Back to Journals » Journal of Inflammation Research » Volume 14
Anti-Inflammatory and Antiviral Osmotic Polymeric Film to Treat Covid-19 Early-Stage Infection
Authors Shrivastava R , Shrivastava R, Johansen B, Allain T
Received 12 February 2021
Accepted for publication 11 March 2021
Published 30 March 2021 Volume 2021:14 Pages 1195—1206
DOI https://doi.org/10.2147/JIR.S306434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ravi Shrivastava,1 Remi Shrivastava,1 Bianca Johansen,1 Thibault Allain2,3
1VITROBIO Research Institute, Issoire, 63500, France; 2Laboratoire SANABEL, Paris, 75011, France; 3UMR 152 Pharma Dev, Université de Toulouse, IRD, UPS, Toulouse, France
Correspondence: Ravi Shrivastava Email [email protected]
Background: Covid-19 infection starts in the nasal cavity when viral S1 and RBD proteins bind to the host cell ACE2 receptors, the virus multiplies, causes cell lysis, and enters the circulation. This triggers a strong cytokine release and inflammation of the nasal mucosa. A multitarget approach of cleaning the nasal mucosa and suppressing chances of nasal and systemic inflammation should minimize severe respiratory consequences. Unfortunately, no such treatments are yet available.
Methods: We describe the conception of an osmotic polymeric film using an in vitro nasal mucosa mimicking model, containing polymers to neutralize Covid-19 specific viral S1, RBD proteins and selected proinflammatory cytokines.
Results: The filmogen barrier forms a stable and osmotic film on the nasal mucosa. Hypotonic liquid exudation from the nasal surface detaches and drains the inflammatory cytokines and other contaminants towards the film where selected polymers bind and neutralize SARS-CoV-2 spike S1 and RBD protein as well as Covid-19 disease-specific key pro-inflammatory IL-6, TNF-α, IL-10, IL-13, and GM-CSF cytokines.
Conclusion: Minimizing the nasal surface concentration of pro-inflammatory cytokines and viruses should help nasal mucosa repair and avoid immune stress. This nearly instant, simple, scientific, safe, and logical approach should help attenuate Covid-19 induced systemic inflammation at an early stage without being affected by viral S1 spike protein mutations.
Keywords: filmogen glycerol, antiviral, anti-cytokines, anti-inflammatory, Covid-19, S1, RBD
Introduction
Severe acute respiratory syndrome (SARS) due to coronavirus (SARS-CoV-2) has become a global pandemic, causing above 1 million deaths, and damaging the global economy.1,2 Current worldwide measures of hand washing, maintaining social distance, and wearing masks are necessary but not sufficient to control infection as the virus spreads through air. Infection can be caused by inhalation of small droplets exhaled by an infected person, that can travel a distance of up to ten meters and attach on the nasal mucosa (NM), which is the first target organ for virus infection in above 90% of cases.3
SARS-CoV-2 possesses multiple surface spike (S1) and RBD (Receptor Binding Domain) proteins which bind to the NM epithelial host cell angiotensin-converting enzyme 2 (ACE2) receptors.4 The binding affinity of the S protein and ACE2 on the host cells was found to be a major determinant of SARS-CoV replication rate and disease severity.5 Viral entry also depends on TMPRSS2 and cathepsin B/L protease activities, which are widely represented on both nasal and bronchial epithelium.6 In most cases, the body’s natural innate immune response controls the infection and virus propagation remains restricted in the nasal cavity or up to the upper respiratory tract (URT) epithelium.7 This is why initial infection is nearly asymptomatic where virus grows in a few NM cells followed by cell death and liberation of a huge quantity of free virus particles on the nasal surface. These newly liberated virus particles then attack new healthy cells, destroy NM cells, intercellular connective tissue and open the gates for free virus entry into the circulation with cough, fever, and fatigue as the main symptoms.
All infected cells undergo apoptosis or necrosis, triggering the inflammatory response with the activation of immune cells such as macrophages, Th2, Th17 and CD8+ cells. To defend the body, these cells release pro-inflammatory cytokines as an inflammation mediated defensive immune response in the early phase of the disease (e.g, IL-2) but if the infection is controlled, anti-inflammatory cytokines are released to suppress inflammation and its consequences. This interplay between cytokines depends upon the disease (disease specific), stage of disease progression, nature of the target cell, nature of activating signal, and pre-existing cytokine concentrations. The key pro-inflammatory cytokines in Covid-19 infection are IL-1, IL-6, IFN-α, IL-10, IL-13, GM-CSF, IFN-γ and many others, depending upon the disease chronicity and the stage of inflammation.8,9 Recent scientific literature on Covid-19 reveals the key role of IL-6 cytokine in modulating inflammation, autoimmunity, and host defense through a number of immune stimulating mechanisms.7,10 If the infection and viral growth is not suppressed at this stage, extensive NM damage may lead to the release of very high concentrations of these pro-inflammatory cytokines with severe nasal inflammation,11 progression of the virus through systemic entry towards other organs, overstimulation of the immune system, lung inflammation and finally immune burn-out with the release of huge quantities of pro-inflammatory cytokines, called Cytokine Release Syndrome (CRS).12–15
This disease physiopathology reveals that the NM plays a key role in nasal and URT infection and systemic disease progression. Therefore, many scientists have already suggested to shift Covid-19 treatment strategy at the level of the NM to control disease dissemination during the early phase of infection.16,17 This approach requires reconstituting the inflamed NM barrier and reestablishing nasal membrane natural defenses to stop systemic virus entry. This should minimize inflammatory cascade and subsequent immune stress. This strategy requires a simple multi-target approach of eliminating contaminants, including free virus particles and pro-inflammatory cytokines from the nasal surface to suppress nasal inflammation as well as stopping new virus entry to minimize further infection. Currently there is no multi-target, physical or chemical treatment capable of protecting and cleaning the NM to reconstitute the natural barrier as well as blocking viral growth and inflammation, essential for controlling Covid-19 infection.
The efficacy of available vaccines and duration of protection offered is also not yet clear. Recent observations in the city of Manaus in Brazil where 76% of residents were seropositive in summer 2020, followed by a new surge of widespread Covid-19 infection in January 2021, raise questions about herd immunity and the vaccine efficacy against new emerging Covid-19 variants.18
We suggest conceiving a long-lasting (4–6 h), absorbent, osmotic, glycerol based, polymeric liquid film, capable of cleaning the nasal surface from contaminants while simultaneously neutralizing viruses and selected pro-inflammatory cytokines.
Polymers, such as plant tannins, are big and inert molecules having a strong affinity for specific proteins, such as the pro-inflammatory cytokines. Initially, the selected polymers can bind with glycerol to stabilize the film. When applied on the nasal surface, such an osmotic film can detach and drain all the proteins towards the film where they can be captured and neutralized through polymeric binding. Such a film can also be used as a cleaning bandage on any hidden body orifices to minimize further infection. The conception of this mechanically acting nasal liquid filmogen bandage to prevent and to treat early-phase cases of Covid-19 is explained. The aim is not to cure the disease but to minimize virus induced cellular destruction and inflammation, to reduce immune stress and let the body defend against Covid-19 infection caused by any strain, during the early phase of the disease.
Materials and Methods
Conception of an in vitro Multicellular, Live Cell Membrane Model Mimicking NM
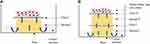
Due to the difficulties in conducting homogeneous experiments in vivo on human or animal NM, we used Episkin®, a modified in vitro reconstituted human epidermis model,19 to assess all the cellular parameters of cytotoxicity, osmosis, and filmogenicity for conceiving and extrapolating eventual in vivo cellular effect of ingredients, used as described by Shrivastava et al.20,21 In short, fresh epidermis cultures were purchased from EPISKIN-SNC (Lyon, France). Each epidermal unit consisted of an organotypic culture made of adult human keratinocytes, obtained from scrapings of normal human skin, kept in an isotonic modified MCDB-153 cell culture medium (Sigma-Aldrich, France), dissociated enzymatically and seeded onto a collagen (type I) matrix-coated polycarbonate filter capable of absorbing culture medium. The filter is kept on a sponge which is placed in a Petri dish containing culture medium (Figure 1A). Liquid medium reaches the skin cell culture by osmotic diffusion through the sponge and nourishes the cells. The polycarbonate filter kept on the sponge creates a barrier between the liquid medium and the epidermis, keeping the outer surface of the epidermis in contact with air, as is the case for human NM in vivo. According to the initial cell seeding count and culture conditions (amount of culture medium or serum), a full multi-layer cell membrane can be obtained within 10–14 days and cells can remain alive for a period of 1–2 weeks.
To evaluate the osmotic properties of glycerol, we added one extra sponge to increase the distance between the live cells and the liquid medium (Figure 1B), leading to poor liquid supply to the cells. Under such conditions, cell mortality increases and almost all the cells die within a period of 2–4 days as liquid medium cannot reach up to the cells, leading to dehydration and cell death, which can be quantified. Applying an osmotic liquid on the epidermal surface, attracts hypotonic medium up to the top and avoids cellular dehydration. Osmotic properties are therefore directly proportional to the cell survival, which can be quantified.22
Cytotoxicity tests were conducted in a standard 1-sponge model while osmotic and filmogen studies were performed using a dehydrated 2-sponge model (n = 6 each test concentration). To study cellular parameters, the test ingredients, liquid or solid, were diluted in culture medium or saline solutions, and applied directly onto the multicellular skin cell cultures (20 µL). Cells were then incubated at 37°C in a 5% CO2 environment for up to 72 h and cell mortality was measured using MTT tetrazolium live cell assay as described by Riss et al.23 The increase or decrease in cellular survival rate compared with the corresponding control cells (1-sponge model as negative control and 2-sponge dehydration model as positive control) indicate cellular effects. The experiments were considered valid only if the negative 1-sponge standard cultures represented >90% cell survival and the positive 2-sponge dehydration cultures <15% live cells.
Evaluation of Cell Membrane and Intracellular Effects
At the end of each experiment, 3/6 epidermis were fixed in a paraffin cassette and the cassettes were transferred into 70% ethanol and stored at 4°C until use. On a day of experiment, epidermis was gradually dehydrated (80% EtOH, 45 min; 90% EtOH, 45 min; 96% EtOH, 45 min, 100% EtOH, 45 min; xylene 100%, 45 min) and vertical cell membrane sections (5 µm) were cut using a standard microtome (Leica) and stained with hematoxylin-eosin stain as described by Fischer et al.24 Sections were examined microscopically to evaluate any eventual cell membrane or intracellular changes.
Selection of Osmotic Filmogen Ingredients
The aim was to find an ingredient which can be used as such or diluted in an appropriate excipient to obtain a filmogen liquid for nasal application which is osmotic, non-cytotoxic, absorbent, and stable for 4–6 h. Due to these selection criteria, all the chemicals which may interact with cellular structures were excluded from the selection. We selected different natural and synthetic ingredients which can be used as liquids and which are commonly used for cleaning injuries, for jellifying food or as medical ingredients, and considered not harmful orally or when applied topically. The cytotoxicity potential was measured initially for all the selected ingredients as per the NF EN ISO 10993 standard: Biological Evaluation of Medical Devices, Part 5 (1999): Tests for in vitro cytotoxicity. Cellular irritation potential was quantified using in vitro Bovine Corneal Opacity Test (BCOP) as described by Schrage et al.25 Any ingredient meeting the maximum defined selection criteria was selected as a filmogen base.
Determining Osmotic and Cytotoxic Potential of Glycerol
Glycerol, either pure or diluted at concentrations of 0, 2, 10, 20, 30, 40, 50, 60, 70, 80, 90% in 0.9% NaCl saline (also served as control) was exposed (20 µL/cell membrane) on a 2-filter dehydration model. Cells were incubated at 37°C for 72 h before determining cell viability. In the dehydrated models, if the test product is osmotic, it continues attracting hypotonic culture medium even if there are 2 sponges, thus keeping the cells alive. Cell viability is therefore proportional to the osmotic potential of the test product applied on the membrane surface.
Adjusting the Thickness and Absorbent Capacity of Osmotic Glycerol Film
The osmotic film must also be thick and absorbent to trap the osmotically drained contaminants entering the film and those entering the nasal cavity from the external environment. Therefore, a few commonly used food-grade thickening agents were added in the selected osmotic ingredient solution at different concentrations to introduce absorbent properties in the film. Only those thickening/jellifying agents having no effects on product osmosis and safe, were retained as absorption enhancing ingredients.
Selection of Glycerol Molecule Binding Polymeric Structures
Glycerol associated with jellifying agents is an excellent slightly filmogen and strongly osmotic solution. However, when applied on a live biological membrane such as the NM, the osmotic activity generated by the film creates a strong hypotonic liquid flow from the mucosa towards the film, leading to instant dilution of the film and loss of osmotic activity within a few minutes. As certain inert and big polymers (e.g. plant tannins or synthetic polymers) are known to bind with selected macromolecules (H, OH binding) and specific proteins,26 after prescreening we selected 82 natural and synthetic polymeric structures to find those which can bind with glycerol molecules to render the glycerol film stable. Glycerol binding polymeric structures were further tested to evaluate their specific protein binding potential.
Selection of Natural (Plant Tannins) or Synthetic Polymers
Eighty-two tannin-rich plants or parts of the plant were used to prepare tannin-rich extracts at different plant/solvent ratios by maceration and tannin fraction was quantified as described by Zhang et al.27 Only those fractions showing glycerol binding were evaluated further. A few synthetic powdered polymers (CMCNa, PVOH, PCM, SPA, PEG, PL127, Klucel and Solagum) were purchased (Sigma-Aldrich, France). These extracts were coded as per their nature, origin, part of the plant used, and extraction technique employed. Further cytotoxicity, pharmacological, analytical, safety, and clinical tests were conducted using these extracts.
Selection of Natural or Synthetic Polymer or Polymeric Associations to Render Glycerol Film Resistant and Flexible
The study design was identical to the 2-sponge cellular dehydration model used to test glycerol osmosis at various concentrations with the exception that an additional group of epidermis was treated with the same concentrations of glycerol premixed with individual polymer or polymeric association (0.01–1.50%) for 1 h. Cell culture membranes were washed by stirring for 1 min at 6 h, 24 h, and 24 h to allow mechanical film detachment. At 72 h, the difference of cell survival, proportional to the osmotic activity, in glycerol alone vs. glycerol plus polymer preparations was compared. An adhered film continues to exert osmotic activity and hydrate cells and thereby improves cellular survival. Cell viability compared with corresponding controls was therefore proportional to the osmotic film adherence to the biological membrane.
Determination of Polymer Binding with Covid-19 S1 Glycoproteins and Viral RBD Proteins
In this study, selected polymers were diluted in saline solution and were tested in non-cytotoxic concentrations, individually (between 0.05–0.30%) or in association (0.05–0.15%). Due to high viscosity of finished formulation containing minimum concentrations of polymeric association (Covispray), only 5% and 10% concentrations were evaluated, employing ACE2:SARS-CoV-2 Spike S1 or RBD inhibitor screening assay kits (BPS Bioscience, ref. 79945, lot 201001-K). Each mean value represents mean of 8 individual data.
These studies were performed using Tebu-bio (USA-French division). Both kits contained a 96-well microplate, purified ACE2 and SARS-CoV-2 Spike S1 or RBD proteins, Streptavidin-HRP or HRP labelled anti-human Fc region antibodies, and assay buffers 2 or 1 for 100 binding reactions. The kits are designed to detect Covid-19 Spike S1-Biotin protein by Streptavidin-HRP or Fc-tagged RBD spike proteins. ACE2 protein was attached to a nickel-coated 96-well plate. Next, SARS-CoV2 Spike S1-Biotin or spike-RBD-Fc were incubated with ACE2 on the plate. Finally, the plate was treated with Streptavidin-HRP or Anti-Fc-HRP, followed by addition of an HRP substrate to produce chemiluminescence, which was measured using a chemiluminescence reader as described by Neufurth et al.28 All experiments were conducted at room temperature. In short, the first step involved coating the microwells with 50 µL ACE2-His (Ref. 11003, batch 200408) at 1 µg/mL in Phosphate Buffered Saline (PBS, Sigma-Aldrich, France) for 1 h under slow agitation. The coated plates were washed three times with 100 µL 1x Immuno Buffer and were blocked by adding 100 µL of Blocking Buffer-2 (or Buffer 1 for RBD) to each well followed by incubation for 10 min under low agitation. The coated plates were then washed thrice with 100 µL 1x Immuno Buffer. The second separate step consisted of inhibiting the binding by adding 20 µL of 1x Immuno Buffer-1 to each well or 40 µL for blank wells of a non-binding pre-incubation plate (Greiner Bio-One, ref. 651901). The positive control and test inhibitor wells were treated with 20 µL SARS-CoV-2 Spike S1-Biotin (5 ng/µL, Ref. 100679, lot 200716) or Spike S1 neutralizing antibodies (Ref. 100793, batch 200617), diluted in 1x Immuno Buffer-1 (approximately 50 nM) followed by 1 h preincubation. All the test products were diluted in PBS at 5x stock solutions and 10 µL of test solution was added in each well (n = 4 each dilution). Positive and blank well controls received 10 µL of PBS inhibitor buffer. The Spike S1 Neutralizing Antibody (Ref. 100793, lot 200617) was tested at 300 nM and 50 nM final concentrations as positive inhibitor control. According to the solubility of test products, a low and a high concentration was tested as follows: solagum (Sg) excipient negative control (0.05 and 0.10%), hydroxypropyl cellulose (HpP) excipient negative control (0.10 and 0.30%), HhP plant extract test control (0.10 and 0.30%), Covispray individual polymer CsL, ClR (0.05 and 0.15%), UdP and TpF (0.10 and 0.30%), association of 4 Covispray polymers (0.05 and 0.15%), and finished Covispray formulation (5% and 10%). Polymeric association called S1 cyanidins (CsL, ClR, UdP, TpF) are derived from tannin-rich whole plant extracts. Due to technical difficulties, it was not possible to test above 10% Covispray finished formulation. The 50 µL preincubated solution was then transferred to the corresponding wells of the coated plates, again incubated for 1 h, then washed with 100 µL 1x immune buffer and the reaction was blocked by adding 100 µL of blocking buffer-2 for 10 min. 50 µL ELISA ECL substrate A and 50 µL ELISA ECL substrate B were mixed and then added to each well followed by chemiluminescence (RLU) readings.
Polymeric Binding with Covid-19 Specific Cytokines
The Covid-19 pathology mobilizes a wide variety of immunological biomolecules, particularly the pro-inflammatory cytokines such as IL-6, TNF-α, IL-10, IL-13, and GM-CSF. The binding potential of Covispray polymers with these key cytokines was evaluated using ELISA tests at fixed polymeric concentration of 0.10% in 5.0% glycerol aqueous solution. The finished Covispray liquid formulation was also tested at 5.0% concentration. Test products were incubated with human purified recombinant cytokine (Invitrogen, 400 pg/mL) for 5 min in PBS. The remaining free and available recombinant cytokine is measured using Specific ELISA kit (Invitrogen, Human IL kits) according to the manufacturer’s instructions. Recombinant cytokines without polymers were used as negative controls. The Optic Density (OD) is measured at 450 nm using an ELISA plate reader (luminometer-Envision, PerkinElmer).
Data analysis and interpretation: The scavenge activity of polymers is inversely proportional to the quantity of cytokine measured. Reduction in quantified cytokine following incubation with polymers compared with the amount in negative controls, indicated cytokine neutralization due to polymeric binding.
Statistical Analysis
Statistical analyses for most experiments were performed in GraphPad Prizm 8.2.1 (GraphPad Software, Inc., CA, USA). Cells survival in glycerol film and cytokines binding were analysed by one-way ANOVA followed by the post hoc Dunnett's multiple comparison test. Inhibition of luminescence due to the binding of polymers or excipients with the S1 or RBD protein were analysed by one-way ANOVA followed by the post hoc Sidak test. A p-value less than 0.05 was considered as statistically significant, with a confidence interval (CI) of 95%.
Results
Selection of Osmotic Filmogen Ingredients
None of the ingredients tested met all initial selection criteria of being cell-friendly, filmogen, non-cytotoxic and osmotically active at once. Only a glycerol (glycerin) - containing solution was found to be highly osmotic yet cell-friendly and not cytotoxic even at concentrations up to 90%.
In vitro Cytotoxic Potential
The concentrations of each ingredient used in the preparation of the finished formulation of Covispray was at least 4 times the concentration which can produce any cellular effects. The finished formulation has no cellular effects.
Osmotic and Cytotoxic Potential of Glycerol in 2-Sponges Dehydration Model
The mean live cell count in negative controls (n = 6 each) was 93.75% while it was <10% in positive 2-sponge controls. Glycerol concentrations at 2, 4, 6, 10, 20, 30, 40, 50, 60, 70, 80, 90% showed a concentration-dependent increase in mean cell survival rate ranging from 21.75%, 22.5%, 26.1%, 24.0%, 30.25%, 35.50%, 34.1%, 38.75%, 39.75%, 42.25%, 50.03%, and 49.75% compared with the positive controls, respectively. No cytotoxic or cellular changes were noticed during histological examination. A low concentration of 2.0% glycerol increased cell survival by 21.75% indicating that even small concentrations can induce osmosis. The glycerol concentration in the finished formulations can be modulated according to the osmotic force required to clean a biological surface. Improved cell survival and the absence of any histological changes in cellular parameters show absence of any cytotoxic effects in concentrations studied. Glycerol can be diluted in water to vary the concentrations between 1 to 98% depending on the low or high osmotic activity required in the film. Applying osmotic glycerol on the NM attracts hypotonic liquid from the inner parts of the nasal tissue. This liquid flow should detach and drain the surface contaminants, including free floating virus particles and pro-inflammatory cytokines, toward the glycerol film. As this glycerol film was not stable and absorbent, it does not form an absorbent and stable film, so further improvements were made to introduce these properties in the glycerol solution.
Selection of Ingredients Improving Absorbent Capacity of Osmotic Glycerol Film
Addition of 0.10 to 0.60% of a thickening and absorbent agent such as HpP, acacia gum, xanthan gum or cellulosic substances, rendered the film thicker and absorbent. A concentration of 9.8% glycerol as an osmotic liquid with 0.30% HpP and 0.25% solagum (an association of acacia gum enrobed in xanthan gum) as film jellifying ingredients, was selected for the conception of the initial Covispray film. Applying this liquid as a spray on the NM helps create a moderately osmotic but highly absorbent film on any live semi-permeable biological membrane but this film is not stable and resistant to the osmotic liquid flow exudating from the biological membrane. The film was rendered resistant to liquid flow by adding specific glycerol binding polymers.
Selection of Natural or Synthetic Polymer or Polymeric Associations to Render Glycerol Film Resistant and Flexible
Preliminary glycerol–polymer binding studies showed that the maximum polymeric concentration to render glycerol filmogen and resistant to liquid flow should not exceed 1.0%, as higher concentrations may agglutinate and cannot be used in a sprayable film. Among 82 natural and synthetic polymeric ingredients tested, only 32 ingredients (27 natural and 5 synthetic) improved glycerol filmogenicity without inducing any cellular effects. Associating 2 or more glycerol binding polymers improves glycerol film stability in a synergistic way. The best glycerol-HpP-Sg absorbent film resistance was obtained by adding an association of 4 natural plant polymers (CsL + ClR + UdP + TpF) at a concentration <1.0%. Only key results are shown in Table 1.
Covispray Polymeric Binding with SARS-CoV-2 Spike Protein
The positive PBS controls have no effect, neither on the binding of Covid-19 S1 protein (Figure 2A and B) or RBD proteins (Figure 2C and D). Similarly, no statistically significant inhibition of S1 or RBD proteins was observed with Sg and HpP excipients, HhP plant extract control, or ClR, UdP, and TpF individual polymers although slight RBD protein binding was observed with 0.30% TpF and HhP polymers. On the contrary, CsL at a concentration of 0.05% blocked nearly 60% S1 spike protein while a slightly higher concentration (0.15%) neutralizes above 90% S1 spike protein (both p<0.001, Figure 2A and B). The same polymer, at concentrations of 0.05% and 0.15% also blocked nearly 70% and 95% RBD proteins (p<0.001, Figure 2C and D). It should be noted that the maximum concentration of CsL + ClR + UdP + TpF which could be tested was 0.15%, nearly 3 times lower than what is incorporated in the finished composition. The Covispray final composition at a concentration as low as 5.0%, blocked on average 50.6% S1 protein and 49.6% RBD protein while at 10% concentration the inhibition was 72.2% for S1 and 72.0% for RBD protein, compared with positive PBS controls. It is understandable that RBD being a fragment protein of SARS-CoV-2 S-protein, shows similar polymeric binding with both proteins. These results clearly show that Covispray at a concentration of only 10% can block up to 70% of Covid-19 S1 and RBD proteins which are involved in virus entry into the host cells through ACE-2 receptor binding.
Covid-19 Disease Specific Pro-Inflammatory Cytokine Binding
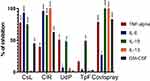
ClR polymer was the most active in blocking nearly 90% IL-6, IL-10 and IL-13 and about 60% GM-CSF while CsL neutralized >75% TNF-α as well as IL-6 and IL-10 (all p<0.001 vs. controls) (Figure 3). At 5% concentration, the final Covispray composition neutralizes nearly 30% IL-6 (p<0.05 vs. controls), 80% TNF-α and GM-CSF (p<0.001) and above 90% IL-10 and IL-13 (p<0.001). Therefore, it can be considered that 100% Covispray could neutralize all the key pro-inflammatory cytokines which are considered responsible for nasal and systemic inflammation.
Discussion
Covid-19 is a multifactorial disease. When symptoms appear, the virus is already developing in the NM cells, nasal mucosa is inflamed, newly generated free virus particles are already pouring on the NM, and the immune system is trying hard to control the infection by releasing multiple disease-related cytokines such as IL-1, IL-6, TNF-α IL-10, IL-13, and GM-CSF.9 The disease becomes systemic which begins by viral inhalation transmission, rapidly involving all the components of the respiratory system. The only way to stop disease progression is therefore to stop inflammation, virus growth and its dissemination in other organs such as lungs, stop cellular destruction and subsequent inflammatory cascade. A specific antiviral drug, acting topically, and capable of blocking virus growth in the nasal cavity and URT would be an ideal approach to avoid side effects but such topical antivirals are not yet available. Using anti-inflammatory drugs would be another alternative but these cannot totally suppress the disease as the virus would continue destroying cells and triggering an immune reaction. Current results with such drugs to treat Covid-19 infection have not shown encouraging results.28 Repairing damaged NM to stop free virus and/or cytokine systemic entry may also help minimize the inflammatory cascade but currently there is no other drug or device to render the NM barrier impermeable. Cell growth needs a contaminant, chemical, and inflammation free environment which cannot be achieved if the virus continues growing, if the nasal surface is inflamed and if the immune activity is maintained in the nasal mucosa. The submucosa of the nasal cavity and the respiratory tract is rich in immune cells, particularly the mast cells, which represent a protective barrier against infection.29 If infection is not controlled during the early phase, continuous virus-induced nasal mucosa cellular destruction over-activates mast cells, releasing large quantities of proinflammatory cytokines, particularly IL-1, IL-6, TNF-α, and IL-33. IL-1 and TNF-α affecthemodynamic parameters which are considered to induce thrombosis, bleeding, edema, thromboembolic disease, alveolar damage, and hypoxemia.30,31 This is the reason why many scientists strongly suggested using anti-IL-1 and IL-6 drugs to suppress Covid-19 induced late phase hemodynamic pathology.32 The combination of these factors induces acute and diffuse alveolar damage (DAD) destroying the alveolar capillary barrier with increased vascular permeability, desquamation of alveolar epithelial cells, alveolar and interstitial inflammatory cells infiltration, hyaline formation, and alveolar edema which may cause severe acute respiratory distress syndrome (ARDS).15,33
Taking into consideration the complexity of the disease involving viral growth, NM damage, free systemic passage of virus and cytokines into the circulation maintaining inflammatory cascade, immune exhaustion, and widespread alveolar damage leading to respiratory distress, only a multitarget drug or medical device which can act simultaneously on all these parameters can help minimize disease pathology once Covid-19 symptoms appear.34 Such an approach should also take into consideration eventual virus mutants due to minor protein changes in S1 level.
Covispray is an osmotic filmogen liquid containing glycerol, two jellifying agents, and an association of plant polymers, all capable of binding with glycerol molecules to render it a filmogen, flexible, and resistant to mechanical pressures exerted by the generated liquid flow. When applied in the nasal cavity, the liquid forms a transparent and slightly osmotic film, like a mask. The thickening/jellifying agents in the film swell when they encounter water and render the film absorbent. This film can capture environmental pollutants entering the nasal cavity like a mask. Being osmotic, the film attracts hypotonic liquid from the inner parts of the NM thereby detaching and draining the contaminants present on the surface of the NM which may then be trapped in the filmogen mask.
Covispray plant tannins are very big polymeric and inert molecules, containing an association of gallotannins, ellagitannins, and condensed tannins where a few H-OH sites always bind with glycerol to render it mechanically resistant. Being bulky, the remaining few sites may also bind to specific proteins such as the Covid-19 virus spike-S1 and RBD proteins and cytokines. To keep Covispray film as a liquid for nasal surface application, it was not possible to add >0.60% plant polymers. Therefore, the association of glycerol binding plant polymers was carefully selected to keep the composition a filmogen, resistant to osmotic liquid flow, and capable of neutralizing the key viral and inflammatory proteins carried towards the absorbent film along with the osmotic liquid flow. For example, the ClR polymer blocks nearly 90% of TNF-α, IL-6 and IL-13 cytokines but has no effect on Covid-19 S1 and RBD proteins while CsL polymer specifically captures only these proteins. The conception of filmogen glycerol and dual acting polymer–protein binding technology is patented worldwide.33,35 Virus particles and cytokines entering the film are permanently trapped by specific polymers while small un-trapped molecules are absorbed in the film or expelled through osmotic liquid flow. This mode of action helps to clean the NM mechanically without being in direct contact with the nasal epithelium. Minimizing virus-induced cellular damage, inflammation, and cleaning the nasal surface, should stimulate cell growth and restores the natural barrier and defensive functions of the NM. Reducing or stopping free entry of pathogens into systemic circulation should help reduce immune stress, offer optimal conditions for immune cells to fight and to neutralize systemic pathogens, and in consequence minimize the risk of diffuse alveolar damage and acute respiratory distress syndrome.
Conclusions
The aim of Covispray is not to act as an antiviral or anti-inflammatory drug but to minimize the concentration of pathogens and the risk factors which continue triggering and maintaining an inflammatory cascade. In the absence of any curative drug, such a multitarget treatment can be used as an effective, safe, and preventive treatment against multiple nasal pathologies involving inflammation, provided that this drug design hypothesis proves its clinical pertinence. It is suggested to verify the clinical efficacy of such a treatment in early stage Covid-19 positive symptomatic patients and in populations continuously exposed to Covid-19, such as health workers and retirement home facilities.
Patents
The conception of filmogen glycerol and dual acting polymer–protein binding technology is patented worldwide.34,35
Abbreviations
ACE2, angiotensin-converting enzyme 2; Covid-19, novel coronavirus disease 2019; CRS, cytokine release syndrome; HpP, hydroxy propyl cellulose; NM, nasal mucosa; OD, Optic Density; PBS, Phosphate Buffered Saline; RBD, receptor-binding domain; S1, spike protein; SARS, severe acute respiratory syndrome; Sg, solagum; URT, upper respiratory tract.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Ms. Genest Anais for her valuable contribution in analyzing the results and preparing this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding and was entirely financed by VITROBIO Pharma Research Institute in France.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Binns C, Low WY, Kyung LM. The COVID-19 pandemic: public health and epidemiology. Asia Pac J Public Health. 2020;32:140–144. doi:10.1177/1010539520929223
2. Dawood FS, Ricks P, Njie GJ, et al. Observations of the global epidemiology of COVID-19 from the prepandemic period using web-based surveillance: a cross-sectional analysis. Lancet Infect Dis. 2020;20:1255–1262. doi:10.1016/S1473-3099(20)30581-8
3. Morawska L, Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139:105730. doi:10.1016/j.envint.2020.105730
4. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117:11727–11734. doi:10.1073/pnas.2003138117
5. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi:10.1016/j.cell.2020.02.052
6. Bertram S, Heurich A, Lavender H, et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7:e35876. doi:10.1371/journal.pone.0035876
7. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13:1619–1629. doi:10.1016/j.jiph.2020.07.001
8. Torabi A, Mohammadbagheri E, Akbari Dilmaghani N, et al. Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. ACS Chem Neurosci. 2020;11:1909–1913. doi:10.1021/acschemneuro.0c00249
9. Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.01446
10. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi:10.1172/JCI137244
11. Bilinska K, Butowt R. Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem Neurosci. 2020;11:2152–2155. doi:10.1021/acschemneuro.0c00406
12. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi:10.1001/jamainternmed.2020.0994
13. Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa449
14. Zhang C, Wu Z, Li JW, Zhao H, Wang G-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi:10.1016/j.ijantimicag.2020
15. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi:10.1186/s40425-018-0343-9
16. Mason RJ. Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J. 2020;55:2000607. doi:10.1183/13993003.00607-2020
17. Sivabakya TK, Srinivas G. Lung barrier function in COVID-19? SN Compr Clin Med. 2020;2:1299–1301. doi:10.1007/s42399-020-00427-5
18. Sabino EC, Buss LF, Carvalho MPS, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. doi:10.1016/S0140-6736(21)00183-5
19. Roguet R, Cohen C, Dossou KG, et al. Episkin, a reconstituted human epidermis for assessing in vitro the irritancy of topically applied compounds. Toxicol in vitro. 1994;8:283–291.
20. Shrivastava R, Delomenie C, Chevalier A, et al. Comparison of in vivo acute lethal potency and in vitro cytotoxicity of 48 chemicals. Cell Biol Toxicol. 1992;8:157–170. doi:10.1007/BF00260565
21. Shrivastava R. In vitro tests in pharmacotoxicology: can we fill the gap between scientific advances and industrial needs? Altern Lab Anim. 2020. doi:10.1177/026119299702500315
22. Shrivastava R, Shrivastava L, Shrivastava RM. Composition for topical application comprising glycerol and tannins PCT. Patent/WO/20141/194966. 2017. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014194966.
23. Riss T, Moravec R, Niles AD. S. Cell viability assays - assay guidance manual - NCBI bookshelf; 2016. Available from: https://www.ncbi.nlm.nih.gov/books/NBK144065/.
24. Fischer AH, Jacobson KA, Rose J, et al. Hematoxylin and Eosin staining of tissue and cell sections. Cold Spring Harb Protoc. 2008;2008:
25. Schrage A, Kolle SN, Moreno MCR, et al. Bovine corneal opacity and permeability test in routine ocular irritation testing and its improvement within the limits of OECD test guideline 437. Altern Lab Anim. 2011;39:37–53. doi:10.1177/026119291103900119
26. Smith AAA, Kryger MBL, Wohl BM, et al. Macromolecular (pro)drugs in antiviral research. Polym Chem. 2014;5:6407–6425. doi:10.1039/C4PY00624K
27. Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review; 2018. Available from: https://pubmed.ncbi.nlm.nih.gov/29692864/.
28. Neufurth M, Wang X, Tolba E, et al. The inorganic polymer, polyphosphate, blocks binding of SARS-CoV-2 spike protein to ACE2 receptor at physiological concentrations. Biochem Pharmacol. 2020;182:114–215. doi:10.1016/j.bcp.2020.114215
29. Kritas SK, Ronconi G, Caraffa A, et al. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents. 2020;34(1):9–14. doi:10.23812/20-Editorial-Kritas
30. Conti P, Caraffa A, Gallenga CE, et al. IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: inhibitory effect of the IL-1 receptor antagonist (IL-1Ra). J Biol Regul Homeost Agents. 2020;34(5):1623–1627. doi:10.23812/20-34-4EDIT-65
31. Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. doi:10.23812/CONTI-E
32. Conti P, Gallenga CE, Tetè G, et al. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J Biol Regul Homeost Agents. 2020;34(2):333–338. doi:10.23812/Editorial-Conti-2
33. Batah SS, Fabro AT. Pulmonary pathology of ARDS in COVID-19: a pathological review for clinicians. Respir Med. 2020;176:106239. doi:10.1016/j.rmed.2020.106239
34. Shrivastava R, Shrivastava C. New synergistic compositions for the treatment of topical viral infections. PCT Patent WO2011082835A1. 2011. https://patents.google.com/patent/WO2011082835A1/en.
35. Shrivastava R, Shrivastava L, Shrivastava R. Dual acting polymers in an osmotic film for topical application to treat inflammatory diseases and cytokine release syndrome. PCT Patent Pending N° R36575WO.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.