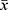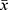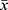Back to Journals » Journal of Inflammation Research » Volume 16
Anti-Inflammatory and Antioxidant Effects of Liposoluble C60 at the Cellular, Molecular, and Whole-Animal Levels
Authors Hui M, Jia X , Li X, Lazcano-Silveira R, Shi M
Received 18 August 2022
Accepted for publication 16 December 2022
Published 7 January 2023 Volume 2023:16 Pages 83—93
DOI https://doi.org/10.2147/JIR.S386381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Mizhou Hui,1 Xiaoxiao Jia,1 Xinrong Li,2 Rayko Lazcano-Silveira,1 Ming Shi3
1College of Life Sciences, Northeast Agricultural University, Harbin, Heilongjiang, People’s Republic of China; 2College of Animal Science and Technology, Qingdao Agricultural University, Qingdao, Shandong, People’s Republic of China; 3Harbin Institute of Technology, Harbin, Heilongjiang, People’s Republic of China
Correspondence: Ming Shi, Harbin Institute of Technology, 2 Yikuang Street, Nangang District, Harbin, Heilongjiang, 150001, People’s Republic of China, Tel +86 13654537645, Email [email protected]
Introduction: Liposoluble carbon-60 (C60) has potential applications in many fields, including cosmetics, medical devices, and medicine, but its specific mechanism of action remains unclear. This study explored whether liposoluble C60 could be delivered to human organs, tissues, and cells through blood, extracellular fluid, and cell culture fluid and whether it exerts anti-inflammatory and antioxidant effects at the molecular, cellular, and whole-animal levels.
Methods: At the cellular level, we mixed C60 dissolved in grape seed oil with cell culture medium containing 10% serum and investigated its effects on tumor necrosis factor-α (TNF-α) release, migration, phagocytosis, respiratory burst, and apoptosis in freshly isolated human neutrophils. At the molecular level, we mixed a trace amount of C60 dissolved in grape seed oil with aqueous and ethanolic solutions and studied its antioxidant effect. At the animal level, we investigated the inhibitory effect of C60 on the serum inflammatory marker C-reactive protein (CRP) in beagle dogs after oral administration of C60 dissolved in grape seed oil.
Results: The results showed that the trace amount of C60 dissolved in grape seed oil significantly inhibited TNF-α release, cell migration, phagocytosis, and respiratory burst in freshly isolated human neutrophils. In addition, the trace amount of C60 dissolved in grape seed oil had a significant scavenging effect on superoxide free radicals and 1,1-diphenyl-2-trinitrophenylhydrazine free radicals. Oral administration of C60 dissolved in grape seed oil markedly reduced the level of the serum inflammatory marker CRP in beagle dogs.
Conclusion: In summary, a trace amount of hydrophobic C60 in hydrophilic media effectively produced anti-inflammatory and antioxidant effects in cells and animals. C60 dissolved in grape seed oil is a novel anti-inflammatory and antioxidant drug candidate.
Keywords: Carbon 60, anti-inflammation, anti-oxidation, C-reactive protein
Introduction
Carbon-60 (C60 or fullerene) is a spherically structured molecule composed of 60 carbon atoms. It has a molecular weight of 720.1–3 In 1985, astronomers used high-energy lasers to sputter graphite placed in a vacuum chamber environment in the laboratory, and accidentally discovered the soccer-ball-like C60.1–3 In 1996, three scientists won the Nobel Prize in Chemistry for the discovery of this new material. C60 is a liposoluble substance. It can be dissolved in vegetable oils (including olive oil and grape seed oil) and is highly soluble in toluene and xylene. Although C60 is insoluble in water, it becomes water-soluble when more than 20 hydroxyl groups are introduced in it.1−3
C60 strongly absorbs free radicals, inhibits the toxicity of chemical toxicants, resists radiation, prevents ultraviolet damage, and prevents heavy metal–induced cell damage. It has potential applications in the fields of cosmetics, medical devices, and medicine.4,5 C60, as an extremely potent antioxidant,6,7 had shown important effects in the fields of skin beauty, hair follicle growth, and anti-aging.8–12 Therefore, C60 has joined the list of cosmetics in Japan, Taiwan, the United States, and China. C60 is an antioxidant “genius” that has officially entered China’s beauty industry.6,7 French scientists dissolved C60 in olive oil and fed it to mice (4 mg of C60 per kg body weight) once every two weeks for 10–17 months. The results showed that the mice in the control group died in 17–38 months, while the life span of the mice in the experimental group was extended to 59–66 months. During the period, the experimental group showed no abnormalities in body weight or physiological manifestations.13 Therefore, C60 is informally sold as a “health food” ingredient in the United States and the European Union.14
C60 dissolved in olive oil functions as a very strong antioxidant and has a therapeutic effect on inflammatory skin diseases, cancers, and intestinal diseases.11–18 The authors of this study discovered that cosmetic-grade C60 dissolved in grape seed oil could quickly and noticeably relieve skin itching and pain in muscles, tendons, and joints when applied and massaged on the skin (patent applied for19). In this study, C60 dissolved in grape seed oil (3 mg/mL) was used as the research object, and its anti-inflammatory and antioxidant effects were investigated at the cellular, molecular, and whole-animal levels. Grape seed oil droplets containing a trace amount of C60 were mixed with culture medium supplemented with 10% bovine serum, and a series of cell-level experiments were conducted to measure the release of tumor necrosis factor α (TNF-α), migration, phagocytosis, respiratory burst, and apoptosis in freshly isolated human neutrophils. We next mixed the grape seed oil droplets containing a trace amount of C60 with aqueous and ethanolic solutions and investigated the antioxidant effect of the C60–grape seed oil at the molecular level. We mainly explored whether the trace amount of water-insoluble, liposoluble C60 could be delivered in water-soluble liquids and produce an anti-inflammatory effect at the cellular level and an antioxidant effect at the molecular level. Finally, we explored the effect of oral administration of C60–grape seed oil on C-reactive protein (CRP), a marker of inflammation, in the blood of dogs.
Materials and Methods
Materials
Experimental Samples
Human blood samples were obtained from the forearm venous blood of 18 healthy volunteers aged 26 ± 5 years. The venous blood samples were obtained from total of six beagles (three males and three females, weighing 7 ± 2 kg) under the same feeding conditions. The documented review and approval from a formally constituted review board (Ethics committee of Qingdao Agriculture University) as well as the written informed consent of all the 18 volunteers in accordance with the Declaration of Helsinki were obtained for collection of all the human blood samples and experimentation of the six beagles.
Experimental Reagents
The following reagents were used in this study: C60 dry powder (Suzhou Dade Carbon Nano Technology Co., Ltd., China), grape seed oil (Pinli Food Co., Ltd., China), fetal bovine serum (FBS, Tianhang Biotechnology, China), penicillin (HyClone, USA), phorbol ester (PMA), lipopolysaccharide (LPS), agarose, molecular probe DHR123, vitamin E (Solarbio life sciences, China), ascorbic acid (Guangcheng Chemical, China), N-formyl-methionyl-leucyl-phenylalanine (fMLP, Shanghai PrimeGene Bio-Tech Company Ltd., China), carboxylate-modified polystyrene (latex beads) (Sigma-Aldrich, USA), Human Neutrophil Isolation Kit (Tianjin Haoyang Huake Biotechnology Co., Ltd., China), TNF-α ELISA kit (R&D Systems, USA), the Annexin V–FITC Cell Apoptosis Detection Kit (Beyotime Biotechnology, China), and RPMI1640 medium.
Methods
Preparation of C60 Stock Solution
C60 dry powder with a purity of >0.99 was dissolved in grape seed oil (hereinafter referred to as C60-Oil). To prepare C60-Oil stock solution with a concentration of 3 mg/mL, C60-Oil was placed in a magnetic stirrer and mixed thoroughly for 9 days at room temperature. The stock solution was sterilized by passing through a 0.22-µM filter and then stored.
Dilution of the C60-Oil Stock Solution
The wrapping method. This method was used to investigate the release of TNF-α, phagocytosis of foreign-body material, and apoptosis in human neutrophils. The C60-Oil stock solution was mixed with RPMI1640 (10% FBS, 1% S/P), resulting in a diluted C60-Oil suspension with a concentration of 20 µg/mL. The C60-Oil suspension was added to cell culture plates (100 µL each well). After repeated pipetting, 80 µL of the C60-Oil suspension was aspirated out, leaving 20 µL of C60-Oil on the bottom of the cell culture plate. Next, 180 µL of human venous blood neutrophils were added to each well. The final concentration of C60-Oil was 2 µg/mL in the culture medium (Figure S1).
The direct dilution method. This method was used to investigate the migration, cellular respiratory burst, and foreign-body material–induced respiratory burst of human neutrophils. The C60-Oil stock solution was mixed with RPMI1640 (10% FBS, 1% S/P) and human venous blood neutrophils into a C60-Oil suspension with a final concentration of 2–10 µg/mL.
Isolation of Neutrophils from Human Venous Blood
The neutrophils were isolated using the Human Neutrophil Isolation Kit in accordance with the manufacturer’s instructions. Cell morphology was examined after staining with a leukocyte classification stain. After counting, the neutrophils were adjusted to the desired density. In each experiment described below, blood was collected from three different volunteers.
Quantification of the Release of TNF-α by Human Neutrophils
In this experiment, the wrapping method was used to treat the bottom surface of a 96-well cell culture plate. The density of human neutrophils was adjusted to 5×105 cells/mL, and 180 µL of cell suspension was added to each well of the plate. The final concentration of the samples in the C60-Oil group was 2 µg/mL. After 24 h of culture, the cell supernatants were collected. The concentration of TNF-α was determined using a human enzyme-linked immunosorbent assay kit following the manufacturer’s instructions.
Examination of the Migration of Human Neutrophils
Five hundred microliters of 0.8% sterile agarose gel was mixed with 500 µL RPMI1640 (20% FBS, 1% S/P). The neutrophils were adjusted to a density of 3×108 cells/mL, mixed thoroughly with the above mixture at a ratio of 1:1, and placed in a 37℃ water bath. The neutrophil/agarose mixture was added to a precooled 96-well plate (2 µL each well), which formed droplets with a diameter of 2 mm on the bottom of the well. The droplets were kept at 4℃ for 15 min. Cell migration was observed after treatment with either 1 ng/mL LPS in grape seed oil, 1 nM fMLP in grape seed oil, or 4 µg/mL C60-Oil for 3 h. The migration distance and area were calculated in ImageJ.
Examination of the Phagocytosis of Foreign Bodies in Human Neutrophils
The wrapping method was used to treat the bottom surface of the 24-well cell culture plate. The human neutrophils were adjusted to a density of 2×106 cells/mL, and 180 µL of the cell suspension was added to each well of the plate. C60-Oil and LPS were then added at the final concentrations of 2 µg/mL and 1 ng/mL, respectively. Then 3.5 µL of latex beads with a diameter of 2 µm were added to stimulate the neutrophils to achieve a fluorescent particle density of 2×107/mL, thereby establishing the optimal phagocytosis model of neutrophils and fluorescent particles. After 1 h at 37℃, the phagocytosis rate of the neutrophils was measured by flow cytometry.
Respiratory Burst Assay of Human Neutrophils (Examination of the Production of Reactive Oxygen Species (ROS))
Human neutrophils were resuspended at a density of 3×106 cells/mL, and 177 µL of the cell suspension was added to each well of a 24-well plate. Then, 3 µL of molecular probe DHR123 was added to the cells at a final concentration of 5 µM. After incubation of cells at 37℃ for 15 min in the dark, C60-Oil was added at a final concentration of 2 µg/mL, 5 µg/mL, or 10 µg/mL. After 30 min at 37℃, 24 µL of PMA (final concentration, 50 nM) was added to stimulate cellular oxidative respiration. The cells were then incubated at 37℃ for 30 min, rinsed once with phosphate-buffered saline (PBS), and resuspended in 800 µL of PBS. The production of ROS was examined by flow cytometry.
In the assay of the respiratory burst induced by foreign-body particles, cells were treated with C60-Oil and LPS at final concentrations of 2 µg/mL and 1 ng/mL, respectively. Finally, 3.5 µL of latex beads with a diameter of 2 µm were added to the cells. After incubation at 37℃ for 40 min in the dark, the cells were washed and resuspended in PBS. Again the amount of ROS was measured.
Examination of the Apoptosis of Human Neutrophils
The wrapping method was used to treat the bottom of a 24-well cell culture plate, leaving 20 µL of C60-Oil suspension in each well. Human neutrophils were resuspended and their cell density adjusted to 1×106 cells/mL. Next, 180 µL of the cell suspension was added to each well of the plate. C60-Oil and LPS were then added at respective final concentrations of 2 µg/mL and 1 ng/mL. After 3 h, the cells were stained using the Annexin V–FITC Apoptosis Detection Kit following the manufacturer’s instructions.
Determination of the Superoxide Radical Scavenging Activity
C60-Oil was suspended in an aqueous solution containing a small amount of PBS, and the scavenging effect of C60-Oil on the superoxide anion radicals in the aqueous solution was examined. The total reaction volume was 5.14 mL (see Table S1 for details). The control groups in this experiment included Oil, Vitamin E+Oil, and Vitamin C groups. The concentration of vitamin E dissolved in grape seed oil and the concentration of vitamin C dissolved in PBS were the same as that of C60.
Determination of the Activity of 1.1-Diphenyl-2-Trinitrophenylhydrazine (DPPH) Free Radical Scavenging
C60-Oil was suspended in an ethanolic solution with PBS, and the scavenging effect induced by C60-Oil on the DPPH free radical in the PBS-containing ethanolic solution was examined. The total reaction volume in this experiment was 4 mL (see Table S2 for details). The control groups of this experiment included Oil, Vitamin E+Oil, and Vitamin C groups. Vitamin E was soluble in grape seed oil, ethanol, and PBS-containing ethanolic solutions. Vitamin C was soluble in both PBS and ethanolic solutions with PBS. The concentrations of vitamin E and vitamin C in the solutions were the same as that of C60.
Investigation of the Effect of Oral Administration of C60-Oil on CRP, a Marker of Inflammation, in Dog Blood
Three male and three female beagle dogs with similar spirit, appetite, body temperature, and other clinical manifestations were selected. The CRP levels in their venous blood were determined (Day 0). Every day for 9 days, 50 mL of C60-Oil at a concentration of 3 mg/mL was mixed with the dog food that the dogs were fed. The CRP levels were measured on the 3rd, 6th, and 9th days.
Statistical Analysis
All data are expressed as 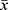 ± s. Two groups of data were compared by the paired-sample Student’s t-test. * P < 0.05 and ** P < 0.01 indicated significant differences and highly significant differences, respectively. Data analysis was conducted in GraphPad Prism 6.0, which was also used to output the tables and graphs.
± s. Two groups of data were compared by the paired-sample Student’s t-test. * P < 0.05 and ** P < 0.01 indicated significant differences and highly significant differences, respectively. Data analysis was conducted in GraphPad Prism 6.0, which was also used to output the tables and graphs.
Results
The Anti-Inflammatory Activity of C60-Oil at the Cellular Level
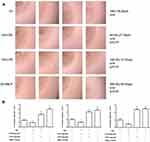
Neutrophils participate in tissue inflammatory responses by migrating to the inflammatory tissues. The migration of neutrophils is also involved in the tissue damage process in inflammatory diseases. This study employed a method of mixing neutrophils with agarose gel to investigate the effect of C60-Oil on the migration of freshly isolated human neutrophils. The results showed that the area and distance of neutrophil migration from the inside to the outside of the agarose gel droplets were lower in the C60 group than the Oil group (P < 0.05, n=4). In contrast, the migration area and distance of neutrophils were significantly increased in the LPS and the fMLP positive control groups (P < 0.01, n=4) (Figure 1). These results indicate that the C60 dissolved in grape seed oil entered the agarose gel droplets through cell culture medium and inhibited the migration of freshly isolated human neutrophils. In addition, compared with the Oil group, the C60-Oil group had less TNF-α release by neutrophils (Figure 2A). These results demonstrate that C60 significantly inhibited the release of TNF-α by freshly isolated neutrophils.
Human neutrophils can be activated by LPS to phagocytize damaged tissue fragments, foreign microorganisms, and foreign bodies. This study examined the ability of the freshly isolated human neutrophils to phagocytize exogenous fluorescent particles. C60-Oil reduced the phagocytic rate of neutrophils regardless of whether LPS was present (Figure 2B). This finding indicated that C60-Oil could enter neutrophils and inhibit their phagocytic function whether or not they were activated. In addition, when C60-Oil was cocultured with the freshly isolated neutrophils, it had no effect on neutrophil apoptosis (Figure 2C).
The inflammatory cell activator PMA induces respiratory burst in neutrophils, which produces a large amount of ROS. This study explored the effect of C60-Oil on respiratory burst in freshly isolated human neutrophils. C60 dissolved in grape seed oil (2–10 µg/mL) inhibited the PMA-induced ROS production by neutrophils in a dose-dependent manner (Figure 3A). Since foreign particles such as latex beads stimulate respiratory burst in freshly isolated neutrophils, we also explored the effect of C60-Oil on the foreign particle–induced respiratory burst in freshly isolated human neutrophils. The results showed that C60-Oil not only inhibited the foreign particle–induced ROS production in neutrophils but also inhibited their LPS-stimulated ROS production (Figure 3B). These results demonstrate that the C60 dissolved in grape seed oil entered neutrophils through cell culture fluid and inhibited the respiratory burst in neutrophils induced by two foreign particles, latex beads and LPS.
In summary, C60 in grape seed oil can enter neutrophils through cell culture fluid to inhibit the ability of neutrophils to migrate, phagocytize, and release TNF-α. In addition, C60 inhibits the ability of neutrophils to generate ROS and induce respiratory burst after stimulation. However, C60 does not affect the apoptosis of neutrophils.
The Antioxidant Activity of C60-Oil at the Molecular Level
C60 is insoluble in water and PBS. However, the scavenging effect of C60-Oil suspended in PBS on superoxide radicals was dose dependent (Table S3). This result demonstrated that C60 entered the PBS solution through an unknown mode that did not involve dissolution and exerted its scavenging effect on the superoxide free radical (O2-). Furthermore, this result suggested that the cytobiological effect of C60-Oil on the freshly isolated human neutrophils was related to its antioxidant activity. The grape seed oil droplets alone had no ability to scavenge superoxide. Vitamin E is insoluble in water and PBS. Therefore, vitamin E–grape seed oil droplets suspended in PBS had no ability to scavenge superoxide. In contrast, the water-soluble vitamin C did scavenge superoxide.
C60 is insoluble in ethanolic reaction solution containing PBS. The experimental results of this study showed that, in ethanol reaction solution containing PBS, C60 dissolved in grape seed oil exerted a scavenging effect on DPPH free radicals in a dose-dependent manner (Figure 4). In other words, C60 entered the PBS-containing ethanolic reaction solution through an unknown transfer mode that did not involve dissolution and exerted a scavenging effect on DPPH free radicals, indicating that the molecular mechanism of the anti-inflammatory biological effect of C60-Oil on the freshly isolated human neutrophils was related to its antioxidant effect. Grape seed oil droplets alone had no ability to scavenge DPPH. Vitamin E is soluble in ethanol. Therefore, vitamin E–grape seed oil droplets had the ability to scavenge DPPH at the high concentrations of 6–8 µg/mL. Vitamin C can be dissolved by PBS. Therefore, in an ethanolic solution containing PBS, vitamin C also exerted a scavenging effect on DPPH in a dose-dependent manner.
 |
Figure 4 Scavenging effect of C60-Oil on DPPH free radicals. |
The Effect of Oral Administration of C60-Oil on the Inflammatory Marker CRP in Canine Blood
After 3 days of oral administration of C60-Oil, the dogs’ serum CRP level was decreased drastically and continued to decline in the following 6 days (Figure 5). These results show that the C60 dissolved in grape seed oil entered the Beagle’s body and significantly reduced the serum level of the inflammatory marker CRP. That is, C60-Oil had a therapeutic effect on inflammation.
Discussion
Under normal conditions, human neutrophils are inactive in blood vessels and do not participate in immune or inflammatory responses. Once activated, human neutrophils migrate to the sites of microbial invasion and inflammation, where their effect is a double-edged sword. Neutrophils not only eliminate the invading pathogenic microorganisms but also participate in the pathological process of inflammatory diseases. For example, ROS released by the activated neutrophils are involved in inflammatory atherosclerosis and brain tissue injury.20 Activated neutrophils migrate to the skin and participate in the pathological processes of inflammatory diseases such as papules and ulcers.21 The above studies suggest that inhibition of the activation and inflammation-promoting effect of neutrophils is a potential treatment for inflammatory diseases.
C60 is insoluble in aqueous solutions, including blood, interstitial fluid, and cell culture fluid. Therefore, we dissolved C60 in grape seed oil and diluted this with serum-containing cell culture medium and wrapped it on the surface of the cell culture medium, forming oil droplets (Figure S1). We also directly diluted the C60-Oil with R1640 cell culture containing 10% bovine serum to form a cell culture liquid containing oil droplets.
The experimental results showed that C60-Oil inhibited TNF-α release, migration, phagocytosis, and ROS production by human neutrophils (Figures 1–3). However, C60-Oil had no significant effect on neutrophil apoptosis or death (Figure 2C). Such results indicate that C60-Oil exerts an inhibitory effect on the immune activity of neutrophils and has no cytotoxic activity. The amount of C60 dissolved in grape seed oil was 2–10 μg/mL, while the concentration of C60 that entered the cultured neutrophils was even lower. This finding suggests that a trace amount of the water-insoluble, liposoluble C60 was effectively delivered in an unknown way and produced an anti-inflammatory effect at the cellular level. This study suggests that liposoluble C60 might be administered through the mucous membrane and the skin to treat the inflammatory diseases related to the release of TNF-α, migration, phagocytosis, and production and release of ROS in human neutrophils. This study used freshly isolated human blood neutrophils, which eliminated the species differences caused by the use of small animals in drug studies and makes our study one step closer to a human clinical trial. At present, there is no report that the liposoluble inorganic C60 used in cosmetics has side effects on the human body.
The water-soluble derivatives of C60 with introduced hydroxyl groups represent a promising therapeutic agent to control ROS-dependent inflammation (including allergic diseases).22 The water-soluble derivatives of C60 may enhance the enzyme activity of superoxide dismutase in microglia, which is involved in inflammatory pain.23 The results of this study showed that C60-Oil had a significant scavenging effect on superoxide free radicals and DPPH free radicals, which effects were dose dependent (Table S3, Figure 4). These results indicate that C60 can be effectively transferred at the molecular level in water-soluble liquids, and the free radical–scavenging effect of C60-Oil is related to the anti-inflammatory mechanism of C60-Oil at the cellular level.24 Clinical drug studies suggest that at the molecular level, transfer of water-insoluble corticosteroids in aqueous liquids is similar to the transfer of C60 dissolved in grape seed oil. In cellular-level studies, corticosteroids are generally dissolved in ethanol first. The corticosteroids dissolved in ethanol will precipitate out tiny crystals after dilution with cell culture fluid, which dissolve into the cell membrane and produce cytological effects. In contrast, C60-Oil does not precipitate out tiny crystals. After entering the human body, C60 binds to blood albumin and is transported in the body, as corticosteroids are.25–27 In addition, the C60 dissolved in vegetable oil can directly enter cell membranes28–30 and the human body.15,31,32 The C60 dissolved in vegetable oil can also enter the human blood circulation after being phagocytized by cells.32 One study has reported on one of the anti-inflammatory mechanisms of C60: C60 may attract human neutrophils and induce them to phagocytize it after entering the human body, rendering the neutrophils unable to damage human tissues and cause diseases.15 The interaction between liposoluble C60 and neutrophils in water-soluble liquids is a new scientific link between physics and cell biology. This study found, through the clear inflammatory cell–regulating function of liposoluble C60 at the cellular level and the antioxidant effect of C60 at the molecular level, that the water-insoluble liposoluble C60 is effectively transferred to cells in water-soluble liquids through an unknown mode.
CRP is a serum marker of inflammation. CRP is synthesized in the liver and released into the blood. Inflammatory cells release inflammatory factors to stimulate the synthesis of CRP and the release of CRP into the blood.33,34 Plasma CRP is significantly increased during acute inflammation (such as that caused by infection, trauma, tissue necrosis, malignant tumors, skin infection, cystitis, bronchitis, and allergic reactions). Plasma CRP is slightly increased in chronic inflammation (such as atherosclerosis, type 2 diabetes, obesity, periodontal disease, colitis, and rheumatoid arthritis).35–37 However, the level of CRP will decrease rapidly after the inflammation subsides.38 This study explored the effect of oral administration of C60-Oil on the blood CRP level in beagle dogs. Their serum CRP level showed a continuous declining trend after treatment (Figure 5). Elevation of serum CRP is common in cardiovascular and cerebrovascular diseases.39 Oral administration of C60-Oil strongly reduced the serum CRP of the dogs, suggesting that C60 may have a therapeutic effect on cardiovascular and cerebrovascular diseases related to elevated CRP.
Conclusion
In summary, a trace amount of hydrophobic C60 in hydrophilic media effectively produced anti-inflammatory and antioxidant effects in cells and animals. C60 dissolved in grape seed oil is a novel anti-inflammatory and antioxidant drug candidate. How water-insoluble C60 was effectively transferred at the molecular level in water-soluble liquids should be further studied.
Acknowledgments
We would like to thank the Science and Technology Center of Qingdao Agricultural University for supporting this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kroto HW, Heath JR, O’Brien SC, et al. C60: buckminsterfullerene. Nature. 1985;318:162–163. doi:10.1038/318162a0
2. Diederich F, Ettl R, Rubin Y, et al. The higher fullerenes: isolation and characterization of C76, C84, C90, C94, and C70O, an oxide of D5h-C70. Science. 1991;252:548–551. doi:10.1126/science.252.5005.548
3. Palit DK, Sapre AV, Mittal JP, et al. Photophysical properties of the fullerenes, C60 and C70. Chem Phys Lett. 1992;195:1–6. doi:10.1016/0009-2614(92)85900-U
4. Kato S, Kikuchi R, Aoshima H, et al. Defensive effects of fullerene-C60/liposome complex against UVA-induced intracellular reactive oxygen species generation and cell death in human skin keratinocytes HaCaT, associated with intracellular uptake and extracellular excretion of fullerene-C60. J Photochem Photobiol B. 2010;98:144–151. doi:10.1016/j.jphotobiol.2009.11.015
5. Kato S, Aoshima H, Saitoh Y, et al. Fullerene-C60/liposome complex: defensive effects against UVA-induced damages in skin structure, nucleus and collagen type I/IV fibrils, and the permeability into human skin tissue. J Photochem Photobiol B. 2010;98:99–105. doi:10.1016/j.jphotobiol.2009.11.010
6. Galvan YP, Alperovich I, Zolotukhin P, et al. Fullerenes as anti-aging antioxidants. Current Aging Sci. 2017;10(1):56–67. doi:10.2174/1874609809666160921120008
7. Mousavi SZ, Nafisi S, Maibach HI. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomedicine. 2017;13(3):1071–1087. doi:10.1016/j.nano.2016.10.002
8. Inui S, Mori A, Ito M, et al. Reduction of conspicuous facial pores by topical fullerene: possible role in the suppression of PGE2 production in the skin. J Nanobiotechnology. 2014;12:6. doi:10.1186/1477-3155-12-6
9. Dellinger A, Zhou Z, Lenk R, et al. Fullerene nanomaterials inhibit phorbol myristate acetate–induced inflammation. Exp Dermatol. 2009;18:1079–1081. doi:10.1111/j.1600-0625.2009.00904.x
10. Inui S, Aoshima H, Nishiyama A, et al. Improvement of acne vulgaris by topical fullerene application: unique impact on skin care. Nanomedicine. 2011;7:238–241. doi:10.1016/j.nano.2010.09.005
11. Ryan JJ, Bateman HR, Stover A, et al. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179(1):665–672. doi:10.4049/jimmunol.179.1.665
12. Kopova I, Lavrentiev V, Vacik J, et al. Growth and potential damage of human bone-derived cells on fresh and aged fullerene C60 films. Int J Mol Sci. 2013;14(5):9182–9204. doi:10.3390/ijms14059182
13. Baati T, Bourasset T, Gharbi T, et al. The prolongation of the lifespan of rats by repeated oral administrtion of fullerene. Biomaterials. 2012;33(19):4936–4946. doi:10.1016/j.biomaterials.2012.03.036
14. Keykhosravi S, Rietveld IB, Couto B, Tmarit JL. [60] Fullerene for medical purposes, A purity criterion towards regulatory considerations. Materials. 2019;12(16):2571. doi:10.3390/ma12162571
15. Fromen CA, Kelley WJ, Fish MB, et al. Neutrophil–particle interactions in blood circulation drive particle clearance and alter neutrophil responses in acute inflammation. ACS Nano. 2017;11(11):10797–10807. doi:10.1021/acsnano.7b03190
16. Saito E, Kuo R, Pearson RM, et al. Designing drug-free biodegradable nanoparticles to modulate inflammatory monocytes and neutrophils for ameliorating inflammation. J Control Release. 2019;300:185–196. doi:10.1016/j.jconrel.2019.02.025
17. Larysa LM, Prylutska SV, Rudyk MP, et al. C60 Fullerene and its nanocomplexes with anticancer drugs modulate circulating phagocyte functions and dramatically increase ROS generation in transformed monocytes. Cancer Nano. 2018;9(1):8. doi:10.1186/s12645-017-0034-0
18. Dellinger AL, Zhou ZG, Kepley CL. A steroid-mimicking nanomaterial that mediates inhibition of human lung mast cell responses. Nanomedicine. 2014;10(6):1185–1193. doi:10.1016/j.nano.2014.02.006
19. Xinrong LI. A novel application of Carbon-60 and its activity assays. Patent application number:202010169138.X; 2020.
20. Jaganjac M, Cipak A, Schaur RJ, et al. Pathophysiology of neutrophil-mediated extracellular redox reactions. Front Biosci. 2016;21:839–855. doi:10.2741/4423
21. Marzano AV, Borghi A, Wallach D, et al. A comprehensive review of neutrophilic diseases. Clin Rev Allergy Immunol. 2018;54(1):114–130. doi:10.1007/s12016-017-8621-8
22. Shershakova N, Baraboshkina E, Andreev S, et al. Anti-inflammatory effect of fullerene C60 in a mice model of atopic dermatitis. J Nanobiotechnology. 2016;14:8. doi:10.1186/s12951-016-0159-z
23. Tzeng SF, Lee JL, Kuo JS, et al. Effects of malonate C60 derivatives on activated microglia. Brain Res. 2002;940(1–2):61–68. doi:10.1016/S0006-8993(02)02592-1
24. Saisavoey T, Sangtanoo P, Reamtong O, et al. Antioxidant and anti-inflammatory effects of defatted rice bran (oryza sativa l.) protein hydrolysates on raw 264.7 macrophage cells. J Food Biochem. 2016;40(6):731–740. doi:10.1111/jfbc.12266
25. Song MY, Liu SF, Yin JF, et al. Interaction of human serum album and C60 aggregates in solution. Int J Mol Sci. 2011;12(8):4964–4974. doi:10.3390/ijms12084964
26. Fu XF, Fang YL, Zhao HL, et al. Size-dependent binding of pristine fullerene (nC60) nanoparticles to bovine/human serum albumin. J Mol Struct. 2018;1166:442–447. doi:10.1016/j.molstruc.2018.04.067
27. Liu QH, Jin L, Mahon BH, et al. Novel treatment of neuroinflammation against low back pain by soluble fullerol nanoparticles. Spine. 2013;38(17):1443–1451. doi:10.1097/BRS.0b013e31828fc6b7
28. Sastre J, Mannelli I, Reigada R. Effects of fullerene on lipid bilayers displaying different liquid ordering: acoarse-grained molecular dynamics study. Biochim Biophys Acta Gen Subj. 2017;1861(11 Pt A):2872–2882. doi:10.1016/j.bbagen.2017.08.004
29. Ha Y, Katz LE, Liljestrand HM. Distribution of fullerene nanoparticles between water and solid supported lipid membranes: thermodynamics and effects of membrane composition on distribution. Environ Sci Technol. 2015;49(24):14546–14553. doi:10.1021/acs.est.5b03339
30. Ikeda A, Kiguchi K, Shigematsu T, Nobusawa K, Kikuchi J-I, Akiyama M. Location of [60] fullerene incorporation in lipid membranes. Chem Commun. 2011;47(44):12095–12097. doi:10.1039/c1cc14650e
31. Dellinger A, Zhou ZG, Norton SK, Lenk R, Conrad D, Kepley CL. Uptake and distribution of fullerenes in human mast cells. Nanomedicine. 2010;6(4):575–582. doi:10.1016/j.nano.2010.01.008
32. Russ KA, Elvati P, Parsonage TL, et al. C60 fullerene localization and membrane interactions in RAW 264.7 immortalized mouse macrophages. Nanoscale. 2016;8(7):134–4144. doi:10.1039/C5NR07003A
33. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi:10.1172/JCI200318921
34. Lau DC, Dhillon B, Yan H, et al. Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol. 2005;288(5):2031–2041. doi:10.1152/ajpheart.01058.2004
35. Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi:10.1001/jama.286.3.327
36. Dehghan A, Kardys I, de Maat MP, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56(3):872–878. doi:10.2337/db06-0922
37. Clearfield MB. C-reactive protein: a new risk assessment tool for cardiovascular disease. J Am Osteopath Assoc. 2005;105(9):409–416.
38. Bray C, Bell LN, Liang H, et al. Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine. WMJ. 2016;115(6):317–321.
39. Avan A, Tavakoly Sany SB, Ghayour-Mobarhan M, et al. Serum C-reactive protein in the prediction of cardiovascular diseases: overview of the latest clinical studies and public health practice. J Cell Physiol. 2018;233(11):8508–8525. doi:10.1002/jcp.26791
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.