Back to Journals » International Journal of Women's Health » Volume 7
Anti-inflammatory, antiangiogenic, and apoptosis-inducing activity of DLBS1442, a bioactive fraction of Phaleria macrocarpa, in a RL95-2 cell line as a molecular model of endometriosis
Authors Tandrasasmita O, Sutanto A, Arifin P, Tjandrawinata R
Received 18 September 2014
Accepted for publication 18 November 2014
Published 3 February 2015 Volume 2015:7 Pages 161—169
DOI https://doi.org/10.2147/IJWH.S74552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Olivia M Tandrasasmita, Adeline M Sutanto, Poppy F Arifin, Raymond R Tjandrawinata
Section of Molecular Pharmacology, Research Innovation and Invention, Dexa Laboratories of Biomolecular Sciences, PT Dexa Medica, Cikarang, West Java, Indonesia
Abstract: DLBS1442 is a bioactive fraction extracted from the fruit of the native Indonesian plant, Phaleria macrocarpa (Scheff.) Boerl (Thymelaceae). This bioactive fraction is a potential treatment for dysmenorrhea and endometriosis. The present study investigated the pharmacological action of DLBS1442 in endometrial cells. The effect of various doses of DLBS1442 (0–200 µg/mL) over 24 hours was studied using the human endometrial RL95-2 cell line to observe its effect on angiogenesis, cell migration, estrogen and progesterone receptor levels, the eicosanoid pathway, cell viability, and apoptosis. The impact of DLBS1442 on nuclear factor kappa B (NFκB) and the eicosanoid pathway was also studied through its marker gene expression using a quantitative real-time polymerase chain reaction method. DLBS1442 showed an ability to inhibit angiogenesis and cell migration in a dose-dependent manner. At a dose of 100 µg/mL, DLBS1442 increased the cell population in sub-G1 phase from 7% to 34%. DLBS1442 also significantly downregulated the estrogen receptor level and upregulated the progesterone receptor level. Further, it inhibited the eicosanoid signaling pathway by reducing the NFκB transcription level and subsequent reduction of inducible nitric oxide synthase. A dose-dependent decrease in viability and increased apoptosis in RL95-2 cells were also evident after exposure to DLBS1442, where the IC50 was obtained at around 100 µg/mL. In conclusion, DLBS1442 is a potential agent for alleviating symptoms of endometriosis via its antiangiogenic, anti-inflammatory, and proapoptotic activity.
Keywords: progesterone receptor, estrogen receptor, eicosanoid pathway, anti-inflammatory
Introduction
Endometriosis is a noncancerous condition in which tissue that normally lines a woman’s uterus appears elsewhere in the body, particularly in the fallopian tubes, ovaries, rectum, bladder, and other pelvic tissues. It is acknowledged to be the most frequent cause of pelvic pain among women in their reproductive years. The disease has been estimated to affect 5%–10% of women of reproductive age and as many as 70% of women with infertility or chronic pelvic pain.1 In a series of 2,080 infertile women, 1,263 (60.7%) were diagnosed with endometriosis.2
In endometriosis, the eutopic endometrium exhibits multiple subtle but biologically important molecular abnormalities, including biosynthetic cascades that result in increased production of cytokines, prostaglandins, and metalloproteinases.1 Upregulation of these proteins may lead to symptoms such as chronic pain, dyspareunia, fatigue, and infertility. Although many attempts have been made to treat endometriosis, many women still suffer from this condition, and the number of endometriosis cases remains high throughout the world. Therefore, a new efficacious agent to help treat this disease is urgently needed. In this regard, the use of natural products would be one promising method for treating endometriosis.
DLBS1442 is a bioactive fraction in Phaleria macrocarpa (Sheff.) Boerl (Thymelaceae). The results of our previous study of the bioactive fractions in P. macrocarpa indicated that these fractions could potentially be used to treat medical conditions such as endometriosis.3,4 Previously, we also conducted a clinical trial demonstrating that DLBS1442 was able to help treat the symptoms of primary dysmenorrhea in premenstrual syndrome.5 In light of our earlier findings, we undertook this study to elucidate further the mechanism of action of DLBS1442 at the cellular level in endometrial cells. We investigated the effects of DLBS1442 on expression of genes that encode critical enzymes associated with the onset of endometriosis in a human endometrial (RL95-2) cell line. Our research focused on investigating a potential bioactive fraction that targets the cell proliferation response, proapoptotic activity, and expression of angiogenesis-regulating factor, and examining its relationship with expression of inflammatory genes and sex hormone receptor-related mechanisms.
Materials and methods
Preparation of DLBS1442
P. macrocarpa was collected from Central Java, Indonesia, identified in the Herbarium Bogoriense, Research Center for Biology, Indonesian Institute of Sciences, and issued with certificate 261/IPH.1.02/If.8/XII/2009. The bioactive fraction (DLBS1442) was prepared in the manner described by Tjandrawinata.5
Cell cultures and treatment with DLBS1442
The human endometrial epithelial cell line (RL95-2) was purchased from the American Type Culture Collection (Rockville, MD, USA). RL95-2 cells were cultured in Dulbecco’s Modified Eagle’s Medium/F12 basal medium (Gibco, Carlsbad, CA, USA) with 10% fetal calf serum (Gibco), 1% penicillin–streptomycin (Gibco), and 0.1% insulin (Gibco). The culture was incubated at 37°C in a 5% CO2 atmosphere. The medium was replaced every 2–3 days until the cells reached 80% confluence. The culture was then subcultivated at a ratio of 1:4-1:6 using 1 mL of trypsin-ethylenediaminetetraacetic acid (Gibco) and plated in 6/24/96 well. For ribonucleic acid (RNA) analysis, cells were plated on 6-well plates. For cell migration scratch assay, cells were plated in 10 cm dishes. While for fluorescence-activated cell-sorting (FACS) analysis, cells were plated in 24-well plate. For the in vitro cell-viability assay, cells were plated on 96-well plates (Iwaki, Tokyo, Japan). Prior to treatment, the cells were maintained in serum-free medium. Treatment with DLBS1442 was given at various concentrations in the range of 0–200 μg/mL. The experiment was repeated twice for each concentration tested. The duration of treatment was 21–24 hours.
RNA analysis
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The RNA concentration was quantified using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Expression of several target genes was determined using a quantitative real-time polymerase chain reaction (RT-PCR) assay in 25 μL solution consisting of 12.5 μL of iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA), 0.8 μM of each targeted primer, and 0.1 μM of each internal control primer. Quantitative RT-PCR was performed using a Mini Opticon MJ Mini™ system (Bio-Rad Laboratories) under optimum conditions for each primer.
Gelatin zymography
The activity of matrix metalloproteinase (MMP) in the total protein isolated from DLBS1442-treated RL95-2 cells was measured by gelatin zymography. The protein sample was separated in nonreducing sodium dodecyl sulfate polyacrylamide gel electrophoresis with 0.2% gelatin substrate incorporated into the gel. The gel was then washed in 2.5% Triton™ X-100 (Sigma, St Louis, MO, USA) for one hour followed by overnight incubation in reaction buffer (50 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 0.15 M NaCl) at a temperature of 37°C. Finally, the gel was stained with 0.25% Coomassie Brilliant Blue R-250 (Merck, Darmstadt, Germany) for 40 minutes before being destained using a mixture of 10% methanol, 10% acetic acid glacial, and water.
Cell migration scratch assay
A scratch assay was done in a RL95-2 cell monolayer cultured in 10 cm dishes. A P10 pipette tip was used to scrape the layer in a straight line. The debris was removed and replaced with 10 mL of growth medium supplemented with 5% serum. After 2 hours, the cells were treated with DLBS1442 in various concentrations. The control cell group was prepared with addition of 15% dimethyl sulfoxide only as a substitute for treatment with DLBS1442. The cells were then incubated at 37°C with 5% CO2, then examined for 6 and 24 hours to examine their migration patterns.
In vitro cell toxicity assays
Cell culture with 80% cell confluence was serum-starved prior to treatment with DLBS1442. The cells were incubated with various concentrations of DLBS1442 (0, 10, 25, 50, and 100 μg/mL) for 24 hours, after which 20 μL of Cell Titer 96® Aqueous One Solution Reagent (Promega, Fitchburg, WI, USA) was added to each well. After 2–4 hours of incubation at 37°C in 5% CO2, the absorbance of each cell culture was measured (λ=490 nm) using a microplate reader (Model 680, Bio-Rad Laboratories).
Flow cytometry analysis
Cells were collected by centrifugation at 4°C for 5 minutes at 3,000 rpm. The cells were then washed with phosphate-buffered saline and collected using the same centrifugation procedure. After centrifugation, the cells were fixed with a mixture containing 300 μL of phosphate-buffered saline and 700 μL of absolute ethanol. The cells were then left to incubate at 4°C overnight. The fixed cells were then collected after a similar centrifugation procedure to remove the supernatant. Cells were stained with 500 μL of propidium iodide solution (Merck) in each culture well and then suspended and incubated at room temperature for 30 minutes in the absence of light. At least 10,000 cells were analyzed for their cell cycle distribution using BD FACSCalibur® and Cell Quest Pro® software (Becton Dickinson, San Jose, CA, USA).
Western blotting of caspase-8 and procaspase-9
Secreted caspase protein in the medium was analyzed by Western blotting assay (Bio-Rad Laboratories). The medium was concentrated up to tenfold by using a filter membrane with a 10 kDa molecular weight cut-off (Millipore, Billerica, MA, USA). The protein concentration was measured using the Bradford method. Next, the protein was separated in sodium dodecyl sulfate polyacrylamide gel electrophoresis using 10% acrylamide gel at 100 volts for 150 minutes. The protein in the gel was then transferred to polyvinylidene difluoride membranes using a blotting system at 500 mA for 75 minutes. Rabbit polyclonal antibodies against human caspase-8 and procaspase-9 were applied to the membrane. After overnight incubation at 4°C, the primary antibodies were removed and replaced with horseradish peroxidase-conjugated secondary antibodies (Abcam, Cambridge, UK). The caspase-8 and procaspase-9 proteins that specifically bind to the antibodies were then detected using luminol chemiluminescence reagent and exposed to a film (Fujifilm, Tokyo, Japan) in the dark room. Antibodies, both primary and secondary, polyvinylidene difluoride membranes, and luminol reagent were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA).
Statistical analysis
Statistical analysis of the comparison between control cells and cells treated with DLBS1442 was done using the Student’s t-test with Stat-View software (Abacus Concepts, Piscataway, NJ, USA). Values are expressed as the mean ± standard deviation of at least two independent experiments.
Results
Effect of DLBS1442 on regulation of angiogenesis
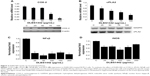
To explore the effects of DLBS1442 on angiogenesis, quantitative RT-PCR analyses were performed to determine the transcription levels of vascular endothelial growth factor (VEGF), hypoxia-inducible factor (HIF)-1α, and MMP-9 in DLBS1442-treated RL95-2 cells. Treatment with DLBS1442 resulted in a significant downregulation of VEGF, HIF-1α, and MMP-9 expression in a dose-dependent manner in RL95-2 cells (Figure 1A–C). The most marked decrease in the transcription level of these genes was seen at a concentration of 75 μg/mL (Figure 1). In addition, expression of MMP-2 and MMP-9 was measured by gelatin zymography. A previous report suggested that eutopic endometrium in women with endometriosis showed greater release of metalloproteases, including MMP-9, when compared with normal women.6 DLBS1442 was found to decrease MMP-9 protein expression in a dose-dependent manner (Figure 1D), while MMP-2 was not affected.
Effect of DLBS1442 on cell migration
Normal endometrial cells are widely known for their ability to migrate actively from one site to another. In endometrial RL95-2 cells, these migrations were investigated in the absence and presence of DLBS1442. The effect of DLBS1442 on cell migration of human endometrial cells at 0, 6, and 24 hours was evaluated using a wound closure assay. The percentage of closure in the wound area with or without the presence of DLRS1442 is shown in black square in Figure 1E. However, this did not occur in cells treated with DLBS1442 at 50, 100, and 200 μg/mL. Therefore, it can be concluded that DLBS1442 is capable of inhibiting the migratory and invasive activity of RL95-2 endometrial cells.
Effect of DLBS1442 on ERβ and PR-B transcription level
The biological roles of the estrogen receptor (ER) and progesterone receptor (PR) in endometrial tissue were examined since they play important roles as signal mediators of estrogen and progesterone. The ER and PR are known to be the most extensively studied biological prognostic markers in endometrial carcinoma.2 In this study, RT-PCR was carried out to evaluate the expression of ERβ and PR-B. After 24 hours of treatment with DLBS1442 (at doses of 25, 50, 75, and 100 μg/mL), the mRNA expression of ERβ (Figure 2A and B) in RL95-2 cells was significantly lowered, while PR was upregulated in a dose-dependent manner (Figure 2C and D).
Effect of DLBS1442 on the eicosanoid pathway
Cyclooxygenase (COX)-2 is known to play a critical role in perpetuating a positive feedback loop in inducing inflammation and promoting proliferation in endometriosis, and is possibly also involved in endometrial cell invasion and angiogenesis.1,2,7–9 Further, overexpression of COX-2 has been identified recently as a putative biomarker for recurrence in endometriosis, and has been reported to correlate with the severity of dysmenorrhea and nonmenstrual chronic pelvic pain in women with endometriosis.10–13 Endometriotic cells are a primary source of cytokine, chemokine, and inflammatory enzyme COX-2 and VEGF secretion.7,14,15 In this regard, COX-2 expression was analyzed by RT-PCR in DLBS1442-treated RL95-2 cells. DLBS1442 was found to significantly decrease transcription levels of COX-2 in a dose-dependent manner (Figure 3A). A similar decrease was found when levels of cytosolic phospholipase A2 (cPLA2) were investigated in DLBS1442-treated cells (Figure 3B). In endometriotic cells, PR-B is downregulated, whereas proinflammatory transcription factors, eg, nuclear factor kappa B (NFκβ), are upregulated, as a consequence of increased expression of proinflammatory cytokines and chemokines.1,14,16,17
Several studies have indicated that expression of COX-2 and cPLA2 is regulated by activation of NFκβ.18 To clarify their relationship with NFκβ (p50/p65), the transcription level of NFκβ was investigated in RL95-2 cells in the presence and absence of DLBS1442. As shown in Figure 3C, administration of DLBS1442 significantly decreased NFκβ transcription levels. Since NFκβ regulates COX-2 and inducible nitric oxide synthase (iNOS), and endometriosis-associated infertility is usually related to higher levels of iNOS and NOS enzyme activity, we also investigated the ability of DLBS1442 to modulate iNOS expression. The results indicate that DLBS1442 suppressed expression of iNOS mRNA in RL95-2 cells (Figure 3D). Taken together, these data suggest that DLBS1442-mediated inhibition of COX-2, cPLA2, and iNOS expression may be attributable to lowered transcriptional levels of NFκβ.
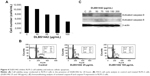
Effect of DLBS1442 on RL95-2 cell viability, flow cytometry, and cellular apoptosis
The effect of DLBS1442 on the viability of endometrial cells was examined in a human endometrial epithelial cell line (RL95-2). The results showed that death of RL95-2 cells occurred in a dose-dependent manner, with more than 50% of cell death occurring at a dose of 100 μg/mL (Figure 4A). Flow cytometry analysis was also conducted on cells treated with DLBS1442 (25 and 100 μg/mL) over 24 hours. DLBS1442 at a dose of 25 μg/mL increased the cell population in the sub-G1 phase of the cell cycle from 7% to 16% (Figure 4B). A similar effect was observed in DLBS1442 cells treated with 100 μg/mL, where the cell population remaining in sub-G1 phase increased from 7% to 34% (Figure 4B). Taken together, these data suggest that DLBS1442 affects cell cycle progression, possibly by inducing cellular apoptosis.
To examine further whether the decrease in cell viability in fact was due to full activation of the apoptotic program, we performed immunoblotting analysis on expression of activated caspase-9 and activated caspase-8, known as markers of apoptosis. As expected, DLBS1442 was able to dose-dependently induce cellular death signal, mostly by activation of caspase-8, but not by activation of caspase-9 (Figure 4C).
Discussion
Endometriosis is a complex medical problem involving a whole array of dysregulation in hormone receptor expression, particularly that of estrogen and progesterone, and chronic inflammation mediated by increased COX-2 expression.1,2 Our present study was performed to investigate the molecular mechanism of DLBS1442 in the treatment of endometriosis.
Angiogenesis is the process by which a new blood-vascular system grows from the existing vascular bed through interaction between cytokines, the cellular matrix, and proteolytic enzymes. This process has a pivotal role in the development of endometriosis because the disease is highly reliant on formation of a new blood-vascular system. In normal case, growth of new endometrial cell is restricted to a few millimeters in diameter due to insufficient nutrients and oxygen that stems from poor vascularization.2 In addition, angiogenesis and vascularization allow endometrial cells to escape into the circulation and lodge in other organs.19 These findings suggest that antiangiogenesis is an important strategy in the treatment of endometriosis. As endometrial tissue expands, local hypoxic conditions induce a molecular response that leads to activation of HIF, a key transcription factor.18 Activation of HIF induces the expression of proangiogenic growth factors, such as VEGF. These proangiogenic growth factors in turn bind to and activate their respective receptors on the surface of endothelial cells, leading to angiogenesis.20 In the present study, treatment with DLBS1442 resulted in downregulation of VEGF and HIF-1α. This result suggests that DLBS1442 inhibited the transcriptional activity of HIF-1α and further reduced cellular and secreted VEGF levels. Therefore, it can be concluded that DLBS1442 exerts its antiangiogenic activity by disturbing the binding of HIF-1α to the VEGF promoter in RL95-2 cells.
In order to allow endothelial cells to migrate and invade into the surrounding tissue, the process of angiogenesis requires degradation of the vascular basement membrane and remodeling of the extracellular matrix. MMPs are a family of proteolytic enzymes that degrade various components of the extracellular matrix and participate in the remodeling of these structures. The activity of MMP-9 increased gradually in endometriotic tissues according to severity. In this study, we investigated the effect of DLBS1442 in treating endometriosis by downregulating MMP activity. Our results demonstrated conclusively that MMP-9, which has a significant effect on endometriotic lesions, was downregulated by DLBS1442 in a dose-dependent manner.
Another factor that is also critical to the development of endometriosis is inflammation. There is mounting evidence suggesting that imbalances in the inflammatory system have a significant role in the development of the disease.1,2 Endometriotic tissue contains high levels of prostaglandin E2 which may contribute to the pain experienced by women with endometriosis. The first step in the synthesis of prostaglandin is hydrolysis of arachidonic acid from phospholipids in the cell membrane, predominantly by the action of cPLA2. The free arachidonic acid is then converted to the intermediates, prostaglandin G2 and prostaglandin H2, by the action of prostaglandin H synthase (alternatively known as COX). The prostaglandin G2 and prostaglandin H2 are further metabolized to prostaglandins, prostacyclin, or thromboxanes.
Several studies have shown that many women with endometriosis overexpress COX-2.7,9,10 There are two isoforms of COX that are encoded by different genes, ie, COX-1, which is constitutively expressed, and COX-2, which is upregulated in response to stimuli such as cytokines and growth factors. Endometriosis patients suffering from moderate to severe chronic pelvic pain (dysmenorrhea) showed significantly higher expression of COX-2 than asymptomatic patients and patients with minimal symptoms.12,13 Inhibition of COX-2 decreases endometriotic epithelial and stromal cell survival, migration, and invasion.21–23 Thus, by inhibiting COX-2, DLBS1442 can help reduce dysmenorrhea in women with endometriosis.5 As high levels of nitric oxide adversely affect sperm, embryos, implantation, and oviductal function, DLBS1442 may improve fertility in women with endometriosis by reducing production of iNOS.
NFκβ has been studied extensively as an inducible transcriptional regulator of the immune and inflammatory responses. Accumulating evidence has demonstrated the essential role of constitutive activation of NFκβ in controlling the initiation and progression of endometriosis. NFκβ contributes to the increased ability of endometriotic cells to invade and adhere to the peritoneal surface by regulating the expression of MMPs.24 Hence, our data indicate that the anti-inflammatory, pain-relieving, and anti-angiogenic actions of DLBS1442 might result from downregulation of NFκB activity, resulting in inhibition of expression of iNOS, COX-2, cPLA2, and MMP-9.
Development of endometriosis is also greatly dependent on proliferation and apoptosis of endometriotic cells. These factors affect the viability of endometriotic cells. It has been reported that endometrial cells in women with endometriosis show resistance to apoptosis.22,23 Our present study demonstrated that DLBS1442 was able to inhibit proliferation of endometriotic cells. Viability of RL95-2 cells decreased in a dose-dependent manner by addition of DLBS1442. Our study also demonstrated that exposure to DLBS1442 within 24 hours leads to a decrease in cell viability and an increase in cell apoptosis, within 24 hours. The ability of DLBS1442 to induce apoptosis in RL95-2 cells was further confirmed with Western blotting of activated cleavage of caspase-8.
Endometriosis is known to be strongly influenced by estrogen and progesterone levels.25 Both estrogen and progesterone exert their effects through intranuclear sex hormone receptors, including the ER and PR. The ER has two isoforms, ie, ERα and ERβ. The two isoforms have different tissue distribution and exert different biological functions.26,27 Bulun1 and Gupta et al2 reported that ERβ mRNA signals were overexpressed in 80% of endometriosis cases.1,2 Endometriosis is also associated with a reduced response to progesterone in the endometrium. The resistance of endometriotic tissue to progesterone can be explained by alterations in the distribution of the ER and PR isoforms and dysregulation of progesterone target genes. PR is present in two isoforms, PR-A and PR-B. These isoforms are translated from the same gene following initiation of transcription by different promoters. However, microarray analyses that express either PR-A or PR-B have confirmed that each of the PR isoforms has a unique set of target genes. PR-B tends to be a stronger activator of progesterone target genes, whereas PR-A has been shown to act as a dominant repressor of PR-B. Alteration in the relative expression of PR-B in endometrial cells may also play a pivotal role in the pathogenesis of endometriosis. Reduced expression of either one or both of the PR isoforms has been observed in the majority of endometrial diseases.1,28 In this study, DLBS1442 was found to have the ability to downregulate ER gene expression and upregulate PR gene expression. The effect of higher levels of PR would have a beneficial effect since progesterone is a growth inhibitory hormone in the endometrium. Increasing PR gene expression will inhibit inflammatory signaling and NFκβ activation. Thus, it can be deduced that the anti-inflammatory and antiproliferative activity of DLBS1442 is mediated via PR of stromal cells.
In summary, the overall data on DLBS1442 suggest a role for this bioactive fraction in decreasing the problems associated with endometriosis. The present study shows that DLBS1442 exerts its anti-inflammatory, proapoptotic, and antiangiogenic activity by reducing NFκβ and inhibiting the eicosanoid pathway. Hence, it can be concluded that DLBS1442 has high potential as a treatment for endometriosis.
Acknowledgments
The authors would like to thank James Sinambela and Irfan Darfiansyah for preparing the DLBS1442 used in this study. They are also grateful to Venni Carolina and Hanna C Rouli for their assistance in editing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. | ||
Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90:247–257. | ||
Tandrasasmita OM, Lee JS, Baek SH, Tjandrawinata RR. Induction of cellular apoptosis in human breast cancer by DLBS1425, a Phaleria macrocarpa compound extract, via downregulation of P13-kinase/AKT pathway. Cancer Biol Ther.2010;10:814–823. | ||
Tjandrawinata RR, Arifin PF, Tandrasasmita OM, Rahmi D, Aripin A. DLBS1425, a Phaleria macrocarpa (Scheff.) Boerl. extract confers anti proliferative and proapoptosis effects via eicosanoid pathway. J Exp Ther Oncol. 2010;8:187–201. | ||
Tjandrawinata RR, Nofiarny D, Susanto LW, Hendri P, Clarissa C. Symptomatic treatment of premenstrual syndrome and/or primary dysmenorrhea with DLBS1442, a bioactive extract of Phaleria macrocarpa. Int J Gen Med. 2011;4:465–476. | ||
Rocha AL, Reis FM, Taylor RN. Angiogenesis and endometriosis. Obstet Gynecol Int. 2013;2013:859619. | ||
Banu SK, Lee J, Speights VO Jr, Starzinski-Powitz A, Arosh JA. Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. J Endocrinol. 2008;149:1180–1189. | ||
Takenaka Y, Taniguchi F, Miyakoda H, Takai E, Terakawa N, Harada T. Lipopolysaccharide promoted proliferation and invasion of endometriotic stromal cells via induction of cyclooxygenase-2 expression. Fertil Steril. 2010;93:325–327. | ||
Machado DE, Berardo PT, Landgraf RG, et al. A selective cyclooxygenase-2 inhibitor suppresses the growth of endometriosis with an anti-angiogenic effect in a rat model. Fertil Steril. 2010;93:2674–2679. | ||
Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16:561–566. | ||
Chishima F, Hayakawa S, Sugita K. Increased expression of cyclooxygenase-2 in local lesions of endometriosis patients. Am J Reprod Immunol. 2002;48:50–56. | ||
Matsuzaki S, Canis M, Pouly JL, Wattiez A, Okamura K, Mage G. Cyclooxygenase-2 expression in deep endometriosis and matched eutopic endometrium. Fertil Steril. 2004;82:1309–1315. | ||
Buchweitz O, Staebler A, Wulfing P, Hauzman E, Greb R, Kiesel L. COX-2 overexpression in peritoneal lesions is correlated with nonmenstrual chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2006;124:216–221. | ||
McLaren J, Prentice A, Charnock-Jones DS, et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–489. | ||
Wieser F, Vigne JL, Ryan I, Hornung D, Djalali S, Taylor RN. Sulindac suppresses nuclear factor-kappaB activation and RANTES gene and protein expression in endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 2005;90:6441–6447. | ||
González-Ramos R, Donnez J, Defrere S, et al. Nuclear factor-kappaB is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. 2007;13:503–509. | ||
Lousse JC, Van Langendonckt A, Gonzalez-Ramos R, Defrere S, Renkin E, Donnez J. Increased activation of nuclear factor-kappaB (NF-kappaB) in isolated peritoneal macrophages of patients with endometriosis. Fertil Steril. 2008;90:217–220. | ||
Lin CC, Lin WN, Wang WJ, et al. Functional coupling expression of COX-2 and cPLA2 induced by ATP in rat vascular smooth muscle cells: role of ERK1/2, p38 MAPK, and NF-κβ. Cardiovasc Res. 2009;8:522–531. | ||
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. | ||
Ikeda E, Achen MG, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270:19761–19766. | ||
Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. | ||
Harada T, Kaponis A, Iwabe T, et al. Apoptosis in human endometrium and endometriosis. Hum Reprod Update. 2004;10:29–38. | ||
Garcia-Velasco JA, Arici A. Apoptosis and the pathogenesis of endometriosis. Semin Reprod Med. 2003;21:165–172. | ||
González-Ramos R, Defrère S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520–528. | ||
Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. | ||
Gustafsson JA. Estrogen receptor beta – a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. | ||
Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–155. | ||
Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma – new development in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54:667–679. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.