Back to Journals » Journal of Pain Research » Volume 15
Anti-Inflammatory and Antinociceptive Effects of Boesenbergia rotunda Polyphenol Extract in Diabetic Peripheral Neuropathic Rats
Authors Wang P, Wen C, Olatunji OJ
Received 24 January 2022
Accepted for publication 3 March 2022
Published 24 March 2022 Volume 2022:15 Pages 779—788
DOI https://doi.org/10.2147/JPR.S359766
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qi Fang
Peng Wang,1 Chaoling Wen,2 Opeyemi Joshua Olatunji3
1Department of Pharmacy, Wuhu Second People’s Hospital, Wuhu City, 241001, Anhui, People’s Republic of China; 2Anhui Traditional Chinese Medicine College, Wuhu City, 241001, Anhui, People’s Republic of China; 3Traditional Thai Medical Research and Innovation Center, Faculty of Traditional Thai Medicine, Prince of Songkla University, Hat Yai, 90110, Thailand
Correspondence: Opeyemi Joshua Olatunji, Traditional Thai Medical Research and Innovation Center, Faculty of Thai Traditional Medicine, Prince of Songkla University, Hat Yai, 90110, Thailand, Email [email protected]
Introduction: Diabetic peripheral neuropathy (DPN) is still one of the most prevailing complication of diabetes and it affects a huge diabetic population. Boesenbergia rotunda is a ginger species that has both culinary and medicinal applications. Recent studies have revealed that B. rotunda has potential applications in diabetes, pain and inflammatory related disorders. As such this study investigated the potential of B. rotunda extract (EBR) in attenuating DPN in rats.
Methods: DPN was induced in male Sprague Dawley rats using a combination of 30% fructose solution and streptozotocin (40 mg/kg). Afterwards diabetic rats were treated with EBR (100 and 400 mg/kg) for 5 weeks. DPN was assessed using thermal hyperalgesia, cold and mechanical allodynia and rotarod test, while nociceptive responses were assessed by formalin and acetic acid test. In addition, serum proinflammatory cytokine levels were determined using ELISA kits.
Results: EBR displayed hypoglycemic effect by significantly reducing the blood glucose concentration of treated diabetic rats, while simultaneously alleviating the reduced body weight. Furthermore, EBR markedly alleviated thermal hyperalgesia, cold and mechanical allodynic responses as well as ameliorated motor coordination in the treated diabetic rats. In addition, EBR significantly reduced nociceptive responses in the formalin and acetic acid test, as well as decreased serum levels of proinflammatory cytokines (TNF-α and IL-1β).
Conclusion: The results suggested that EBR exerted anti-inflammatory and anti-nociceptive effects, thus alleviating diabetic painful neuropathy.
Keywords: diabetic peripheral neuropathy, diabetes, Boesenbergia rotunda, polyphenols, anti-inflammatory
Introduction
Diabetes mellitus (DM) is a serious, lifelong and incurable metabolic disease with devastating consequences. Almost half a billion people were estimated to be living with DM in 2019 and an additional 200 million people have diabetes but undiagnosed.1,2 DM occurs due to abnormalities in the production of insulin from the pancreas or when the body becomes insensitive and unable to effectively utilize the insulin produced by the pancreas,3 resulting in hyperglycemia, hyperlipidemia as well as alteration in lipids, carbohydrates and protein metabolism.4 The increase in endogenous glucose production in DM accompanied by excessive oxidative stress has been identified as major culprits in several diabetes induced comorbidity including neuropathy, nephropathy, retinopathy, cardiovascular diseases and neurodegenerative diseases.5–7
Diabetic peripheral neuropathy (DPN) is a common symmetrical degenerative comorbidity in diabetic patients and it affects approximately 30–50% of DM patients.8,9 DPN is clinically characterised by sensory, autonomic and motor nerve function defects, which includes hyperalgesia, limb numbness, loss of reflexes and muscle atrophy. All these may eventually lead to infections, foot ulcers and amputations.10,11 Although DPN on its own may not be life threatening, however it can significantly affect the quality of life of diabetic patients and create huge financial burden.12,13
Boesenbergia rotunda (fingerroot) belongs to the family Zingiberaceae and it is a ginger species that is indigenous to many southeast Asian countries and notable for its culinary purposes. B. rotunda is traditionally used in the treatment of fever, rheumatism, muscle pain, peptic ulcer, stomach disorders and bacterial infections.14,15 Aside its folk use, B. rotunda have shown several bioactivities including biofilm formation inhibition, antiobesity, antioxidant, anticancer, antiviral, anti-inflammatory activities and in vitro antidiabetic activities.16–18 Furthermore, B. rotunda is particularly rich in polyphenols notably chalcones and flavonoids, which are majorly responsible for its in vitro antidiabetic effects of the plant.16,19,20 Consequently, we hypothesized that B. rotunda rhizome extract could exert ameliorative effects against diabetes and diabetic complications. However, there are no report on the effect of B. rotunda in in vivo antidiabetic models or its protective effects against diabetic induced comorbidity. This study investigated the effects of B. rotunda rhizome extract on diabetic peripheral neuropathy in high fructose/streptozotocin induced diabetic rats.
Materials and Methods
Chemicals and Reagents
Streptozotocin and fructose were purchased from Alfa Aesar (Massachusetts, United States) and Kemaus (New South Wales, Australia). The kit for measuring proinflammatory cytokines determination were purchased from Abcam (Cambridge, United Kingdom). All other reagents and solvents are of analytical grade and purchased from RCI Labscan.
Plant Material
The rhizomes of B. rotunda was purchased from a Traditional Medicinal Herbs Store in Hat Yai, Songkla, Thailand. Authentication of the plant material and deposition of voucher specimen (BR-TT-050) was done at the Faculty of Thai Traditional Medicine, Prince of Songkla University.
Preparation of Extract
The chopped and dried rhizomes of B. rotunda (1 kg) was pulverised with a mechanical grinder and the powdered samples was macerated exclusively with 5 L of 95% ethanol for 24 h. The pooled ethanolic extracts was filtered and dried using reduced pressure. The dried extract was resuspended in distilled water and extracted with n-hexane and ethyl acetate. The EtOAc soluble portion of B. rotunda (EBR) was dried and stored at 4°C until use.
Isolation of Compounds from EBR
The ethyl acetate fraction (EBR, 5 g) was loaded on sephadex LH-20 column and eluted with MeOH-CH2Cl2 (1:1) to obtain 40 collected fractions which was pulled together to obtain 8 (Fr 1–8) fractions based on their TLC profiles. Panduratin A (650 mg) was purified from sub fractions Fr 4 by recrystallization using a mixture of MeOH/CH2Cl2. Fraction Fr 5 was also subjected to column chromatography eluting with stepwise gradient of EtOAc/MeOH to afford 15 subfractions. The combinations of subfractions 8–12 was recrystallized with MeOH to afford pinostrobin (900 mg). Another portion of EBR (10 g) was separated on silica column chromatography using hexane/EtOAc and EtOAc/MeOH in a gradient elution manner to afford 20 fractions (F1–F20). Fractions 6–9 (485 mg) was chromatographed on Sephadex LH-20 (MeOH), silica column chromatography (hexane–CH2Cl2) and recrystallization (hexane–EtOAc) to afford panduratin A (480 mg), pinocembrin (150 mg) and cardamonin (25 mg). The combined fractions 10–11 was subjected to column chromatography on silica using gradient elution of CH2Cl2/EtOAc/MeOH and sephadex LH-20 (MeOH) and recrystallized in CHCl3/MeOH mixture to afford alpinetin (58 mg) and boesenbergin A (76 mg).
Animals
Twenty four six weeks old male Sprague Dawley rats (160 ± 20 g) were randomly divided into four groups and housed in stainless steel cages under standard environmental and experimental animal husbandry conditions. The animals were acclimatized for 7 days with access to standard rat chows and normal water ad libitum. Animal maintenance procedures was in conformity with the approved procedures of the Research Ethics Committee of Wuhu Second People's Hospital, Wuhu, China (Ethical protocol approval number: WuhueyLLWYH/2021/0928). In addition, the procedures of the National Institute of Health on experimental use of animals were strictly followed.
Animal Groupings, Induction of DM and Treatment
The rats were randomly allotted into four experimental groups (six rats each) as stated below:
Normal Healthy Control (NHC): treated with vehicle (5% DMSO),
Diabetic Control (DC): diabetic rats treated with vehicle,
DLEBR: diabetic rats treated with low dose (100 mg/kg) of EBR extract,
DHEBR: diabetic rats treated with high dose (400 mg/kg) of EBR extract.
T2DM was induced in the animals following previously described method.4 Briefly, the animals in the DC, DLEBR and DHEBR groups were administered with 30% fructose solution ad libitum for four weeks, while the NHC animals were simultaneously given normal water during the same period. After a 12 h overnight fast, streptozotocin (STZ, 40 mg/kg) dissolved in sodium citrate buffer (0.1 M; pH 4.5) was intraperitoneally injected to the animals in the DC, DLEBR and DHEBR groups, while the NHC rats received the same volume of citrate buffer. After three days, blood glucose concentration was measured in all the animals with a portable glucometer (Accu-Chek Guide) using blood obtained from the tail tip. T2DM was affirmed in rats with fasting blood glucose level above 250 mg/dL. Five days after confirmation of T2DM, the animals were administered with their respective treatment as indicated above on a daily basis by oral gavage for 5 weeks. Weekly fasting blood glucose level and body weight changes were determined during the treatment period. After the treatment, all the animals were subjected to series of pain behavioural experiments.
Evaluation of Hyperalgesia
Hot Plate and Tail Flip Test
Thermal hyperalgesia was evaluated in all the experimental animal groups using hot plate and tail flip test. Briefly, the hot plate apparatus used was set at a temperature of 55 ± 1 °C. The animals were individually placed on the apparatus and the time it takes for the rats to display the first sign of nociceptive response including jumping and paw licking (paw withdrawal latency, PWL) was adjudged as pain threshold and recorded. A cut-off time of 35s was used to avoid paw tissue damage.
For the tail flip test, the temperature of the hot water bath was set at 50 ± 1 °C and the tail of the rats was immersed into the hot water bath until the animals showed the first sign of struggle, tail flicking or withdrawal (tail withdrawal latency, TWL). A cut off time of 12s was set to avoid tissue damage. A decrease in the tail withdrawal time signifies hyperalgesia.
Cold Allodynia Test
The sensitivity of the rats hind paw to cold allodynia was measured by inserting the hind paw of the rats in cold water set at 4.5 ± 1°C. The hind paw withdrawal latency (CPWL) was measured in seconds. A limit of 20 secs was set to avoid tissue damage.
Mechanical Allodynia
Mechanical allodynia was determined in the hind paws of all the rats with the aid of von Frey filaments. Briefly, the rats were individually positioned on top of a wired meshed surface and von Frey filaments with varying bending forces was applied on the plantar surface of the right hind paw for 5 s. The paw withdrawal threshold was evaluated by increasing the stimulus strength of the filament until paw withdrawal was displayed by the animals.
Motor Coordination Test
Motor coordination was evaluated using a rotarod apparatus. The rats were trained twice a day for three consecutive days, during this period the animals were positioned on a rotating rod accelerating from 0–25 rpm. The time it takes for the animals to dismount from the rotarod was recorded in seconds. Three trials was performed on the test day.
Acetic Acid-Induced Writhing
Acetic acid nociception was induced in the animals by intraperitoneal injection of 10 mL/kg of 1% acetic acid to induce characteristic abdominal writhing and stretching. The number of abdominal writhing and stretching was counted from 0–30 min after acetic acid injection.
Formalin-Induced Paw Licking Test
The formalin-induced paw licking test was performed in the rats after treatment by injecting 50 µL of 2.5% formalin solution subcutaneously into the right hand paw of the animals. The time spent licking the injected paws was recorded during the neurogenic pain phase (0–5 mins after injection) and the inflammatory pain response (15–30 min after the injection).
Animal Sacrifice
Upon completion of the behavioural experiment, the rats were anesthetized using thiopental and bled through cardiac puncture. The blood collected blood was processed to obtain the serum. Proinflammatory cytokine levels (TNF-α and IL-1β) were quantified in the serum using the ELISA kits.
Statistical Analysis
All data represents mean ± SD (n=6). For comparisons among multiple groups, one-way ANOVA with Bonferroni post hoc analysis was used. P < 0.05 was defined as statistically significant. Data analysis was performed on GraphPad Prism (version 5.0; GraphPad Software, USA).
Results
Effects of EBR on Blood Glucose Concentration and Body Weight
There was a significant increase in the initial blood glucose concentration of rats in the DC, DLEBR and DHEBR groups compared with the HNC group (Figure 1A). Whereas, treatment of diabetic rats with EBR (100 or 400 mg/kg) resulted in a dose dependent decrease in blood glucose concentration at the end of the treatment, while the DC maintained a higher blood glucose concentration at the end of the treatment (Figure 1A).
 |
Figure 1 Effect of EBR on (A) fasting blood glucose concentration, (B) body weight gain. Data was presented as the mean ± SD (n = 6). **P<0.05 vs HNC group, ##P<0.05 vs DC group. |
In addition, as shown in Figure 1B, the body weight gain of the DC group was significantly lowered when compared to the HNC group, while treatment with EBR (100 or 400 mg/kg) significantly improved the body weight gain compared to the DC group (Figure 1B).
Effects of EBR on Thermal Hyperalgesia in Diabetic Rats
As shown in Figure 2A and B, there was marked increase in thermal hyperalgesia in the DC rats as observed by decrease in the pain latency of the DC group in the hot plate and tail flip tests compared to the HNC group (Figure 2A and B). However, in the diabetic rats treated with EBR (150 or 400 mg/kg), significant increase was observed in their pain latency response, indicating reduced thermal hyperalgesia compared to the untreated DC group (Figure 2A and B).
Effects of EBR on Cold and Mechanical Allodynia in Diabetic Rats
In the cold water test, the rats in the DC group exhibited significantly reduced paw withdrawal latency (CPWL) compared to normal control group (Figure 2C). In the EBR treated groups, the CPWL of the rats were increased significantly in comparison with DC group (Figure 2C). Furthermore, the effect of EBR on mechanical hyperalgesia in diabetic rats is as shown in Figure 2D. There was obvious decrease in mechanical threshold in the DC group as compared to HNC group. Whereas, treatment of diabetic rats with EBR significantly increased the mechanical pain threshold in the treated rat groups compared to the untreated DC group (Figure 2D).
Effects of EBR on Motor Coordination in Diabetic Rats
The DC rats showed signs correlated with motor coordination dysfunction as evidenced by shorter dismount latency time on the rotarod apparatus when compared to the HNC group that had longer dismount latency time (Figure 2E). Whereas, treatment of diabetic rats with EBR significantly increased the duration time of the treated rats on the rotarod apparatus when compared to the DC animals (Figure 2E).
Effects of EBR on Formalin Paw Licking in Diabetic Rats
As shown in Figure 3A, the response of the DC rats was observed to be significantly increased as observed by marked increase in the paw licking time in both phases (neurogenic and inflammatory phases) compared to the HNC group. Whereas, treatment of diabetic rats with EBR significantly decreased the neurogenic and inflammatory phases paw licking time when compared to the DC group (Figure 3A).
Effects of EBR on Acetic Acid-Induced Writhing in Diabetic Rats
As shown in Figure 3B, the number of abdominal writhes was markedly increased in the untreated DC group compared to HNC group. Compared to DC control group, ERB treatment (100 and 400 mg/kg) significantly decreased the number of abdominal writhing in the treated animals when compared to the untreated DC group (Figure 3B).
Effects of EBR on Serum Proinflammatory Cytokines in Diabetic Rats
As shown in Figure 4, the DC rats showed significant increases in the serum concentration of TNF-α and IL-1β compared to the normal control rats. However, treatment of diabetic rats with EBR significantly and dose dependently reduced serum levels of these proinflammatory cytokines when juxtaposed with the DC control group (Figure 4A and B)
Chemical Characterization of EBR
The phytochemical investigation of EBR using various chromatographic separation techniques and recrystallization afforded the isolation and identification of six compounds, all polyphenols including panduratin A, pinostrobin, pinocembrin, cardamonin, boesenbergin A and alpinetin (Figure 5). All the isolated compounds were identified via spectroscopic data analyses and comparison with previous literature data.
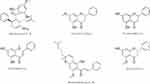 |
Figure 5 Chemical structure of the isolated polyphenolic compounds from EBR extract. |
Discussion
Diabetes is a chronic metabolic disorder associated with increase in blood glucose concentration, paving the way for the development of multiple diabetic associated comorbidity such as cardiovascular diseases, neuropathy, neurodegenerative diseases and nephropathy.5,21 Unfortunately most of the synthetic antidiabetic drugs commonly used for treating diabetes have not shown efficacy in reversing diabetic complications, in addition to the reported side effects of these drugs.3 The development of DPN is largely influenced by low blood flow and hyperglycemia, as such maintaining strict glycaemic control have been proposed as a viable option in the prevention or treatment of DPN.10,22
Several studies have highlighted the relevance of medicinal plants in the treatment of diabetic complications including DPN.23 Polyphenols are one of the major bioactive components in medicinal plants and they have been reported as multitarget agents with powerful antidiabetic, antioxidant and anti-inflammatory activities.24 Furthermore, polyphenols have been shown to be effective in the treatment of a variety of diseases which include diabetes and diabetes-associated problems. In this regard, B. rotunda a culinary and medicinal plant rich in polyphenolic constituents is widely used as a remedy for the treatment diabetes, rheumatic and muscle pain.14 As such this study evaluated the ameliorative effects of B rotunda polyphenol extract against diabetes induced peripheral neuropathic pain.
This study established a high fructose/STZ diabetic model characterized by insulin resistance and hyperglycemia. The combination of high fructose and streptozotocin induces insulin resistance and destroys pancreatic beta cells leading to decrease in insulin production causing excessive increase in circulating blood glucose concentration.3,4,25 The results from this study indicated that the administration of EBR significant reduced blood glucose level in the treated diabetic rats. Furthermore, our study observed a dramatic weight loss in the diabetic animals which was attenuated in the EBR treated rats. Previous literatures have reported that diabetes induces body weight loss, polyphagia and polydipsia.3,26
DPN results in painful sensation, reduced motility and may eventually lead to amputation in extreme cases. Hyperglycemia induces oxidative stress in the neurons and sciatic nerves leading to nerve destruction.27 Studies have shown that DPN reduces pain threshold and hypersensitivity to different pain stimulus which has been strongly associated with marred myelinated C fibres in diabetes resulting in hyperalgesia and pricking pain sensations.28,29 Pain related behavioral parameters including thermal and mechanical hyperalgesia have employed for evaluating DPN. Diabetic animals displayed significant hyperalgesia and increased sensitivity to pain stimulus in the hot plate, tail flick and von Frey test, which was consistent with previous studies.5,28,30 Administration of EBR to diabetic rats markedly improved thermal, cold and mechanical hyperalgesia in the treated diabetic rats.
Excessive hyperglycemia has been unequivocally shown to instigate progressive decline in motor coordination and nociceptive responses.5,31 In the current study, the diabetic rats exhibited significantly impairment in motor coordination on the rotarod test. In addition, in line with the results obtained from both the thermal and mechanical hyperalgesia, diabetes caused marked increase in nociception. Moreover, previous studies have shown that hyperglycemia through the action of ROS and oxidative stress leads to the release of inflammatory mediators including pro-inflammatory cytokines and prostaglandins from macrophages, Schwann cells and mast cells of the nociceptive neurons leading to increase in nociceptive responses.5,31 In line with earlier studies, diabetic rats showed amplified nociceptive responses in the acetic acid (abdominal stretching) and formalin tests (paw licking or biting behaviour).30,32 The result obtained from this study demonstrated that ERB significantly attenuated acetic acid and formalin induced abdominal stretching and paw licking time, respectively in the treated diabetic rats.
The role of inflammation instigated by hyperglycemia induced ROS has been extensively reported in diabetic complications including neuropathy.10,33 Numerous studies have shown that inflammatory cytokines have a significant impact on the functioning of the nervous system. Proinflammatory cytokines including TNF-α and IL-1β are critically involved in pain and hyperalgesia. In addition, the occurrence and severity of diabetic neuropathic pain is aggravated by increase in cytokines generation.34,35 Prolonged hyperglycemia can induce the glycosylation of nerve myelin protein, resulting in the activation of lymphocytes and macrophages leading to damages in peripheral nerve sheaths.34,36 In the present study it was observed that diabetic rats showed significantly increased levels of TNF-α and IL-1β, whereas treatment with EBR alleviated the increased cytokine levels.
Understanding the phytochemical profile of B. rotunda is critical in the identification of bioactive constituents responsible for the observed antidiabetic, anti-inflammatory and analgesic effects. This study isolated and characterized six polyphenolic constituents as pinostrobin, pinocembrin, panduratins A cardamonin, boesenbergin A and alpinetin. The findings of these polyphenols in EBR is in line with previous studies, as well as with their antidiabetic and anti-inflammatory properties. Pinocembrin and its O-methylated derivative pinostrobin have been demonstrated to have significant antinociceptive and anti-inflammatory effects.37 Potipiranun et al also demonstrated that pinocembrin, panduratin A, cardamomin, and alpinetin displayed significant anti-glycation effect, while panduratin A and pinocembrin showed good α-glucosidase inhibition.16,20 Besides, pinostrobin and pinocembrin have both demonstrated extensive neuroprotective effects by in several disease models.38–40 These previous studies together with the results from our study suggests that these metabolites may play significant roles in the observed anti-neuropathic activity of EBR.
Conclusion
The results from this study suggested that EBR displayed ameliorative effects against hyperglycemia-induced DPN, through its anti-inflammatory and antinociceptive effects, thus minimizing pain hypersensitivity and exaggerated nociception. Further mechanistic studies is required.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Song P, Sun C, Li J, et al. Tiliacora triandra extract and its major constituent attenuates diabetic kidney and testicular impairment by modulating redox imbalance and pro-inflammatory responses in rats. J Sci Food Agric. 2021;101:1598–1608. doi:10.1002/jsfa.10779
2. International Diabetes Federation. IDF Diabetes Atlas.
3. Makinde EA, Radenahmad N, Adekoya AE, Olatunji OJ. Tiliacora triandra extract possesses antidiabetic effects in high fat diet/streptozotocin-induced diabetes in rats. J Food Biochem. 2020;44(6):e13239. doi:10.1111/jfbc.13239
4. Olatunji OJ, Zuo J, Olatunde OO. Securidaca inappendiculata stem extract confers robust antioxidant and antidiabetic effects against high fructose/streptozotocin induced type 2 diabetes in rats. Exploration of bioactive compounds using UHPLC-ESI-QTOF-MS. Arch Physiol Biochem. 2021. doi:10.1080/13813455.2021.1921811
5. Pang X, Makinde EA, Eze FN, Olatunji OJ. Securidaca inappendiculata polyphenol rich extract counteracts cognitive deficits, neuropathy, neuroinflammation and oxidative stress in diabetic encephalopathic rats via p38 MAPK/Nrf2/HO-1 pathways. Front Pharmacol. 2021;12:737764. doi:10.3389/fphar.2021.737764
6. Zimath PL, Dalmagro AP, Mota da Silva L, Malheiros A, Maria de Souza M. Myrsinoic acid B from Myrsine coriacea reverses depressive-like behavior and brain oxidative stress in streptozotocin-diabetic rats. Chem Biol Interact. 2021;347:109603. doi:10.1016/j.cbi.2021.109603
7. Olatunji OJ, Chen H, Zhou Y. Lycium chinense leaves extract ameliorates diabetic nephropathy by suppressing hyperglycemia mediated renal oxidative stress and inflammation. Biomed Pharmacother. 2018;102:1145–1151. doi:10.1016/j.biopha.2018.03.037
8. Zhang YJ, Liu FR. Effectiveness of acupuncture for treatment of diabetic peripheral neuropathy. Medicine. 2019;98:e17282. doi:10.1097/MD.0000000000017282
9. Iqbal Z, Azmi S, Yadav R, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. 2018;40(6):828–849. doi:10.1016/j.clinthera.2018.04.001
10. Alkhalaf MI, Hussein RH, Hamza A. Green synthesis of silver nanoparticles by Nigella sativa extract alleviates diabetic neuropathy through anti-inflammatory and antioxidant effects. Saudi J Biol Sci. 2020;27(9):2410–2419. doi:10.1016/j.sjbs.2020.05.005
11. Shoaib A, Dixit RK, Ganash M, Barreto G, Ashraf GM, Siddiqui HH. Beneficial effects of n-hexane bark extract of Onosma echioides L. on diabetic peripheral neuropathy. J Cell Biochem. 2019;120:16524–16532. doi:10.1002/jcb.28912
12. Alleman CJ, Westerhout KY, Hensen M, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diabetes Res Clin Pract. 2015;109:215–225. doi:10.1016/j.diabres.2015.04.031
13. Bril V. Treatments for diabetic neuropathy. J Peripher Nerv Syst. 2012;17:22–27. doi:10.1111/j.1529-8027.2012.00391.x
14. Saah S, Siriwan D, Trisonthi P. Biological activities of Boesenbergia rotunda parts and extracting solvents in promoting osteogenic differentiation of pre-osteoblasts. Food Bio sci. 2021;41:101011. doi:10.1016/j.fbio.2021.101011
15. Kanchanapiboon J, Kongsa U, Pattamadilok D, et al. Boesenbergia rotunda extract inhibits Candida albicans biofilm formation by pinostrobin and pinocembrin. J Ethnopharmacol. 2020;261:113193. doi:10.1016/j.jep.2020.113193
16. Chatsumpun N, Sritularak B, Likhitwitayawuid K. New biflavonoids with alpha-glucosidase and pancreatic lipase inhibitory activities from Boesenbergia rotunda. Molecules. 2017;22:1862. doi:10.3390/molecules22111862
17. Ruttanapattanakul J, Wikan N, Okonogi S, et al. Boesenbergia rotunda extract accelerates human keratinocyte proliferation through activating ERK1/2 and PI3K/Akt kinases. Biomed Pharmacother. 2021;133:111002. doi:10.1016/j.biopha.2020.111002
18. Mohan S, Hobani YH, Shaheen E, et al. Ameliorative effect of Boesenbergin A, a chalcone isolated from Boesenbergia rotunda (Fingerroot) on oxidative stress and inflammation in ethanol-induced gastric ulcer in vivo. J Ethnopharmacol. 2020;261:113104. doi:10.1016/j.jep.2020.113104
19. Nguyen MTT, Nguyen HX, Dang PH, et al. dimeric metabolites from Boesenbergia rotunda and their antiausterity activities against the PANC-1 human pancreatic cancer cell line. Phytochemistry. 2021;183:112646. doi:10.1016/j.phytochem.2020.112646
20. Potipiranun T, Adisakwattana S, Worawalai W, Ramadhan R, Phuwapraisirisan P. Identification of pinocembrin as an anti-glycation agent and alpha-glucosidase inhibitor from fingerroot (Boesenbergia rotunda): the tentative structure-activity relationship towards MG-trapping activity. Molecules. 2018;23:3365. doi:10.3390/molecules23123365
21. Raish M, Ahmad A, Bin Jardan YA, et al. Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats. Biomed Pharmacother. 2022;145:112412. doi:10.1016/j.biopha.2021.112412
22. Balaha M, Kandeel S, Kabel A. Phloretin either alone or in combination with duloxetine alleviates the STZ-induced diabetic neuropathy in rats. Biomed Pharmacother. 2018;101:821–832. doi:10.1016/j.biopha.2018.02.135
23. Kabir MT, Tabassum N, Uddin MS, et al. Therapeutic potential of polyphenols in the management of diabetic neuropathy. Evid Based Complement Alternat Med. 2021;2021:9940169. doi:10.1155/2021/9940169
24. Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016;8:33–42. doi:10.1016/j.cofs.2016.02.002
25. Erukainure OL, Oyebode OA, Ijomone OM, Chukwuma CI, Koorbanally NA, Islam MS. Raffia palm (Raphia hookeri G. Mann & H. Wendl) wine modulates glucose homeostasis by enhancing insulin secretion and inhibiting redox imbalance in a rat model of diabetes induced by high fructose diet and streptozotocin. J Ethnopharmacol. 2019;237:159–170. doi:10.1016/j.jep.2019.03.039
26. Ibrahim MA, Habila JD, Koorbanally NA, Islam MS. Butanol fraction of Parkia biglobosa (Jacq.) G. Don leaves enhance pancreatic β-cell functions, stimulates insulin secretion and ameliorates other type 2 diabetes-associated complications in rats. J Ethnopharmacol. 2016;183:103–111. doi:10.1016/j.jep.2016.02.018
27. Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int. 2015;2015:515042. doi:10.1155/2015/515042
28. Fajrin FA, Nugroho AE, Nurrochmad A, Susilowati R. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J Ethnopharmacol. 2020;249:112396.
29. Jain D, Bansal MK, Dalvi R, Upganlawar A, Somani R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J Integr Med. 2014;12(1):35–41. doi:10.1016/S2095-4964(14)60001-7
30. Saraswat N, Sachan N, Chandra P. Anti-diabetic, diabetic neuropathy protective action and mechanism of action involving oxidative pathway of chlorogenic acid isolated from Selinum vaginatum roots in rats. Heliyon. 2020;6(10):e05137. doi:10.1016/j.heliyon.2020.e05137
31. Pandhare RB, Sangameswaran B, Mohite PB, Khanage SG. Attenuating effect of seeds of Adenanthera pavonina aqueous extract in neuropathic pain in streptozotocin-induced diabetic rats: an evidence of neuroprotective effects. Rev Bras Farmacogn. 2012;22(2):428–435. doi:10.1590/S0102-695X2012005000008
32. Abo-Salem OM, Ali TM, Harisa GI, Mehanna OM, Younos IH, Almalki WH. Beneficial effects of (-)-epigallocatechin-3-O-gallate on diabetic peripheral neuropathy in the rat model. J Biochem Mol Toxicol. 2020;34:e22508. doi:10.1002/jbt.22508
33. Abdelkader NF, Ibrahim SM, Moustafa PE, Elbaset MA. Inosine mitigated diabetic peripheral neuropathy via modulating GLO1/AGEs/RAGE/NF-κB/Nrf2 and TGF-β/PKC/TRPV1 signaling pathways. Biomed Pharmacother. 2022;145:112395. doi:10.1016/j.biopha.2021.112395
34. Baka P, Escolano-Lozano F, Birklein F. Systemic inflammatory biomarkers in painful diabetic neuropathy. J Diabetes Complicat. 2021;35:108017. doi:10.1016/j.jdiacomp.2021.108017
35. Sun JJ, Tang L, Zhao XP, Xu JM, Xiao Y, Li H. Infiltration of blood-derived macrophages contributes to the development of diabetic neuropathy. J Immunol Res. 2019;2019:7597382. doi:10.1155/2019/7597382
36. Okdahl T, Brock C, Fløyel T, et al. Increased levels of inflammatory factors are associated with severity of polyneuropathy in type 1 diabetes. Clin Endocrinol. 2020;93:419–428. doi:10.1111/cen.14261
37. Déciga-Campos M, Mata R, Rivero-Cruz I. Antinociceptive pharmacological profile of Dysphania graveolens in mouse. Biomed Pharmacother. 2017;89:933–938. doi:10.1016/j.biopha.2017.02.096
38. Gong LJ, Wang XY, Gu WY, Wu X. Pinocembrin ameliorates intermittent hypoxia-induced neuroinflammation through BNIP3-dependent mitophagy in a murine model of sleep apnea. J Neuroinflammation. 2020; 17: 337.
39. Li C, Tang B, Feng Y, et al. Pinostrobin exerts neuroprotective actions in neurotoxin-induced Parkinson’s disease models through Nrf2 induction. J Agric Food Chem. 2018;66(31):8307–8318. doi:10.1021/acs.jafc.8b02607
40. Patel NK, Jaiswal G, Bhutani KK. A review on biological sources, chemistry and pharmacological activities of pinostrobin. Nat Prod Res. 2016;30:2017–2027. doi:10.1080/14786419.2015.1107556
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



