Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 7
Anti-inflammaging and antiglycation activity of a novel botanical ingredient from African biodiversity (Centevita™)
Authors Maramaldi G, Togni S, Franceschi F, Lati E
Received 15 June 2013
Accepted for publication 11 September 2013
Published 12 December 2013 Volume 2014:7 Pages 1—9
DOI https://doi.org/10.2147/CCID.S49924
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Giada Maramaldi,1 Stefano Togni,1 Federico Franceschi,1 Elian Lati2
1Indena SpA, Milan, Italy; 2Laboratoire BIO-EC, Longjumeau, France
Purpose: The aim of this study was to investigate the topical efficacy of a new purified extract from Madagascar, Gotu Kola (Centella asiatica [L.] Urban), both on human explants and on human volunteers, in relation to skin wrinkling and skin protection against ultraviolet light exposure. The extract, with a peculiar content of biologically active molecules, was investigated as a novel anti-inflammaging and antiglycation agent. Its typical terpenes, known as collagen synthesis promoters, represent at least 45% of the extract. It also contains a polyphenolic fraction cooperating to the observed properties.
Methods: C. asiatica purified extract was assayed on human skin explants maintained alive, and several parameters were evaluated. Among the most relevant, the thymine dimerization was evaluated by immunostaining. Malondialdehyde formation was evaluated as free-radical scavenging marker by enzyme-linked immunosorbent assay. The expression of interleukin-1a was observed by enzyme-linked immunosorbent assay as well. The product was further evaluated as an antiglycation agent, being glycation quantified by the advanced glycation product carboxymethyl lysine. C. asiatica purified extract was also evaluated as an antiwrinkling agent in a single-blind, placebo-controlled study. Formulated in a simple oil-in-water emulsion, the extent of wrinkling was assessed by skin replicas, skin firmness, skin elasticity, and collagen density measurements.
Results: C. asiatica purified extract could protect DNA from ultraviolet light-induced damage, decreasing the thymine photodimerization by over 28% (P<0.05). A reduced (26%, P<0.01) expression of interleukin-1α was also observed, supporting its anti-inflammatory potential. C. asiatica purified extract showed in vitro a total inhibition of carboxymethyl lysine formation induced by the glycating agent methylglyoxal. A clear epidermal densification of collagen network in the papillary dermis was observed. These in vitro data have been confirmed by clinical results.
Conclusion: These results qualify C. asiatica purified extract as an antiaging ingredient, addressing skin damage caused by inflammaging and glycation by relying on the synergy of triterpens and polyphenolics.
Keywords: Centella asiatic, glycation, inflammaging, skin aging, collagen, triterpenes
Introduction
Centella asiatica is a perennial herbaceous plant widely used in Indian Ayurvedic medicine and as a traditional herbal remedy in Asia, and especially India, to treat a wide range of indications.1
In the traditional medicine of Madagascar, C. asiatica has been used since time immemorial as an agent favoring cicatrization, as well as for managing dermatological conditions such as ulcers and small wounds and chaps.2 In addition, in several Asian countries, C. asiatica leaves are consumed as vegetables.
Common names of C. asiatica include Gotu Kola, India Pennywort, and Luei Gong Gen. Traditionally, the leaves of C. asiatica are hand collected in the wild, with Madagascar being the most reliable area of collection. In Madagascar, C. asiatica grows spontaneously all over the island except for the arid regions in the southwest.
C. asiatica contains a triterpenic fraction composed of asiaticoside, madecassoside, asiatic acid, and madecassic acid (Figure 1), and all these molecules have been reported to promote collagen synthesis.
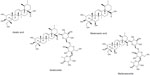  | Figure 1 Chemical structure of Centella asiatica triterpenes. |
However, madecassoside is the only centella triterpenic compound that has shown the capability to promote collagen type III production, whereas asiaticoside, asiatic acid, and madecassic acid are reported to promote the synthesis of collagen type I,3 with a major potency being reported for madecassoside.4
The triterpene components ratio of centella is known to vary depending on its growth location5 and diverse environmental conditions. In addition to that, the manufacturing processes may isolate different chemical fractions.6 The regular triterpenic extracts available on the market are normally composed of asiaticoside, asiatic acid, and madecassic acid. The triterpenic fraction of C. asiatica has been shown to promote collagen synthesis in fibroblast cultures,7 and more recently it was reported to exert action on factors controlling the regulation of inflammation, normalizing keratinocyte hyperproliferation and re-establishing the natural epidermal homeostasis.8 The active compounds are known for their beneficial effects when topically applied on the skin, specifically on wound healing and chronic venous insufficiency.9
The C. asiatica extract that was used in the described trials is commercially available at Indena SpA (Milan, Italy) and was obtained as a free sample from the manufacturing company.
The form of extract is a greenish powder containing all four typical centella terpenoids (not less than 45%) as well as a polyphenolic fraction (over 7%). The product specifications are provided as an addendum in the additional material. The extract has been tested in vitro and clinically with the aim of investigating its overall biological activity on human skin explants maintained alive as well as clinically in healthy volunteers.
Material and methods
Product characterization
To produce this extract, the biomass is sourced exclusively from Madagascar. It is grinded, extracted, and concentrated; diluted with water, centrifuged, concentrated, and purified with a resin column; and finally filtrated, concentrated, and dried. This novel purified extract is obtained only with food-grade solvents (ie, water and ethanol – it has been Ecocert validated) and is then purified by chromatography. The final result is a standardized extract containing all four triterpenic molecules (over 45%) and a polyphenolic fraction representing 7%–8% of the extract in weight. The extract is characterized by a high-performance liquid chromatography method for the determination of madecassoside, asiaticoside, madecassic acid, and asiatic acid (Indena SpA, data on file, 2009).
Human skin explants
The first investigations refer to the biological activity of an aqueous solution containing the extract (at 1%) on human skin explants maintained alive. The study was performed in accordance with the Declaration of Helsinki after the patient had given informed consent. The explants were obtained as full thickness human skin biopsies from an abdominoplasty of a 58-year-old Caucasian woman. Punch biopsies removed from the patient were immediately sent to Laboratoire BIO-EC (Longjumeau, France), where the hypodermis was removed from the skin and circular samples were excised using a punch instrument. The samples, with the dermis face down, were immediately placed in a liquid–air interface in Laboratoire BIO-EC’s Explant Medium (BEM®) and cultured under classical cell-culture conditions (37°C in 5% CO2), and half of the medium (1 mL) was refreshed every other day.
Several parameters have been evaluated, such as the ultraviolet light (UV)-induced photodimerization of thymine, the expression of the proinflammatory cytokine interleukin (IL)1-α, the free-radical scavenging properties by quantification of the biomarker of oxidative stress malondialdehyde (MDA),10,11 as well as the antiglycation activity.10,12 The general cells’ morphology was also evaluated prior to all other instrumental observations. Statistical significance is assigned by the Student’s t-test.
Photoaging, anti-inflammatory, and DNA protective activity
C. asiatica purified extract was applied on six human skin explants of an average diameter of 11 mm maintained alive. The applied product was an aqueous solution of the extract at 1% and the application was performed on day 0 and from day 2 to day 5 with a standardized quantity of 2 mg/explant. Half of the medium was partially renewed on days 2 and 4 and the totality was renewed on day 5. The control explants did not receive any treatment.
On day 5 the explants were irradiated by a UV irradiator (RMX 3V, Vilbet Lomart) after the culture medium was replaced by Hanks’ balanced salt solution receiving a dose equivalent to 4 MED (UVA 18 J · cm2 and UVB 0.54 J · cm2). The regular consequence of such skin exposure is normally an increase in so-called sunburnt cells. Changes in morphology as well as the formation of thymine dimers and the overexpression of proinflammatory cytokines also occur.
On day 5 the supernatants of irradiation were collected immediately after irradiation and frozen in expectation of the MDA assay.
On day 6 three explants from each batch were collected and processed the same way.
MDA was assayed in each sample of the irradiation’s supernatant on day 4, collected after UV irradiation, and evaluated by enzyme-linked immunosorbent assay.
IL1-α was assayed in each sample of culture medium and evaluated by enzyme-linked immunosorbent assay. Thymine dimers were stained on paraffinized sections with a monoclonal thymine dimer antibody (ref MC-062, clone KTM53, Kamiya Biomedical Company, Seattle, WA, USA) diluted 1/1,000. The staining was enhanced with a streptavidin/biotin system (PK-7200, Vector Laboratories, Inc, Burlingame, CA, USA) and revealed using Vector® VIP (SK-4600, Vector Laboratories, Inc).
Antiglycation activity
C. asiatica purified extract was applied on 12 human skin explants of an average diameter of 11 mm maintained alive. The preparation of the explants was from an abdominoplasty of a 50-year-old African woman. The average diameter of each explant was 11 mm (±1 mm) and they were kept in survival in BEM culture medium at 37°C in a humid, 5% CO2 atmosphere.
The applied product was in the form of an aqueous solution of the extract at 1% and the application was performed every day with a standardized application of 2 mg/explant. Methylglyoxal (MG) was applied as a glycation promoter at 500 μmol on days 3, 5, and 7.
Carboxymethyl lysine (CML) immunostaining was realized on frozen sections with an anti-CML monoclonal clone PEN (Ref KH012, Trans Genic Inc, Kobe, Japan) and evaluated by microscopical observation. The explants were distributed in five batches as follows: D0, no treatment, sampling on day 0; B, no treatment, sampling on day 9; P, treatment with C. asiatica 1% solution, sampling on day 9; MG, treatment with MG, sampling on day 9; and PMG, treatment with both C. asiatica 1% solution and MG, sampling on day 9.
Clinical trial on healthy volunteers
In addition to the in vitro activities on human explants maintained alive, the efficacy of the extract has also been clinically validated as an antiwrinkling agent in a single-blind, placebo-controlled study.10,13 The aim of this study was to test the efficacy of a C. asiatica purified extract containing cream versus a placebo cream on 20 volunteers. Volunteers were selected according to previously agreed inclusion criteria (age 40–70 years) and signed the informed consent (in compliance with the Declaration of Helsinki and the 1988 Act of the Code de la Santé Publique, France) prior to the beginning of the study on day 0. They were stabilized in a controlled room atmosphere for 10 minutes prior to all basal measurements. An oil-in-water emulsion was used (formulation shown in Table 1, pH 5.5, density 0.98) and the extent of wrinkling was assessed by skin replicas (DermaTOP, Eotech SA, Marcoussis, France), skin firmness, and skin elasticity (Cutometer® MPA 580 by Courage + Khazaka Electronic GmbH, Cologne, Germany) and collagen density measurements (by Siascope [Siascope Siametric™, Aston Clinical Research Limited, Hertfordshire, UK]). The Siascope measurement is actually based on several images taken in quick succession. Light from controlled illumination sources shines on to the selected skin area through a window at the end of the probe, which is placed in contact with the skin surface. The instrument-specific software performs calculations of the different wavelength images using data from the layered nature of skin tissue and optical characteristics. It then produces images for melanin, blood, and collagen, which are then quantified and analytically evaluated.
Each volunteer represented their own placebo, applying either the placebo or the test emulsion on each half of the face (randomly selected). The minimal erythemal dose on the forearm was also assessed on 20 volunteers aged 55±9 years. For the antiwrinkle evaluations, the same volunteers were required to apply the tested emulsion and the placebo emulsion (randomly selected) each on one half of the face for 6 weeks. Basal measurements as well as final evaluations were carried out at the beginning and at the end of the study. Again, the statistical significance of results was evaluated by the Student’s t-test.
Results
Photoaging, anti-inflammatory, and DNA protective activity
The general morphology of the explants was observed on paraffinized sections after staining with Masson’s trichrome, Goldner variant. The batch treated with C. asiatica on day 6 presents a moderately thick stratum corneum that is moderately laminated and slightly keratinized on the surface. Its epidermis presents four to five cellular layers with quite a good morphology, and the relief of the dermal–epidermal junction is moderate. The papillary dermis presents thick collagen bundles forming quite a dense network. After UV irradiation, the sunburnt cells were evaluated per cm2 of epidermis and although a slight reduction was observed, it showed no statistical significance.
The MDA assay was performed to evaluate the free-radical scavenging capacity of the extract following UV irradiation. MDA is, in fact, a terminal degradation product of UV-induced lipoperoxydation. It was assayed in the irradiation’s supernatants on day 4 and collected after UV irradiation. The pretreatment of the explants with the extract prior to UV irradiation provided a decrease in the formation of MDA by 38% compared with untreated batches. Unfortunately, this result cannot be defined as statistically significant, due to damage occurring to one of the control samples.
The formation of thymine dimers, evaluated by antithymine dimer antibody, has been quantified by image analysis and decreased by 28% (P<0.05) (Figures 2–4). The formation of the proinflammatory cytokine IL1-α induced by UV irradiation decreased by 26% in the treated samples (P<0.01) (Figure 5).
  | Figure 2 Immunostaining of thymine dimers in the epidermis (sample batch, day 6). |
  | Figure 3 Immunostaining of thymine dimers in the epidermis (batch with Centella asiatica extract, day 6). |
  | Figure 4 Surface occupied by thymine dimers in the epidermis. |
  | Figure 5 IL1-α concentration in the culture medium on day 6. |
Antiglycation activity
This C. asiatica purified extract in the antiglycation assay completely prevented physiological glycation and also efficiently counteracted the action of the potent glycating agent MD.
On day 0, CML expression is slight on collagen bundles in the superior reticular dermis (Figure 6). On day 8, in the untreated batch, CML expression is slightly increased (Figure 7). MG induces a clear increase in CML expression on day 8 (Figure 8). The C. asiatica-treated batch (not stimulated with MG) shows a total inhibition of physiological CML expression (Figure 9). The C. asiatica-treated batch induced by MG shows a total inhibition of CML expression induced by MG (Figure 10). C. asiatica purified extract has shown a clear inhibition of CML expression both in the MG-treated and in the non-MG-treated batches. The C. asiatica-treated batch has shown a very slight dermal stimulation with a clear increase of the collagen network density in the papillary dermis (Figure 11) compared with the untreated batch (Figure 12).
  | Figure 6 Carboxymethyl lysine (CML) immunostaining of control batch on day 0. |
  | Figure 7 Carboxymethyl lysine (CML) immunostaining of control batch on day 8. |
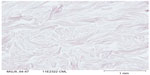  | Figure 8 Carboxymethyl lysine (CML) immunostaining of methylglyoxal-treated batch on day 8. |
  | Figure 9 Carboxymethyl lysine (CML) immunostaining of Centella asiatica-treated batch on day 8. |
  | Figure 10 Carboxymethyl lysine (CML) immunostaining of Centella asiatica-treated batch stimulated with methylglyoxal on day 8. |
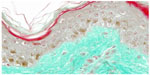  | Figure 11 Skin section of one sample explant on day 8 (Centella asiatica-treated batch). |
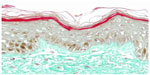  | Figure 12 Skin section of one sample explant on day 8 (untreated batch). |
Glycation occurs when glucose or other endogenous reducing sugars bind nonenzymatically to proteins or lipids. This process may also be amplified by UV exposure, impairing the functioning of biomolecules and eventually leading to less firm and less elastic skin that is more prone to wrinkling. In fact, an excessive amount of sugars transported by blood flow leads to the formation of the so-called advanced glycation end products, which, once reacting with tissutal proteins, determine a hardening process of the tissue, similar to “caramelization.”
Clinical trial on healthy volunteers
The formulation containing C. asiatica purified extract at 0.5% was applied, together with the control emulsion, on one side of the faces of 20 volunteers over 6 weeks. Collagen redensification, evaluated by Siascope, was observed in 70% of the tested volunteers (P<0.05), and a significant decrease in wrinkle depth and volume was observed.
The half of the face treated with the centella-containing formulation also showed improved biomechanical elasticity of the skin (evaluated by Cutometer, parameter R5, Figure 13A and 13B) and skin firmness (evaluated by Cutometer parameter R7) by 29% and 17%, respectively (P<0.05) (Figure 14A and 14B).
  | Figure 13 Variation of skin elasticity (R5 parameter) in placebo-treated area (A) and Centella asiatica-treated area (B). |
  | Figure 14 Variation of skin elasticity (parameter R7) in placebo-treated area (A) and Centella asiatica-treated area (B). |
The assessment of the mechanical properties of the skin enables assessment of the functional state of the elastic structures (such as elastic fibers, curvature of the connective bundles, wrinkles of the stratum corneum) and of the viscous-behaving structures (as interstitial fluids and internal adherences). The measuring principle of the Cutometer is based on the suction method. A negative pressure of 500 mbar is created in the suction probe and the skin is drawn into the cylindrical opening (diameter 2 mm) of the probe. Once inside the probe, the skin penetration depth is determined by an optical measuring system. Each suction phase, lasting 2 seconds, is followed by a relaxation phase of the same duration. The area of the face where this measurement is taken is the cheekbone. The resistance of the skin to be sucked up by the negative pressure and its ability to return to its original position are displayed as curves at the end of each measurement. During the suction phase, the skin deformation measures, first, the elastic resistance of the skin and then the viscous component, which, taken together, represent firmness. During the relaxation phase, the immediate skin recovery represents sheer cutaneous elasticity, whereas the delayed return of the skin to its initial position measures the viscoelastic component. Parameter R5 is the ratio Ur (recovery during relaxation)/Ue (elastic sheer during suction) and represents elasticity in the clinical sense: the closer to the 1 value, the better the elasticity. Parameter R7 is the ratio Ur (recovery during relaxation)/Uf (total cutaneous deformation) and represents firmness. The closer to the 1 value, the firmer the skin.
Conclusion
The investigated C. asiatica purified extract (Centevita™) has shown antiaging topical efficacy acting on free-radical-induced oxidative stress and cytokine-induced skin inflammaging, whereas no statistical significance was achieved in the SCB count. As per the MDA assay, although the observed reduction of the formation of MDA was quite remarkable, it has shown no statistical significance, due to the damage that occurred to one of the control samples. It would be necessary to repeat the MDA assay, if possible with a higher sample numerosity, in order to correctly define the free-radical scavenging properties of the extract.
On the other side, the extract has shown a clear prevention of the phenomenon of glycation, targeting in a pleiotropic and complementary way the biochemical and cellular bases of skin aging. Although several publications confirm the biological activity of C. asiatica compounds,14–16 the underlying mechanisms involved in the skin’s physiological effects are not yet fully understood,17,18 even if gene expression changes have been reported in human fibroblasts.17–20
The clinical evaluations have further confirmed the observed in vitro properties. Additional investigations are needed to further confirm the observed results as well as to elucidate the molecular mechanisms at the base of the biological activity.
Acknowledgments
This study was funded by the company Indena SpA, Milan, Italy. Laboratoire BIO-EC possesses an authorization from the French Minister for Health. A special acknowledgment goes to Professor Giovanni Appendino from the University of Piemonte Orientale, Italy, who loves to share his deep knowledge on natural compounds. We also thank Mr P Gasser and Mr L Peno-Mazzarino for their help in the microscopical analysis of skin samples and for the interpretation of the results, and Miss M Daniel for her help with clinical trials on healthy volunteers.
Disclosure
The authors report no conflicts of interest in this work.
References
Nadkarni KM. Indian materia medica. Popular Prakash Bombay. 1986:662. | |
Brinkhaus B, Lindner M, Schuppan D, Hahn EG. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomed. 2000;7:427–448. | |
Bonte F, Dumas M, Chaudagne C, Meybeck A. Activité compare de l’asiaticoside et du madecassoside sur la synthèse des collagènes I et III par des fibroblastes humains en culture [Comparative activity of asiaticoside and madecassoside on type I and III collagen synthesis by cultured human fibroblasts]. Ann Pharm Fr. 1995;53(1):38–42. French. | |
Wu F, Bian D, Xia Y, et al. Identification of major active ingredients responsible for burn wound healing of Centella asiatica herbs. Evid Based Complement Alternat Med. 2012;2012:848093. | |
Randriamampionona D, Diallo B, Rakotoniriana F, et al. Comparative analysis of active constituents in Centella asiatica samples from Madagascar: application for ex situ conservation and clonal propagation. Fitoterapia. 2007;78:482–489. | |
Rao PS, Seshadri TR. Variation in the chemical composition on Indian samples of Centella asiatica. Curr Sci. 1970;38:77–79. | |
Maquart FX, Bellon G, Gillery P, Wegrowski Y, Borel JP. Stimulation of collagen synthesis in fibroblasts cultures by a triterpene extracted form Centella asiatica. Connect Tissue Res. 1990;24:107–120. | |
Segond C, Théron E, Petit V, Loiseau A. Innovative natural active ingredient with anti-inflammatory properties. Antiaging: Physiology to Formulation. 2006:189–196. | |
Pointel JP, Boccalon MD, Cloarec M, Ledevehat MD, Joubert M. Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology. 1987;38:46–50. | |
Togni S, Maramaldi G, Franceschi F, Lati E. Centevita™: a novel inflammaging and anti-glycation agent. Proceedings of the IFSCC, Johannesburg, South Africa, October 15–18, 2012. | |
Laboratoire BIO-EC report 11E2321P1. Highlighting the free radical, anti-inflammatory and DNA protection activities of one cosmetic ingredient after UV irradiation on human explants maintained alive. 2011. | |
Laboratoire BIO-EC report 11E2322. Evaluation of the anti-glycation activity of an active ingredient on living human skin explants. 2011. | |
Laboratoire BIO-EC report 11E2402. Evaluation of the anti-ageing activity and activity on the MED of a product vs placebo on a panel of volunteers. 2011. | |
Cristoni A. Cosmetic applications of natural pentacyclic triterpenes. Nutracos. 2005:2–4. | |
Sampson JH, Raman A, Karlsen G, Navsaria H, Leigh IM. In vitro keratinocyte antiproliferant effect on Centella asiatica extract and terpenoid saponins. Phytomedicine. 2001;8(3):230–235. | |
Hashim P, Sidek A, Helan MH, Sabery A, Devi Palanisamy U, Ilham M. Triterpene composition and bioactivities of Centella asiatica. Molecules. 2011;16:1310–1322. | |
Zheng C-J, Qin L-P. Chemical components of Centella asiatica and their bioactivities. Chin J Integr Med. 2007;5:348–351. | |
Liu J, Henkel T. Traditional Chinese medicine: are polyphenols and saponins the key ingredients triggering biological activities. Cur Med Plant Biol. 2002;9:1483–1485. | |
Coldren CD, Hashin P, Ali JM, Oh SK, Sinskey AJ, Rha C. Gene expression changes in human fibroblast induced by Centella asiatica terpenoids. Planta Med. 2003;69:725–732. | |
Lu L, Ying K, Wei S, et al. Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int J Dermatol. 2004;43(11):801–807. |
 © 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

