Back to Journals » Drug Design, Development and Therapy » Volume 10
Anti-inflammatory effects of guggulsterone on murine macrophage by inhibiting LPS-induced inflammatory cytokines in NF-κB signaling pathway
Authors Zhang J, Shangguan Z, Chen C, Zhang H, Lin Y
Received 19 January 2016
Accepted for publication 14 March 2016
Published 1 June 2016 Volume 2016:10 Pages 1829—1835
DOI https://doi.org/10.2147/DDDT.S104602
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Jin-Hua Zhang,1,2,* Zhao-Shui Shangguan,3,* Chao Chen,4 Hui-Jie Zhang,4 Yi Lin1
1College of Chemical Engineering, Huaqiao University, 2Department of Pharmacy, Xiamen Medical College, 3Central Laboratory, The First Affiliated Hospital of Xiamen University, 4Xiamen Diabetes Institute, The First Affiliated Hospital of Xiamen University, Xiamen, People’s Republic of China
*These authors contributed equally to this work
Abstract: The present study was aimed to investigate the effects of guggulsterone (GS) on proinflammatory responses as well as the underlying molecular mechanisms in macrophage upon lipopolysaccharide (LPS) stimulation. Effects of GS on viability of Raw264.7 cells were examined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Real-time polymerase chain reaction (PCR) was employed to examine the mRNA expression of cytokines, including interleukin 1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and inducible nitric oxide synthase (iNOS). Phosphorylations of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinases (p38), and inhibitor of nuclear factor kappaB (IκB) were determined using immunoblotting. The results revealed that GS was not toxic to Raw264.7 cells at designated concentrations. We demonstrated that GS significantly suppressed the elevated mRNA expression of proinflammatory cytokines, including IL-1β, TNF-α, and iNOS in a dose-dependent manner. GS treatment reduced the level of IκB phosphorylation in LPS-stimulated macrophages in a dose-dependent manner. Use of BAY 11-7082, an inhibitor of nuclear factor-kappaB (NF-κB), led to significantly suppressing effects on IL-1β and TNF-α expression similar as that of GS-treated cells. Our findings suggest that GS possesses anti-inflammatory activity, which may be attributed to downregulation of iNOS and inhibition of NF-κB activity in LPS-stimulated Raw264.7 cells.
Keywords: Anti-inflammatory effects, guggulsterone, lipopolysaccharides, NF-κB, IL-1β, TNF-α
Introduction
Inflammation is considered as a protective response against diverse pathogens or deteriorating stimuli. It is tightly regulated by an orchestra of cellular and soluble mediators. Inflammatory responses are initiated and propagated by cellular sensing systems such as toll-like receptor system (TLR) and production of inflammatory mediators such as inducible nitric oxide (NO), interleukin 1β (IL-1β), and tumor necrosis factor-alpha (TNF-α).1 These soluble mediators play crucial role in controlling inflammation and tissue repair; however, aberrant production may exacerbate the damages.
Macrophages play a pivotal role in inflammatory process. Upon inflammation, these phagocytic cells are activated depending on stimuli and molecular pattern of recognition.2 Activation of macrophage through pattern recognition receptor such as TLR leads to the production of a variety of mediators, including NO, TNF-α, and IL-1β.3 Macrophage-derived NO is synthesized by inducible NO synthase (iNOS). Excessive production of NO contributes to the pathogenesis of chronic inflammatory disorders.4,5 Additionally, TNF-α and IL-1β are produced in activated macrophages, in turn, to facilitate and amplify cytokines and chemokine production in chronic inflammatory setting. Lipopolysaccharide (LPS), a component of Gram-negative bacteria cell wall, is known as the ligand of TLR4. Recognition of LPS by TLR4 in macrophages initiates downstream signaling pathways including nuclear factor-kappaB (NF-κB) complex and mitogen-activated protein kinases (MAPKs), such as p38 MAPKs (p38), c-Jun N-terminal kinase (JNK), and extracellular-signal regulated kinase (ERK).6,7 NF-κB is reported to play a critical role in acute and chronic inflammatory conditions. It is considered as a potential target for anti-inflammatory drug development.
Guggulsterone (GS) is a phytosterol that is found enriched in Commiphora mukul. It is reported as an antagonist of farnesoid X receptor and demonstrated hypolipidemic activity.8 GS has been demonstrated to exert a range of pharmacological activities, including antineoplastic, antioxidation, antidiabetic, and cardioprotection.9–13 GS attenuates colitis in mice through inhibition of NF-κB activation.14,15 Researches have shown that GS inhibits proliferation of tumor cells through induction of apoptosis and inhibition of NF-κB signaling pathway.16–18 It is of interest to determine the effects of GS on LPS-induced inflammation in lymphocytes.
In this study, we investigated the anti-inflammatory effects and the underlying mechanism of GS, in particular gene expression of iNOS, IL-1β, and TNF-α in LPS-stimulated Raw264.7 cells. We also examined the role of NF-κB in LPS-induced inflammatory response in macrophages.
Materials and methods
Cell culture
Murine macrophage-like cell line (Raw264.7) was obtained from ATCC and incubated in complete Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) containing 0.1% sodium bicarbonate, 2 mM glutamine, 100 U/mL penicillin G, streptomycin (100 μg/mL), and 10% fetal bovine serum (FBS) at 37°C. For GS treatments, Raw264.7 cells were seeded and incubated overnight prior to the treatments. Cells were treated with GS (0, 1, 5, 10, and 25 μM) for 24 hours (cell viability assay), 2 hours (real-time polymerase chain reaction [PCR] analysis), and 4 hours (immunoblotting), respectively, with or without a subsequent exposure to 1 μg/mL LPS. GS samples were prepared and added to the culture medium at a final concentration of 0.1% (v/v) in dimethyl sulfoxide (DMSO). DMSO with a final concentration of 0.1% was used as blank control.
Cell viability
Raw264.7 cells were seeded and incubated overnight prior to the treatments and then was followed by a treatment with GS (0, 1, 5, 10, and 25 μM) for 24 hours. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. In brief, 10 μL of MTT solution (5 mg/mL in complete DMEM) was added to the medium followed by an incubation time of 4 hours at 37°C. Following aspiration of the medium, cells were lyzed with 2-propanol which solubilized intracellular formazan. The optical density of formazan was measured using a microplate reader at a wavelength of 570 nm.
Real-time PCR
Raw264.7 cells were seeded at a concentration of 1×106 cells/mL and incubated overnight prior to the treatments. Cells were treated with GS (0, 1, 5, 10, and 25 μM) for 2 hours followed by an exposure to 1 μg/mL LPS for 2 hours. Total RNA was isolated from each sample using RNA simple Total RNA Kit (Tiangen, BJ, People’s Republic of China) as per the instruction of manufacturer. The resulting RNA was used as a template for generating first-strand cDNA synthesis using ReverTra Ace Kit (Toyobo, Osaka, Japan). The sequences of primers used for reverse transcription PCR (RT-PCR) are shown in Table 1. RT-PCR experiments were carried out using real-time PCR Master Mix (Toyobo) in triplicates for each sample. The threshold cycle numbers were determined based on the ΔΔCT relative value and the cycle number was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The PCR products were examined and confirmed for the size using agarose gel electrophoresis.
Immunoblot assay
Raw264.7 cells were seeded at a concentration of 1×106 cells/mL and incubated overnight prior to the treatments. Cells were treated with GS (0, 1, 5, 10, and 25 μM) for 4 hours followed by an exposure to 1 μg/mL LPS for 30 minutes. After treatments, cells were harvested and washed twice with ice-cold PBS, followed by lysis using RIPA buffer (Thermo Fischer Scientific, Waltham, MA, USA). The resulting lysates were subjected to centrifugation at 13,000 rpm for 10 minutes at 4°C. The supernatants were obtained and protein concentrations were measured using BCA protein assay kit (Pierce, Rockford, IL, USA). Thirty micrograms of protein was loaded in each lane and was subjected to 12.5% SDS-PAGE followed by a transfer onto a polyvinylidene difluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA). Resulting blots were incubated with 5% (w/v) skimmed milk in PBS followed by incubation with 1/1,000 dilution of antibodies against inhibitor of NF-κB (IκB), phosphorylated IκB (p-IκB), JNK, phosphorylated-JNK (p-JNK), p38, phosphorylated p38 (p-p38), ERK1/2, and phosphorylated ERK1/2 (p-ERK1/2) (Cell Signaling Technologies, Danvers, MA, USA) as well as GAPDH (Santa Cruz Biotechnology Inc., Dallas, TX, USA). The antigen–antibody complexes were unveiled using 1/2,000 dilution of peroxidase-conjugated secondary antibodies (Abcam, Cambridge, UK) and a chemiluminescence substrate (EMD Millipore).
Statistical analyses
All the data were presented as mean ± standard deviation (SD) of triplicate experiments. A one-way analysis of variance (ANOVA) with a Duncan multiple-comparison test was utilized to determine statistical differences among the groups. P-values <0.05 were considered statistically significant.
Results
Effects of GS on cell viability of murine macrophage Raw264.7 cells
To determine the cytotoxic effects of GS, Raw264.7 cells were exposed to GS at serial concentrations (0, 1, 5, 10, and 25 μM) and determined for the viability using MTT assay. We found that GS exerted nontoxic effects on Raw264.7 cells at designated concentrations as high as 25 μM (data not shown).
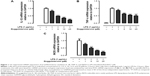
GS inhibited LPS-induced mRNA expression of IL-1β, TNF-α, and iNOS in Raw264.7 cells
We next investigated whether treatment with GS alters the expression of proinflammatory cytokines in LPS-stimulated macrophage. mRNA expressions of IL-1β, TNF-α, and iNOS in LPS-treated Raw264.7 cells were determined using real-time PCR. Results of real-time PCR analysis revealed that mRNA expressions of IL-1β, TNF-α, and iNOS in Raw264.7 cells were significantly enhanced in the presence of LPS, and the elevated mRNA expressions were suppressed in response to GS pretreatment in a dose-dependent manner (Figure 1).
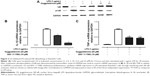
GS inhibited LPS-induced NF-κB pathway in Raw264.7 cells
It is evident that GS exerts its pharmacological effects through manipulating NF-κB pathway. Phosphorylation of IκB, NF-κB inhibitor, is concomitantly required with nuclear translocation of NF-κB. We hence investigated the effect of GS on LPS-induced phosphorylation of IκB in Raw264.7 macrophages. The results showed that Raw264.7 cells exhibited an increased phosphorylation of IκB in response to LPS stimulation (Figure 2A). The elevated IκB phosphorylation was inhibited by GS in a dose-dependent manner. Inhibition of NF-κB using BAY 11-7082 (10 μM), a commercially available inhibitor of NF-κB, led to a significant decrease in the LPS-induced IL-1β expression, whereas GS showed comparatively less suppression effects (Figure 2B). GS treatment significantly decreased the expression of TNF-α in LPS-stimulated macrophages, while BAY 11-7082 also exerted similar inhibitory activity (Figure 2C).
Effects of GS on phosphorylation of ERK1/2, JNK, and p38 in LPS-stimulated Raw264.7 cells
Activation of ERK1/2, JNK, and p38 is suggested to be involved in excessive production of proinflammatory cytokines in presence of LPS. We examined the effects of GS on phosphorylation of ERK1/2, JNK, and p38 in LPS-stimulated Raw264.7 cells. As shown in Figure 3, exposure to LPS led to significantly increased phosphorylation of ERK1/2, JNK, and p38 in Raw264.7 cells. Treatment of LPS-stimulated Raw264.7 cells with GS at 1, 2.5, 5, 10, 12.5, and 25 μM concentrations resulted in no change in the levels of p-ERK1/2, p-JNK, and p-p38 in comparison to LPS alone.
Discussion
In the present study, we demonstrated that GS significantly suppressed elevated production of proinflammatory mediators in LPS-stimulated Raw264.7 cells. The GS-induced downregulation of chemokine expression was associated with inhibition of NF-kB activation induced by LPS in macrophages. Moreover, GS had no effects on the LPS-induced phosphorylation of MAP kinase family members, including ERK1/2, JNK, and p38.
Macrophages are known to play a fundamentally critical role in the inflammatory response through producing a variety of mediators and proinflammatory cytokines depending on stimuli. A prolonged activation of macrophages results in a dysregulated inflammatory mediator production, leading to a vicious cycle of chronic inflammation.19 LPS is recognized by TLR4 in association with MD2 and CD14 in macrophages. LPS-induced activation of TLR4 signaling is known to trigger the release and mRNA accumulation of critical proinflammatory cytokines, including IL-1β, TNF-α, and iNOS.20–22 We showed that the upregulated mRNA expression of IL-1β, TNF-α, and iNOS in LPS-stimulated Raw264.7 cells was abolished in the presence of GS. The finding is consistent with previous research in which GS suppresses the activation of transcription factor IRF3 induced by LPS.23 The LPS-induced activation of TLR4 leads to both early and late activation of NF-κB through MyD88- and TRIF-dependent signaling pathways, respectively.24,25 Our data showed that GS was unable to fully exert its inhibitory effects on TNF-α expression in LPS-stimulated Raw264.7 cells. It is indicated that the inhibitory effect of GS in LPS-stimulated macrophages might be attributed to a later step in the NF-κB activation cascade.
NF-κB is a ubiquitous transcription factor, which has a central role in LPS-induced inflammatory responses. In resting macrophages, NF-κB is inactive and sequestered in the cytoplasm through binding with IκB. Interaction of LPS with TLR4 leads to the activation of NF-κB through phosphorylation and degradation of IκB, followed by nuclear translocation of NF-κB.26 Phosphorylation of IκB is catalyzed by IκB kinase (IKK) complex. We found that LPS induced a significantly increased level of p-IκB in the Raw264.7 cells, which was restored by GS treatment. Corresponding to the changes in IκB phosphorylation, LPS-induced elevation of IL-1β and TNF-α mRNA expression was reduced in response to GS treatment. These data suggest that GS exerts anti-inflammatory activity through inhibition of NF-κB activation. Use of IκB inhibitor, BAY 11-7082, which blocks phosphorylation of IκB, resulted in a relatively low expression of IL-1β compared with that of GS-treated cells, whereas GS and BAY 11-7082 shared similar suppression effects on TNF-α expression. It is known that LPS induces biphasic activation of NF-κB.26 LPS-induced early NF-κB activity initiates production of proinflammatory cytokines such as TNF-α and IL-1β, which in turn induce the late NF-κB activation.26,27 Our results suggest that GS suppressed LPS-induced inflammatory response through interfering with late NF-κB activation.
MAPK signaling pathway has been reported to be involved in the regulation of proinflammatory cytokine expression in activated macrophages. In the presence of LPS, signal transduction is initiated with the formation of LPS/TLR/MD2 complex, leading to activation of MAPK pathway cascade.7,28 Researches have shown that p38 MAPK pathway is associated with expression of TNF-α, IL-1β, IL-6, and IL-8.29–31 In our study, ERK1/2, JNK, and p38 were activated in LPS-stimulated Raw264.7 cells in parallel with elevated expression of proinflammatory cytokines. GS treatment showed no alteration in levels of p-ERK, p-JNK, or p-p38, but increased expression of cytokines was abolished. It is suggested that anti-inflammatory property of GS is not mediated by ERK, JNK, or p38 MAPK pathways in part.
Conclusion
In conclusion, we provide evidence highlighting the immunomodulatory activity of GS via the suppression of NF-κB activation but not ERK, JNK, or p38 MAPK pathways in LPS-treated Raw264.7 cells. Our results are expected to contribute to the understanding of mechanism of regulating chronic inflammation, such as sepsis, by using a natural plant component.
Acknowledgments
This study was supported by grants from the Xiamen Medical College, Young Foundation of Fujian Provincial Health Department (2010-2-68), Young Talent Foundation of Fujian Provincial Health Department (2013-ZQN-ZD-31), and Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (ZQN-YX205).
Disclosure
The authors report no conflicts of interest in this work.
References
Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147:199–207. | ||
Moonis M, Ahmad I, Bachhawat BK. Macrophages in host defence – an overview. Indian J Biochem Biophys. 1992;29:115–122. | ||
Schwartz Y, Svistelnik AV. Functional phenotypes of macrophages and the M1-M2 polarization concept. Part I. Proinflammatory phenotype. Biochemistry (Mosc). 2012;77:246–260. | ||
Castranova V. Role of nitric oxide in the progression of pneumoconiosis. Biochemistry (Mosc). 2004;69:32–37. | ||
MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. | ||
An H, Xu H, Yu Y, et al. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-kappaB, ERK and p38 MAPK signal pathways. Immunol Lett. 2002;81:165–169. | ||
Bode JG, Ehlting C, Haussinger D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 2012;24:1185–1194. | ||
Urizar NL, Liverman AB, Dodds DT, et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–1706. | ||
Samudio I, Konopleva M, Safe S, et al. Guggulsterones induce apoptosis and differentiation in acute myeloid leukemia: identification of isomer-specific antileukemic activities of the pregnadienedione structure. Mol Cancer Ther. 2005;4:1982–1992. | ||
Wang X, Greilberger J, Ledinski G, et al. The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis. 2004;172:239–246. | ||
Wang WC, Uen YH, Chang ML, et al. Protective effect of guggulsterone against cardiomyocyte injury induced by doxorubicin in vitro. BMC Complement Altern Med. 2012;12:138. | ||
Chander R, Rizvi F, Khanna AK, et al. Cardioprotective activity of synthetic guggulsterone (E and Z-isomers) in isoproterenol induced myocardial ischemia in rats: a comparative study. Indian J Clin Biochem. 2003;18:71–79. | ||
Sharma B, Salunke R, Srivastava S, et al. Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food Chem Toxicol. 2009;47:2631–2639. | ||
Kim JM, Kim SH, Ko SH, et al. The guggulsterone derivative GG-52 inhibits NF-kappaB signaling in gastric epithelial cells and ameliorates ethanol-induced gastric mucosal lesions in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G193–G202. | ||
Mencarelli A, Renga B, Palladino G, et al. The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Biochem Pharmacol. 2009;78:1214–1223. | ||
Noh EM, Chung EY, Youn HJ, et al. Cis-guggulsterone inhibits the IKK/NF-kappaB pathway, whereas trans-guggulsterone inhibits MAPK/AP-1 in MCF7 breast cancer cells: guggulsterone regulates MMP9 expression in an isomer-specific manner. Int J Mol Med. 2013;31:393–399. | ||
Yang MH, Lee KT, Yang S, et al. Guggulsterone enhances antitumor activity of gemcitabine in gallbladder cancer cells through suppression of NF-kappaB. J Cancer Res Clin Oncol. 2012;138:1743–1751. | ||
Macha MA, Matta A, Chauhan SS, et al. Guggulsterone (GS) inhibits smokeless tobacco and nicotine-induced NF-kappaB and STAT3 pathways in head and neck cancer cells. Carcinogenesis. 2011;32:368–380. | ||
Morimoto Y, Izumi H, Kuroda E. Significance of persistent inflammation in respiratory disorders induced by nanoparticles. J Immunol Res. 2014;2014:962871. | ||
Rossol M, Heine H, Meusch U, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. | ||
Ferwerda B, McCall MB, Verheijen K, et al. Functional consequences of toll-like receptor 4 polymorphisms. Mol Med. 2008;14:346–352. | ||
Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. | ||
Youn HS, Ahn SI, Lee BY. Guggulsterone suppresses the activation of transcription factor IRF3 induced by TLR3 or TLR4 agonists. Int Immunopharmacol. 2009;9:108–112. | ||
Kizaki T, Shirato K, Sakurai T, et al. Beta2-adrenergic receptor regulate Toll-like receptor 4-induced late-phase NF-kappaB activation. Mol Immunol. 2009;46:1195–1203. | ||
Kang YJ, Kim SO, Shimada S, et al. Cell surface 4-1BBL mediates sequential signaling pathways ‘downstream’ of TLR and is required for sustained TNF production in macrophages. Nat Immunol. 2007;8:601–609. | ||
Han SJ, Ko HM, Choi JH, et al. Molecular mechanisms for lipopolysaccharide-induced biphasic activation of nuclear factor-kappa B (NF-kappa B). J Biol Chem. 2002;277:44715–44721. | ||
Han Y, Brasier AR. Mechanism for biphasic rel A. NF-kappaB1 nuclear translocation in tumor necrosis factor alpha-stimulated hepatocytes. J Biol Chem. 1997;272:9825–9832. | ||
Rao KM. MAP kinase activation in macrophages. J Leukoc Biol. 2001; 69:3–10. | ||
Yougbare I, Boire G, Roy M, et al. NCS 613 exhibits anti-inflammatory effects on PBMCs from lupus patients by inhibiting p38 MAPK and NF-kappaB signalling pathways while reducing proinflammatory cytokine production. Can J Physiol Pharmacol. 2013;91:353–361. | ||
Armstrong J, Harbron C, Lea S, et al. Synergistic effects of p38 mitogen-activated protein kinase inhibition with a corticosteroid in alveolar macrophages from patients with chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2011;338:732–740. | ||
Neuder LE, Keener JM, Eckert RE, et al. Role of p38 MAPK in LPS induced pro-inflammatory cytokine and chemokine gene expression in equine leukocytes. Vet Immunol Immunopathol. 2009;129:192–199. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.