12 Pages 575—587
DOI https://doi.org/10.2147/JEP.S282407
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bal Lokeshwar
Zemene Demelash Kifle, Seyfe Asrade Atnafie
University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Department of Pharmacology, Gondar, Ethiopia
Correspondence: Zemene Demelash Kifle Tel +251918026724
Email [email protected]
Background: Malaria is among the most devastating and widespread tropical parasitic diseases which is more prevalent in developing countries. Acanthus polystachyus (Acanthaceae) leaves are traditionally used for the treatment of malaria in Ethiopia. This study aimed to investigate the in vivo antimalarial and in vitro antioxidant activity of the leaves extract of Acanthus polystachyus.
Materials and Methods: The leaves part of A. polystachyus were extracted using 80% methanol. The antioxidant effect of Acanthus polystachyus extract was evaluated by using (DPPH) diphenyl-2-picrylhydrazyl assay model. To evaluate the antimalarial effect of the A. polystachyus extract, Plasmodium berghei ANKA strain (chloroquine sensitive) was used to infect the mice in three different experimental protocol: suppressive, curative, and prophylactic test models.
Results: The leaves extract of Acanthus polystachyus significantly (P< 0.05– 0.0001) suppressed the parasitemia in all experimental protocol. The extract exhibited a chemosupression of 27.64%, 33.67% and 49.25% in 4-day suppressive test; 33.76%, 39.24% and 53.59% in curative test, and 25%, 32.84% and 50% in prophylactic tests at dose of 100, 200, and 400mg/kg, respectively. The extract also extended the mean survival time, prevented the bodyweight loss and body temperature drop, and packed cell volume significantly (P< 0.05) as compared to the negative control. Dose-dependent antioxidant activity was shown by the leaf extract of A. polystachyus with an IC50 value of 9.37μg/mL.
Conclusion: The current finding showed that the leaves extract of Acanthus polystachyus has revealed promising antimalarial effects. Thus, this finding supports the traditional use of A. polystachyus for the treatment of malaria.
Keywords: antimalarial activity, antioxidants, Plasmodium berghei, Acanthus polystachyus
Introduction
Malaria is a lethal disease caused by Plasmodium parasites transmitted to humans via bites of Plasmodium-infected female Anopheles mosquito. P. falciparum is the most fatal malaria parasite in Africa and accounts for most malarial deaths worldwide.1,2 About 219 million cases and 435, 000 deaths of malaria were reported in the world by 2017. Among these, 92% of the cases and 93% of the death report due to malaria were from Africa. Globally, more than two-third of the malaria deaths were reported in under-five children.3 According to WHO 2019 malaria report, roughly 228 million cases and 405,000 deaths of malaria were reported worldwide.4 The cases of P. falciparum and P. vivax were 66% and 34%, respectively, in Ethiopia.5 According to a 2015 report, a total of 621,345 cases and 1561 deaths were reported in Ethiopia.6 Nearly 6% of global malaria cases and 12% of global deaths due to P. vivax have occurred in Ethiopia. About 75% of global malarial deaths were from four countries: India, Pakistan, Indonesia, and Ethiopia.3
In Ethiopia, malaria was the foremost cause of outpatient visits, accounting for 17% of all outpatient visits, and 8% of health facility admissions among all age groups and one of the top ten causes of inpatient deaths among children aged less than five years and in older individuals.7 The socioeconomic impact of malaria is striking, although the country’s economy is based on agriculture and peak malaria transmission coincides with the planting and harvesting season. About 75% of the country is malicious, with about 68% of the country’s total population living in areas at risk of malaria.8,9
Plant-based traditional medicines have been the core source for the management of malaria in different countries.10 Traditionally claimed anti-malarial plants are among the most potential areas to search antimalarials drugs in African flora. It is well known that about 80% of the Ethiopian population depend on herbal medicine for the management of numerous diseases, including malaria.11 Although most of the medicinal plants that have potential anti-malarial activity have been studied, there still exist potentially useful medicinal plants awaiting evaluation for therapeutic applications against various groups of pathogens.12
Acanthus is a genus belonging to the family Acanthaceae. The Acanthaceae family has several pharmacological activities such as cytotoxic, immunomodulatory, anti-inflammatory, antiviral, insecticidal, antiplatelet, antipyretic, hepatoprotective, antifungal, and antioxidant activities.13,14 Traditionally, the leaves and root parts of Acanthus polystachyus have been used for the treatment of different illnesses such as pneumonia, bleeding, scorpion sting, stabbing pain, and anthrax.15 The leaves part of Acanthus polystachyus Delile have been used in the treatment of malaria in Ethiopia.16–18 So far in vivo antimalarial activity of Acanthus polystachyus has not been reported. Thus, this study is intended to evaluate the in vivo antimalarial and in vitro antioxidant activities of the leaves extract of Acanthus polystachyus.
Materials and Methods
Drugs and Reagents Used
Chloroquine (Addis Pharmaceuticals Factory, Ethiopia), giemsa (ScienceLab, USA), trisodium citrate (Deluxe Scientific Surgico, India), hydrochloric acid (Supertek Chemicals, India), halothane (Rotexmedica, Germany), normal saline (Epharm, Ethiopia), 2,2-Diphenyl-1-picrylhydrazyl, 3.5 (Sigma Aldrich, Germany), chloroform (Avishkar Lab Techchemicals, India), methanol (Avishkar Lab Tech Chemicals, India), ethyl acetate (Loba chemie, India), sulfuric acid (Supertek, India), acetic anhydride (Central Drug House, India), nitric acid (Supertek, India), ferric sulfate (BDH Ltd, UK), lead acetate (BDH Ltd, UK), benzene (Nice laboratory reagent, India), ferric chloride (Fisher Scientific Co, USA), Mayer’s reagent (Avishkar Lab Tech Chemicals, India), ascorbic acid (Blulux Laboratories, India), Wagner’s Reagent (BDH Ltd, UK) were used. All reagents were analytically graded and procured from certified local and international suppliers.
Plant Materials
Fresh leaves of Acanthus polystachyus were collected from Bahir Dar city, Ethiopia in February 2017. The botanical identification and authentication of the plant material were done by a botanist from Department of Biology, University of Gondar, Gondar, Ethiopia. The voucher specimen (D/W-0/0/1) was deposited in Herbarium at University of Gondar.
Methods of Extraction
The leaves of Acanthus polystachyus were washed with distilled water to remove dirt and dried at room temperature (25–27°C). The plant material was coarsely grounded into powder by the electrical mill. Then, the coarse powder of the plant material was macerated with methanol for about 72 hours (three times) and filtered via Whatman filter paper. The filtrate of the plant material obtained from the successive maceration was concentrated via a rotary evaporator followed by a hot air oven (40°C). Finally, the dried extract of Acanthus polystachyus was kept separately in a desiccator until used for the experiment. The extraction yield was calculated using the following formula:19
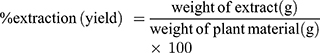
Experimental Animals and Parasites
A total of 95 Swiss albino mice of both sexes (weighing 24–30g and age 6–8 weeks) were used for the experiment. The mice were kept at room temperature with a 12h light/12h dark cycle and given food and water ad labium. After randomization into various groups and before initiation of the experiment, the mice were acclimatized to the laboratory conditions of the Department of Pharmacology, College of Medicine & Health Sciences, University of Gondar. Animal handling and care was carried out throughout the experiment according to international laboratory animal use and care guidelines.20 The euthanasia procedure was conducted in a chemical fume hood to prevent inhalation of halothane by workers. Finally, cervical dislocation was used on mice to ensure that they have been properly euthanized. The Plasmodium berghei ANKA strain (Chloroquine sensitive) was obtained from EPHI_ Addis Ababa.21
Preliminary Phytochemical Screening of Leaf Crude Extract
Qualitative tests were done for the leaves extract of Acanthus polystachyus for the presence of phytoconstituents such as flavonoids, steroidal compounds, terpenes, alkaloids, saponins, tannins, and phenolic compounds, as described in a standard procedure. Test for alkaloids: extract (0.2 g) was dissolved in dilute hydrochloric acid. The solution was filtered and a few drops of Dragendroff’s reagent added. The treated solutions were observed for any precipitation. Similarly, to a portion of filtered solution, a few drops of Mayer’s reagent were added. The treated solutions were observed for any precipitation; test for flavonoids: five mL of ethyl acetate was added to a solution of 0.5 g of the extract in water. The mixture was shaken, allowed to settle and inspected for the production of yellow color in the organic layer which is taken as positive for free flavonoids; test for saponins: the hydro-alcoholic extract (0.5 g) was dissolved in 10 mL of distilled water in a test tube. The test tube was corked and shaken vigorously for about 30 sec. The test tube was allowed to stand vertically and observed over a 30-min period of time. If froth above the surface persists after 30 min the sample is suspected to contain saponins; test for tannins: a portion of the alcoholic extract was dissolved in water. The solution was clarified by filtration. 10% ferric chloride solution was added to the clear filtrate. This was observed for a change in color to bluish black; test for glycosides: 0.5 g of crude extracts was dissolved separately in 5 mL of methanol. 10 mL of 50% HCl was added to 2 mL of each extract in test tubes. The mixtures were heated in a boiling water bath for 30 min. 5 mL of Fehling’s solution was added and the mixtures were boiled for 5 min to give a brick red precipitate as an indication for the presence of glycosides; test for Phenols: 0.5 g of each of crude extracts were put in a different test tube and treated with a few drops of 2% of FeCl3; bluish green or black coloration were indicated the presence of phenols; test for steroids: 0.5 g of each crude extracts were dissolved in 5 mL of methanol. 1 mL of the extract was treated with 0.5 mL of acetic acid anhydride and cooled in ice. This mixed with 0.5 mL of chloroform and 1 mL of concentrated sulphuric acid was then added carefully by means of a pipette. At the separations level of the two liquids, reddish-brown rings were formed, as indication of the presence of steroids; test for terpenoids: 0.5 g of each crude powders were separately dissolved in 5 mL of methanol. 2 mL of the extract was treated with 1 mL of 2,4-dinitrophenyl hydrazine were dissolved in 100 mL of 2M HCl. Yellow-orange colorations were observed as an indication of Terpenoids; and test for anthraquinones: the extract of the plant material (equivalent to 100 mg) was shaken vigorously with 10 mL of benzene, filtered and 5 mL of 10% ammonia solution added to the filtrate. The mixture was shaken and observed for the presence of a pink, red or violet color in the ammonia (lower) phase that indicates the presence of free anthraquinones.22–24
In-vitro Antioxidant Activity in DPPH Assay Model
The antioxidant activity of the leaves extract of Acanthus polystachyus was evaluated by using DPPH free radical scavenging assay. 0.004 gm of DPPH was dissolved in 100mL methanol in the dark and 3.9 mL of a 0.1 mM methanolic solution of DPPH was mixed with a 0.1mL methanolic solution of different concentrations of the extract ranging from 50 to 1000 μg/mL and incubated in the dark for thirty minutes at room temperature. Ascorbic acid served as a standard antioxidant for this experiment. After 30 minutes, the absorbance of the mixture and the control at 517 nm was read by a UV spectrophotometer.25 The test was carryout in triplicate so that the average value was taken. The percent inhibition of free scavenging was calculated as:
where Abs Control was the absorbance without sample, Abs samples was the absorbance of sample latex or ascorbic acid.
Acute Oral Toxicity Study
Acute oral toxicity test was done according to the OECD (Organization for Economic Cooperation and Development) 425 guideline for the crude extracts.26 Following the acclimatization of the mice for one week, five female mice (weighing 25–28 gram, 6–8weeks of age) were employed for the acute toxicity study. Following the period of fasting, the first one mouse was weighed and the test substance 2000mg/kg bodyweight of the extract of Acanthus polystachyus was administered by the oral route. The animal was observed at least once during the first thirty minutes, intermittently during the first 24 hours, with special attention given during the first four hours and daily thereafter, for a total of 14 days. Observations include changes in skin, eyes, mucous membranes, respiratory, circulatory, autonomic, and central nervous system changes, and behavioral patterns. The mouse in the sighting test did not show any sign of toxicity for 4 days and the toxicity test of the remaining four mice for methanolic crude extract was given and followed as usual for 14 days.27,28
Grouping and Dosing of Animals
In all mouse models, the positive control group was received Chloroquine 25 mg/kg and the negative control group was received distilled water 10 mL/kg; while the test groups were received three doses (100mg/kg, 200mg/kg, and 400mg/kg) of the Acanthus polystachyus crude extract. The leaves extract of Acanthus polystachyus was determined based on the result of the acute oral toxicity test. The middle doses of the solvent fractions of Acanthus polystachyus were 1/10 of the limit dose, the higher doses of the solvent fractions Acanthus polystachyus were twice of the middle doses of the solvent fractions of Acanthus polystachyus, and a half does of the middle doses of Acanthus polystachyus were the lower doses of the extract.29,30
Peter’s Test (4-Day Suppressive Test)
By using chloroquine-sensitive rodent malaria parasite, namely; Plasmodium berghei, the standard four-day suppressive method was used in this study.31 Treatment of infected mice was started after 3 hours of infection on day 0 and continued daily for four days (ie, from day 0 to day 3). On the fifth day (day 4) blood samples were collected from the tail snip of each mouse. Thin smears were prepared and stained with 10% Giemsa solution. Then, each stained slide was examined under the microscope with an oil immersion objective of 100x magnification power to evaluate the percent suppression of each extract with respect to the control groups.
Rane’s Test/Curative Test
Evaluation of the curative antimalarial potential of the extract was done by using a method described previously.27,32 On the first day, blood containing approximately the inoculum of 1 x 107 Plasmodium berghei ANKA strain (Chloroquine sensitive) was injected into 25 Swiss Albino mice, intraperitoneally (IP). After 72 hours, the mice were randomly divided into 5 groups of 6 mice each. Subsequently, 3 different doses of the extract (100 mg/kg, 200 mg/kg, and 400 mg/kg/day), chloroquine phosphate (25 mg/kg/day), and distilled water (1 mL/100 g/day) were given orally to the respective groups. These treatments were administered once daily for 3 consecutive days. For each mouse, thin blood film stained with 10% Giemsa was prepared from the tail blood of each mouse daily for 5 consecutive days to monitor the levels of parasitemia. The mice were further observed for 30 days. Any death that occurred during this period was recorded and used to determine the mean survival time. Similar to the chemosuppressive test, the average % parasitemia suppression was calculated.
Prophylactic Activity Test
Evaluation of the prophylactic potential of the active compounds in a 4-day suppressive test of the extract was done by methods described previously.33 30 Swiss Albino mice were randomly divided into 5 groups of 6 mice each. They were administered orally with 100, 200, and 400 mg/kg/day of the crude extract, chloroquine phosphate (25 mg/kg/day), and distilled water (1 mL/100 g/day) for 3 consecutive days of the respective groups. On the fourth day, a standard inoculum of 1 x 107 Plasmodium berghei ANKA strain (Chloroquine sensitive) infected erythrocytes were administered by the IP route to each mouse. After 72 hours (on the seventh day), thin blood smears were prepared from the tail blood. Percentage parasitemia and the percentage of chemosuppression of parasitemia were calculated using the formula described in the chemosuppressive test.
Determination of the Level of Parasitaemia and % Suppression
Tail blood smearing of each mouse was made for both the suppressive and curative tests. After fixation with absolute alcohol and staining with 10% Giemsa at pH 7.2 for 15 min, the slides were washed gently with distilled water followed by air drying at room temperature. By using Olympus microscope oil immersion objective of power, the number of parasitized erythrocytes in random fields of the microscope was counted. Three separate fields on each of the slides were used to calculate percent parasitemia and percent suppression using the following formula:34
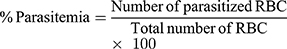

Determination of Mean Survival Time
From the time of infection until death, the mortality of each mouse was monitored and recorded regardless of the group in which the mouse was allocated throughout the follow-up period (15 days). The mean survival time of mice of each group was determined using the following formula:35
Determination of Packed Cell Volume
Blood was collected from the tail of each mouse in heparinized micro-hematocrit capillary tubes. The capillary tubes were filled to 3/4th of their height with blood and sealed at one end with sealing clay. The tubes were then placed in a micro-hematocrit centrifuge, with the sealed end outwards and centrifuged for 5 minutes at 11,000 rpm. The tubes were taken out of the centrifuge and PCV was determined using a standard Micro-Hematocrit Reader. It was measured before inoculating the parasite (day 0) and day 4 in Peter’s 4-day suppressive test and D0 and D7 in the prophylactic activity test. In the case of Rane’s test, PCV was measured on the third day after the infection was established, and on the last day of treatment on the seventh day. PCV is a measure of the proportion of RBCs to plasma in the whole blood and determined using the relation shown below.28
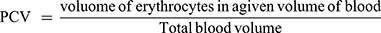
Monitoring of Body Weight and Temperature Changes
The body weight of each mouse in all groups was recorded on day 0 and after infection on day 4. The rectal temperature of mice was measured with a digital thermometer to see the effect of the extract on the prevention of body temperature reduction due to malaria.36
Statistical Analysis
All data values are expressed as mean ± SEM for six mice per group. Statistical analyses were carried out by using SPSS Statistical software version 24. Statistical significance of mean of parasitemia suppression, weight, PCV, and survival time differences between groups was computed by one-way ANOVA, followed by post hoc Turkey’s Multiple Comparison Test. P-value of less than 0.05 was considered as statistically significant.
Results
The Percentage Yield of Plant Material Extraction
A total of 187 grams of dried leaves extract of Acanthus polystachyus was collected. In the preparation of the extract from the dried leaves of Acanthus polystachyus Delile (Acanthaceae), a yield of 14% was obtained.
Preliminary Phytochemical Screening
The phytochemical screening finding for the leaves extract of Acanthus polystachyus revealed the presence of tannins, phenols, flavonoids, terpenoids, glycosides, anthraquinones, saponins, and steroids. However, steroids and alkaloids were not present in the phytochemical screening (Table 1).
 |
Table 1 Phytochemical Constituent of Methanolic Crude Leaf Extract of A. polystachyus |
Antioxidant Activity of Hydromethanolic Leaves Extract of A. polystachyus
In the present study, the antioxidant activity of the leaves extract of Acanthus polystachyus was tested using DPPH free radical. The extract exhibited antioxidant activity as summarized in Figure 1. There was concentration-dependent free radical scavenging activity by the extract and the standard drug. Ascorbic acid was used as a standard drug for the determination of the antioxidant activity by DPPH method. The concentration of ascorbic acid and the Acanthus polystachyus varied from 50 to 1000µg/mL. The percentage inhibitions of the extract were: 27.43%, 35.67%, 50.02%, 65.43%, 72.32%, 79.86%, and 82.54% at 50, 100, 200, 400, 600, 800, and 1000μg/mL concentration of the extract, respectively. Likewise, the percentage inhibitions of ascorbic acid were: 35.21%, 50.21%, 65.43%, 77.53%, 86.43%, 90.42%, 96.83% at 50, 100, 200, 400, 600, 800, and 1000μg/mL concentration of the standard drug, respectively. Furthermore, the IC50 values of the extract and ascorbic acid were: 9.37μg/mL, and 2.31μg/mL, respectively (Figure 1).
Acute Toxicity Test
In acute oral toxicity test, the leaves extract of A. polystachyus revealed no mortality 2 gm/kg bodyweight concentration in mice. The mice did not also demonstrate any toxic effects like changes in behavioral activities such as anxiety, polyuria, diarrhea, seizures, and coma. Thus, the median lethal dose (LD50) of the extract of A. polystachyus is >2gm/kg.
Effect of the Extract on Blood Parasitemia and Mean Survival Time in 4-Day Suppressive Test
The current finding revealed that all tested doses (100mg/kg, 200mg/kg and 400mg/kg) of A. polystachyus extract exhibited significant parasitemia suppression when compared with the negative control group. The maximum suppression (49.25%, p < 0.0001) was revealed at the highest dose of the crude extract (400mg/kg) then by the middle dose of the extract (200mg/kg) (33.67%, p < 0.0001) and 100mg/kg (27.64%, p < 0.01) (Table 2). All doses of the extract (100mg/kg, 200mg/kg and 400mg/kg) were shown significant (p < 0.001, p < 0.0001, and p < 0.0001, respectively) variance in mean survival time when compared with the negative control group (Table 2).
 |
Table 2 Effect of A. polystachyus on Percentage Parasitemia and Survival Time of P. berghei Infected Mice in the 4-Day Suppressive Test |
Effect of the Extract on Packed Cell Volume, Body Weight, and Rectal Temperature in the 4-Day Suppressive Test
As summarized in Table 3, the crude extract at the doses of 200mg/kg and 400mg/kg significantly (p<0.05) prevented the loss of packed cell volume as compared to the negative control group. However, there was no significant difference at 100mg/kg of crude extract (p>0.05) as compared to the negative control.
 |
Table 3 Effect of A. polystachyus on the Body Weights, PCV, and Temperature of P. berghei Infected Mice in the 4-Day Suppressive Test |
The lower (100mg/kg), and the middle (200mg/kg) doses of A. polystachyus failed to protect the weight loss as compared to the negative control group. However, the higher (400mg/kg) dose of the extract and the standard drug exhibited a significant (p<0.05) protection of body weight loss as compared to the negative control group. The extract also produced a dose-dependent effect on preventing a drop in body temperature on day 4 after the infection. The middle (200mg/kg) and highest (400mg/kg) crude extract-treated group had showed significant (p<0.01, p < 0.0001, respectively) activity in prevention against rectal temperature reduction when compared to negative control group. Similarly, chloroquine 25mg/kg had shown significant (p<0.001) activity in prevention against body temperature reduction when compared to the negative control (Table 3).
Effect of the Extract on Blood Parasitemia and Mean Survival in Curative Test
In the curative test, the extract of A. polystachyus showed a dose-dependent parasitemia suppression (33.76%, 39.24%, and 53.59% at 100mg/kg, 200mg/kg and 400mg/kg, respectively). As summarized in Table 4, the extract of A. polystachyus exhibited a significant (p< 0.0001) curative action at the doses of 100mg/kg, 200mg/kg, and 400mg/kg on the 7th day from a thin blood smear. The mean survival time in the curative test was significant at the middle (200mg/kg, p<0.001) and highest dose (400mg/kg, p<0.0001) of crude extract as compared to the negative control. Similarly, the mean survival time of mice treated with the standard drug significantly (p<0.0001) extended when compared to the untreated group (Table 4).
 |
Table 4 Effect of A. polystachyus Crude Extract on Percentage Parasitemia and Survival Time of P. berghei Infected Mice in Rane’s Test |
Effect of the Extract on Packed Cell Volume, Body Weight, and Rectal Temperature in the Curative Test
All doses of the extract of A. polystachyus (100mg/kg, 200mg/kg, and 400mg/kg) significantly (p<0.0001) prevented the PCV reduction when compared to the untreated group (Table 5). Similarly, chloroquine 25 mg/kg significantly (p<0.0001) prevented the packed cell volume reduction when compared to the negative control group. Excluding the lower dose (100mg/kg), both the middle (200mg/kg) and higher (400mg/kg) doses of the extract exhibited a significant (p<0.01 and p<0.0001, respectively) increment of body weight as compared to the untreated group. Similarly, the standard drug showed significant (p<0.0001) increment of body weight change between the seventh and fourth day as compared to the untreated group. The extract at the dose of 200 mg/kg, 400 mg/kg, and chloroquine 25 mg/kg significantly (p<0.05, p<0.01, and p<0.001, respectively) prevents the drop of temperature compared to the untreated group. Though, the lower dose (100mg/kg) of the extract failed to prevent the drop of temperature as compared to the negative control group (Table 5).
 |
Table 5 Effect of A. polystachyus Crude Extract on the Body Weights, PCV, and Temperature of P. berghei Infected Mice in Rane’s Test |
Effect of the Crude Extract on Blood Parasitemia and Mean Survival Time in Prophylactic Test
The crude extract of A. polystachyus showed a significant parasitemia suppression (25%, p<0.001; 32.84%, p<0.0001; and 50%, p<0.0001), at 100mg/kg, 200mg/kg, and 400mg/kg doses the extract, respectively, as compared to the negative control. Likewise, the standard drug significantly (p<0.0001, 100%) suppressed the parasitemia level when compared with the negative control group (Table 6). Regarding the mean survival time, mice that received the middle dose (200 mg/kg) and the higher dose (400mg/kg) of the extract had longer MST (p<0.001, p<0.0001, respectively) as compared to the negative control group. However, the lower dose (100 mg/kg) of the extract of A. polystachyus did not show a significant difference in MST when compared with the negative control (Table 6).
 |
Table 6 Effect of A. polystachyus on Percentage Parasitemia and Survival Time of P. berghei Infected Mice in the Prophylactic Test |
Effect of the Crude Extract on Packed Cell Volume, Body Weight and Rectal Temperature in Prophylactic Test
All doses of the extract of A. polystachyus (100mg/kg, 200mg/kg, and 400mg/kg) significantly (p<0.01, p<0.001, and p<0.0001, respectively) prevented the loss of body weight when compared with the negative control group. Similarly, chloroquine 25 mg/kg significantly (p<0.0001) prevented weight loss as compared to the negative control. The higher (400mg/kg) dose of the crude extract showed a significant (p<0.01) effect on preventing a drop in body temperature as compared to the negative control. However, a drop in body temperature was not significantly prevented at 100mg/kg and 200mg/kg doses of the crude extract as compared to the negative control (Table 7). The standard drug still had a significant effect on preventing a drop in body temperature compared to the negative control All doses of the crude extract (100 mg/kg, 200mg/kg, and 400mg/kg) showed a significant (p<0.01, p<0.0001 and p<0.0001), respectively, effect on preventing the loss of packed cell volume when compared with the negative control group. Likewise, the standard drug showed a significant (p<0.0001) prevention of packed cell volume reduction when compared to the negative control group (Table 7).
 |
Table 7 Effect of A. polystachyus Crude Extract on the Body Weights, PCV, and Temperature of P. berghei Infected Mice in the Prophylactic Test |
Discussion
In-vivo models are usually applied in antimalarial studies since they allow the possible prodrug effect and likely boosting of the immune system of the body against the pathogen.37,38 The study conducted currently explored the phytochemical screening, antioxidant activity, acute oral toxicity study, and in vivo antimalarial activities of the crude extract of A. polystachyus.
In acute oral toxicity test, leaves extract of A. polystachyus revealed no mortality at the 2 gm/kg bodyweight concentration in mice. The mice did not also demonstrate any toxic effects like changes in behavioral activities such as anxiety, polyuria, diarrhea, seizures, and coma which received the extract. Thus, the median lethal dose (LD50) of the extract of A. polystachyus is >2gm/kg. This finding supports the tough evidence of the non-toxic outcome of the plant.39 This extract is therefore safe and this could explain the safe use of this plant by the local people who have been using it in the traditional treatment of malaria in Ethiopia.
Medicinal plants with antimalarial activity considered to be active should reduce parasitemia at least by 30%, which supports the finding of parasite inhibition in the current study.40 In 4-day suppressive test, the extract of A. polystachyus showed significant parasite suppression: 27.64% (p<0.001), 33.67% (p<0.0001), and 49.25 (p<0.0001) at doses of 100, 200 and 400mg/kg/day, respectively. In the curative test, significant (p<0.0001) parasitemia suppression: 33.76%, 39.24%, and 53.59% at 100mg/kg, 200mg/kg and 400mg/kg, respectively. In prophylactic test, 25%, p<0.001; 32.84%, p<0.0001; and 50%, p<0.0001, at 100mg/kg, 200mg/kg, and 400mg/kg doses of the extract, respectively, as compared to the negative control. Secondary metabolites such as terpenoids, flavonoids, saponins, alkaloids, tannins, glycosides, phenols, and anthraquinone have been implicated for their antiprotozoal and antimalarial activities in many pharmacological studies.41–44 The current phytochemical screening result revealed that the methanolic extract of A. polystachyus leaves possesses phytochemical constituents namely saponins, tannins, terpenoids, phenols, flavonoids, glycosides, steroids, and anthraquinones. Thus, the significant parasitemia suppression detected in the current study could be due to the presence of these phytoconstituents. The present finding is in line with previous similar studies.45–47 Furthermore, it has been conveyed that antioxidant activity can inhibit heme polymerization (the unpolymerized heme is very toxic for the Plasmodium species).48 In the current study, the leaves extract of A. polystachyus showed potent antioxidant activity. Hence, the observed antimalarial activity of the extract might be due to the antioxidant activity of A. polystachyus leaves extract. In the present study, the percentage suppression was higher in the curative test than in prophylactic test. This may be because of a rapid hepatic metabolism or clearance of the bioactive compounds accountable for ant plasmodial activities because of the administration of the extract initially for 4 days before the inoculation of the parasite. Compared to the standard drug, the extract of A. polystachyus showed less antimalarial activity. This could be because of low selectivity, unpurified/crude nature, poor bioavailability, slow absorption, other pharmacokinetics and pharmacodynamics parameters of the extract of A. polystachyus.
In all experimental protocol, the mean survival time was extended by the extract of A. polystachyus demonstrating suppression of the parasitemia which was due to the P.berghei infection. However, all doses of the extract (100, 200, and 400 mg/kg) failed to cure the P.berghei infection. The bioactive compound of medicinal plants that prolonged survival time greater than twelve days, it is considered as active.49
Bodyweight loss is one of the most common features in P. berghei infected mice.50 Therefore, a potential antimalarial plant product is expected to preserve these parameters in p. berghei infected mice due to the rise in the level of parasitemia.28,41,51 In the curative test and prophylactic test model, the middle (200mg/kg) and the higher (400mg/kg) doses of the extract exhibited significant (p<0.05) protective effect against weight loss as compared to the negative control. This effect could have been contributed from the overall improvement of packed cell volume and parasite clearance among extract-treated groups.27,52 However, the lower doses of the extract did not prevent body weight loss in all experimental protocol, although there was significant suppression of parasitemia. This indicates the contribution of other factors for these reductions beyond malaria infection. The weight loss could be due to catabolic action on stored lipids or anorexigenic effect of the extract that may lead to reduced food intake because of the presence of appetite-suppressant phytoconstituents in the extract.53 Thus, the extract of A. polystachyus may possess appetite-suppressing activity which could be attributed to flavonoids, saponins, and phenolic compounds.45,54,55 Notably, the present finding is in agreement with the previous finding.45 However, the finding of the current study on body weight was not consistent with previous similar studies.52,56,57
Hematological abnormalities and body temperature reduction are the common features of P. berghei infected rodents.58,59 P. berghei infection increases erythrocyte fragility and significantly reduces packed cell volume in mice.60 In all test models, the higher doses of the crude extract (200 and 400mg/kg) showed a significant (p<0.05) protective effect against temperature reduction as compared to the negative control. The possible mechanism for the prevention of body temperature reduction could be the reduced level of parasitemia as a reduction of temperature is directly related to the increased level of parasitemia. The packed cell volume was determined to assess the effectiveness of the extract of A. polystachyus in preventing hemolysis as a result of a rising parasitemia level. The basic causes of anemia in humans and rodents comprise, the clearance of uninfected RBC, dyserythropoiesis, erythropoietic suppression, and the clearance and/or destruction of infected RBCs. In the negative control group, the parasitemia level was increased, while the hematocrit packed cell volume was decreased noticeably from day to day till the death of the mice, which was also reported in previous similar studies.61,62
In vivo antimalarial activity can be classified as very good, good, and moderate if the plant extract showed the percent of parasite suppression ≥50% at the dose of 100mg, 250mg, and 500mg/kg body weight per day, respectively.63 According to this classification, the leaves extract of A. polystachyus revealed a moderate antimalarial activity.
Conclusion
The results obtained from the present study revealed that the extract of A. polystachyus has antiplasmodial activity as evidenced by their ability to suppress P. berghei infection in mice in a dose-dependent manner. The results also revealed free radical scavenging activity by the extract which may contribute to the antimalarial activity. This confirms the traditional antimalarial claim of A. polystachyus. Thus, the extract of A. polystachyus leaves might serve as a new source for the development of a novel antimalarial agent. However, further fractionation, isolation, and characterization of the extract are required to identify the accountable lead compound (s) with the potential of antimalarial activity.
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval
Ethical clearance was obtained from the research and ethics committee, department of pharmacology, University of Gondar to conduct the study in animal model with a Reference number of SOP 3/81/10. Experimental procedures were done on Swiss albino mice according to the internationally accepted laboratory animal use and care guideline (Guide for the care and use of laboratory animals). However, no consent was needed for this study.
Acknowledgment
The authors would like to acknowledge University of Gondar for funding and for allowing to use the laboratory facility.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Hailesilase GG, Rajeshwar Y, Hailu GS, Sibhat GG, Bitew H. In vivo antimalarial evaluation of crude extract, solvent fractions, and TLC-isolated compounds from olea europaea Linn subsp. cuspidata (Oleaceae). Evid Based Complement Alternat Med. 2020;2020:1–12. doi:10.1155/2020/6731485
2. Mace KE, Arguin PM, Tan KR. Malaria surveillance – United States, 2015. MMWR Surveill Summ. 2018;67(7):1. doi:10.15585/mmwr.ss6707a1
3. Barber BE, Rajahram GS, Grigg MJ, William T, Anstey NM. World Malaria Report: time to acknowledge Plasmodium knowlesi malaria. Malar J. 2017;16(1):1–3. doi:10.1186/s12936-017-1787-y
4. Organization WH. World Malaria Report. Geneva, Switzerland: World Health Organization; 2019.
5. Organization WH. Global Tuberculosis Report 2013. World Health Organization; 2013.
6. Deribew A, Dejene T, Kebede B, et al. Incidence, prevalence and mortality rates of malaria in Ethiopia from 1990 to 2015: analysis of the global burden of diseases 2015. Malar J. 2017;16(1):1–7. doi:10.1186/s12936-017-1919-4
7. Beyene HB, Telele NF, Mekuria AH. Knowledge, attitude and practice on malaria and associated factors among residents in Pawe district, north west Ethiopia: a cross-sectional study. Sci J Public Health. 2015;3(3):303–309. doi:10.11648/j.sjph.20150303.11
8. Jima D, Getachew A, Bilak H, et al. Malaria indicator survey 2007, Ethiopia: coverage and use of major malaria prevention and control interventions. Malar J. 2010;9(1):58. doi:10.1186/1475-2875-9-58
9. Nigatu T, Haileselassie B, Hailu S, Seyum D. Involving communities in the fight against malaria in Ethiopia. Afr Med Res Foundation Case Stud. 2009:1–26.
10. Deressa T, Mekonnen Y, Animut A. In vivo anti-malarial activities of Clerodendrum myricoides, Dodonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiop J Health Dev. 2010;24(1). doi:10.4314/ejhd.v24i1.62941
11. Ndiege I, Wanyonyi A. Chemical and Biological Studies on Traditional Anti-Malarial Plants from Meru and Kilifi Districts. Mst Plant and Microbial Sciences. 2012
12. Silva J, Ramos A, Machado M, et al. A review of antimalarial plants used in traditional medicine in communities in Portuguese-speaking countries: Brazil, Mozambique, Cape Verde, Guinea-Bissau, São Tomé and Príncipe and Angola. Mem Inst Oswaldo Cruz. 2011;106:142–158. doi:10.1590/S0074-02762011000900019
13. Assefa E, Alemayhu I, Endale M, Mammo F. Iridoid glycosides from the root of Acanthus sennii. J Pharm Pharmacogn Res. 2016;4(6):231–237.
14. Bukke S, Raghu P, Sailaja G, Kedam TR. The study on morphological, phytochemical and pharmacological aspects of Rhinacanthus nasutus. (L) kurz (A review). J Appl Pharm Sci. 2011;1(8):26–32.
15. Vlietinck A, Van Hoof L, Totte J, et al. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J Ethnopharmacol. 1995;46(1):31–47. doi:10.1016/0378-8741(95)01226-4
16. Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M Antimalarial medicinal plants used by Gumuz people of mandura woreda, benishangul-gumuz regional state, Ethiopia; 2016.
17. Meragiaw M, Asfaw Z. Review of antimalarial, pesticidal and repellent plants in the Ethiopian traditional herbal medicine. Res Rev J Herbal Sci. 2014;3(3):21–45.
18. Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110(3):516–525. doi:10.1016/j.jep.2006.10.011
19. Anza M, Worku F, Libsu S, Mamo F, Endale M. Phytochemical screening and antibacterial activity of leaves extract of Bersama abyssinica. J Adv Bot Zool. 2015;3(2):1–5.
20. Care IoLARCo, Animals UoL, Resources NIoHDoR. Guide for the Care and Use of Laboratory Animals. National Academies; 1985.
21. Leary SL, Underwood W, Anthony R, et al. AVMA Guidelines for the Euthanasia of Animals. American Veterinary Medical Association: 2013 Edition. 2013.
22. Trease G, Evans M. Text Book of Pharmacognosy.
23. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem. 2014;2(5).
24. Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106.
25. Brand-Williams W, Cuvelier M-E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi:10.1016/S0023-6438(95)80008-5
26. Woodward R. The Organisation for Economic Co-Operation and Development (OECD). Routledge; 2009.
27. Nureye D, Assefa S, Nedi T, Engidawork E. In vivo antimalarial activity of the 80% methanolic root bark extract and solvent fractions of Gardenia ternifolia Schumach. & Thonn. (Rubiaceae) against Plasmodium berghei. Evid Based Complement Alternat Med. 2018;2018:1–10. doi:10.1155/2018/9217835
28. Fentahun S, Makonnen E, Awas T, Giday M. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement Altern Med. 2017;17(1):13. doi:10.1186/s12906-016-1529-7
29. OCDE O. Acute oral toxicity: up and down procedure. In: OECD Guideline for the Testing of Chemicals. Vol. 425; 2008:1–2
30. Habte BM, Kebede T, Fenta TG, Boon H. Explanatory models of adult patients with type 2 diabetes mellitus from urban centers of central Ethiopia. BMC Res Notes. 2016;9(1):441. doi:10.1186/s13104-016-2248-3
31. Fidock DA, Rosenthal PJ, Croft SL, Brun§ R, Nwaka S Antimalarial drug discovery: efficacy models for compound screening (supplementary document). Antimalarial efficacy screening: in vitro and in vivo protocols Supplemental file. Available from: https://www.mmv.org/sites/default/files/uploads/docs/publications/8_-_supplementary_info_SCREENING_PDF_7.pdf.
32. Gebrehiwot S, Shumbahri M, Eyado A, Yohannes T. Phytochemical screening and in vivo antimalarial activity of two traditionally used medicinal plants of Afar Region, Ethiopia, against Plasmodium berghei in Swiss Albino Mice. J Parasitol Res. 2019;2019:1–8. doi:10.1155/2019/4519298
33. Peters W. Drug resistance in Plasmodium berghei Vincke and Lips, 1948. III. Multiple drug resistance. Exp Parasitol. 1965;17(1):97–102. doi:10.1016/0014-4894(65)90014-7
34. Zeleke G, Kebebe D, Mulisa E, Gashe F. Vivo antimalarial activity of the solvent fractions of fruit rind and root of Carica papaya Linn (Caricaceae) against Plasmodium berghei in Mice. J Parasitol Res. 2017;2017:1–9. doi:10.1155/2017/3121050
35. Belay WY, Endale Gurmu A, Wubneh ZB. Antimalarial activity of stem bark of periploca linearifolia during early and established plasmodium infection in Mice. Evid Based Complement Alternat Med. 2018;2018.
36. Dikasso D, Makonnen E, Debella A, et al. Anti-malarial activity of withania somnifera L. Dunal extracts in mice. Ethiop Med J. 2006;44(3):279.
37. Hilou A, Nacoulma O, Guiguemde T. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J Ethnopharmacol. 2006;103(2):236–240. doi:10.1016/j.jep.2005.08.006
38. Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Supplementary documents. Trends Parasitol. 2004;15:19–29.
39. Organization WH. The guidebook to the registration of public health pesticides and repellents against vectors: hazard classification-acute LD50 values of formulated products. In:2019.
40. Carvalho L, Brandao M, Santos-Filho D, Lopes J, Krettli A. Antimalarial activity of crude extracts from Brazilian plants studied in vivo in Plasmodium berghei-infected mice and in vitro against Plasmodium falciparum in culture. Braz J Med Biol Res. 1991;24(11):1113–1123.
41. Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern Med. 2014;14(1):79. doi:10.1186/1472-6882-14-79
42. Alkandahri M, Berbudi A, Utami NVU, Subarnas A. Antimalarial activity of extract and fractions of Castanopsis costata (Blume) A. DC. Avicenna J Phytomed. 2019.
43. Abdela J, Engidawork E, Shibeshi W. In vivo antimalarial activity of solvent fractions of the leaves of justicia schimperiana hochst. Ex Nees against Plasmodium berghei in Mice. Ethiop Pharm J. 2014;30(2):95–108.
44. Frederich M, Tits M, Angenot L. Potential antimalarial activity of indole alkaloids. Trans R Soc Trop Med Hyg. 2008;102(1):11–19. doi:10.1016/j.trstmh.2007.10.002
45. Kifle ZD, Adinew GM, Mengistie MG, et al. Evaluation of antimalarial activity of methanolic root extract of Myrica salicifolia A Rich (Myricaceae) against Plasmodium berghei–infected mice. J Evid-Based Integr Med. 2020;25:2515690X20920539. doi:10.1177/2515690X20920539
46. Tadesse SA, Wubneh ZB. Antimalarial activity of Syzygium guineense during early and established Plasmodium infection in rodent models. BMC Complement Altern Med. 2017;17(1):21. doi:10.1186/s12906-016-1538-6
47. Girma S, Giday M, Erko B, Mamo H. Effect of crude leaf extract of Osyris quadripartita on Plasmodium berghei in Swiss albino mice. BMC Complement Altern Med. 2015;15(1):184. doi:10.1186/s12906-015-0715-3
48. Vial H. Recent developments and rationale towards new strategies for malarial chemotherapy. Parasite. 1996;3(1):3–23. doi:10.1051/parasite/1996031003
49. Ural IO, Kayalar H, Durmuskahya C, Cavus I, Ozbilgin A. In vivo antimalarial activity of methanol and water extracts of Eryngium thorifolium Boiss (Apiaceae Family) against P. berghei in infected mice. Trop J Pharm Res. 2014;13(8):1313–1317. doi:10.4314/tjpr.v13i8.16
50. Oluwakanyinsola A, Tijani A, Babayi H, Nwaeze A, Anagbogu R, Agbakwuru V. Anti-malarial activity of ethanolic stem bark extract of Faidherbia Albida (Del) a. Chev (Mimosoidae) in mice. Arch Appl Sci Res. 2010;2:261–268.
51. Hintsa G, Sibhat GG, Karim A. Evaluation of antimalarial activity of the leaf latex and TLC isolates from Aloe megalacantha Baker in Plasmodium berghei infected mice. Evid Based Complement Alternat Med. 2019;2019:1–9. doi:10.1155/2019/6459498
52. Mengiste B, Makonnen E, Urga K. Invivo antimalarial activity of Dodonaea Angustifolia seed extracts against Plasmodium berghei in mice model. Momona Ethiop J Sci. 2012;4(1):47–63. doi:10.4314/mejs.v4i1.74056
53. Basir R, Rahiman SF, Hasballah K, et al. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran J Parasitol. 2012;7(4):62.
54. Philippe G, Christiane D, Isabelle F. Advances in antimalarial drug evaluation and new targets for antimalarials. Malaria Parasites. 2012:320–350.
55. Asrade S, Mengesha Y, Moges G, Gelayee DA. In vivo antiplasmodial activity evaluation of the leaves of Balanites rotundifolia (Van Tiegh.) Blatter (Balanitaceae) against Plasmodium berghei. J Exp Pharmacol. 2017;9:59. doi:10.2147/JEP.S130491
56. Eyasu M, Shibeshi W, Giday M. In vivo antimalarial activity of hydromethanolic leaf extract of Calpurnia aurea (Fabaceae) in Mice infected with chloroquine sensitive Plasmodium berghei. Int J Pharm. 2013;2(9):131–142.
57. Chinchilla M, Guerrero OM, Abarca G, Barrios M, Castro O. An in vivo model to study the anti-malaric capacity of plant extracts. Revista De Biología Tropical. 1998;46(1):35–39.
58. Adugna M, Feyera T, Taddese W, Admasu P. In vivo antimalarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in mice. Global J Pharmacol. 2014;8(3):460–468.
59. Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. Malarial anemia: of mice and men. Blood. 2007;110(1):18–28.
60. Iyawe H, Onigbinde A. Impact of Plasmodium berghei and chloroquine on haematological and antioxidants indices in mice. Asian J Biochem. 2009;4(1):30–35. doi:10.3923/ajb.2009.30.35
61. Okokon J, Ofodum K, Ajibesin K, Danladi B, Gamaniel K. Pharmacological screening and evaluation of antiplasmodial activity of Croton zambesicus against Plasmodium berghei berghei infection in mice. Indian J Pharmacol. 2005;37(4):243. doi:10.4103/0253-7613.16571
62. Ayodele T Studies on Azadricha indica in malaria.
63. Deharo E, Bourdy G, Quenevo C, Munoz V, Ruiz G, Sauvain M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part V. Evaluation of the antimalarial activity of plants used by the Tacana Indians. J Ethnopharmacol. 2001;77(1):91–98. doi:10.1016/S0378-8741(01)00270-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



