Back to Journals » Journal of Asthma and Allergy » Volume 13
Anti-IL5 Therapies for Severe Eosinophilic Asthma: Literature Review and Practical Insights
Authors Menzella F , Ruggiero P, Ghidoni G, Fontana M, Bagnasco D , Livrieri F, Scelfo C , Facciolongo N
Received 3 August 2020
Accepted for publication 22 August 2020
Published 9 September 2020 Volume 2020:13 Pages 301—313
DOI https://doi.org/10.2147/JAA.S258594
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Francesco Menzella,1 Patrizia Ruggiero,1 Giulia Ghidoni,1 Matteo Fontana,1 Diego Bagnasco,2 Francesco Livrieri,1 Chiara Scelfo,1 Nicola Facciolongo1
1Pneumology Unit, Arcispedale Santa Maria Nuova, Azienda USL di Reggio Emilia-IRCCS, Reggio Emilia 42123, Italy; 2Allergy & Respiratory Diseases, University of Genoa, Genoa 16132, Italy
Correspondence: Francesco Menzella
Pneumology Unit, Santa Maria Nuova Hospital, Azienda USL-IRCCS di Reggio Emilia, Via Amendola 2, Reggio Emilia 42122, Italy
Email [email protected]
Abstract: Severe refractory asthma (SRA) still has a high economic and social impact, including a reduction in quality of life (QoL), productivity, a greater risk of exacerbations and emergency department (ED) visits. Another major issue is the need of oral corticosteroids (OCS), often due to a poor response to standard therapies or the lack of indication for currently available biological drugs. A thorough understanding of the immunological pathways and eosinophilopoietic processes allows a correct application of the new pharmacological strategies and leads to better clinical responses. For these unmet needs, several monoclonal antibody (mAb) drugs have been introduced over the past few years. These are mainly available for allergic and especially eosinophilic uncontrolled refractory asthma. As the number of therapeutic options increases, the choice of biological drugs can be made only after careful considerations of the particular asthma endotype, patients’ comorbidities and clinical data. The selection of the correct therapeutic option can therefore be guided after a careful evaluation of the particular endotype and phenotype, from the combined evaluation of inflammatory biomarkers, clinical picture and comorbidities. The careful evaluation of all these parameters can therefore help the physician in the optimal management of these complex patients, for whom it is often possible to achieve exceptional results by managing the available options in the best possible way. The aim of this review is to define the positioning of the biological drugs currently available for type 2 asthma, with a special focus on options for eosinophilic asthma in the context of the most recent knowledge of immunological pathways.
Keywords: severe refractory asthma, eosinophilia, IL-5, biomarkers, oral corticosteroids, pandemic
Introduction
In the last few years, we witnessed a great increase in knowledge on etiopathogenesis and immunological pathways of severe asthma. This happened in particular for type 2 asthma, due to a better definition of phenotypes and endotypes and to the identification of biological drugs aimed at different targets and able to respond to the needs of many patients.
Despite these aspects, severe refractory asthma (SRA) still has a high economic and social impact, including a reduction in quality of life (QoL), productivity, a greater risk of exacerbations and emergency department (ED) visits.1,2 Another major issue is the need of oral corticosteroids (OCS), often due to a poor response to standard therapies or the lack of indications for currently available biological drugs. In literature, up to 60% of patients with severe or uncontrolled asthma need long-term therapy with OCS.3 These patients are treated with increasing dosages of OCS, due to the unsatisfactory response to OCS therapy or to the untargeted action of these drugs.
As a consequence of the lack of predictive biomarkers, it is not easy to precisely choose the right drug in case of a phenotypic overlap. This occurs especially in patients with allergic and eosinophilic asthma, where it is difficult to choose between different biologic therapies available.
The aim of this review is to define the positioning of the biological drugs currently available for type 2 asthma, with a special focus on options for eosinophilic asthma in the context of the most recent knowledge of immunological pathways.
Methods
We carried out a comprehensive literature research using validated keyword filters to select articles related to allergic and eosinophilic asthma and to biological treatments. To date, from 1 January 1990 until 31 July 2020 a thorough and selective search has been conducted on biomedical bibliographic databases (PubMed and Embase) and research documents. International guidelines and meta-analyzes have also been taken into consideration, as well as articles published “ahead of print”. The following keywords were used: refractory asthma, eosinophilia, allergy, therapies, inflammation, oral corticosteroids, cytokines, interleukins. In addition, the best and most authoritative documents were considered based on chronological parameters, specifically the date of publication, in order to define a clear research path and a coherent development of the arguments in support to the conclusions.
IL-5, Eosinophils and Other Target Cells
Recruitment and survival of eosinophils in airways as well as maturation of granules are promoted by IL-3, granulocyte-macrophage colonies stimulating factor (GM-CSF) and in particular IL-5, which represents the most important interleukin responsible for eosinophilic airways inflammation in asthmatics.4
IL-5 is related to both GM-CSF and IL-3 and is produced by CD4+ Th2 cells, mast cells, eosinophils and basophils and it is involved in different stages of eosinophil development and function.5 IL-5 stimulates final differentiation and activation of B cells in antibody-forming cells and acts by stimulating proliferation and differentiation of eosinophil precursors.6
The accumulation of group 2 innate lymphoid cells (ILC2) is present in sites of eosinophilic inflammation.7 Furthermore, sputum IL-5 +, IL-13 + and ILC2 are significantly increased in patients with severe asthma whose eosinophilia sputum persists high despite peripheral blood eosinophils (PBE) in normal range.8 This occurs as a consequence of uncontrolled localized production of IL-5, IL-4, IL-13 and ILC2, which contributes to airway eosinophilia in patients with severe eosinophilic asthma refractory to OCS.
It is important to remember that the systemic increase in IL-5 does not always result in the appearance of a pathology mediated by eosinophils. For example, transgenic IL-5 mice remain physically normal, except for the development of splenomegaly.9 In familial eosinophilia (FE), a rare autosomal dominant disorder characterized by marked eosinophilia and progression to organ damage, some clinical manifestations related to this disease are not common.10 At molecular level, the signaling of IL-5 in mature eosinophils activates several signaling molecules, such as JAK-STAT and MAPK, inducing priming (increase of effector functions), chemokinesis/chemotaxis, activation of integrins and prolongation of cell survival through inhibition of apoptosis.11
There are now numerous data confirming how eosinophils regulate homeostatic processes in a steady state, going beyond the concept of eosinophils considered solely as a destructive and inflammatory cells.12,13 The vast majority of these tissue resident eosinophils (rEos) is found in the non-esophageal portions of the gastrointestinal tract, where they perform numerous functions.14,15 Pulmonary rEos have morphological and phenotypic characteristics peculiar to inflammatory eosinophils (iEos), which are recruited into the lungs as an inflammatory Th2 response. It is assumed that rEos, unlike iEos, are not affected by IL-5 during allergen-induced inflammation, because they are not reached by IL-5 due to their specific parenchymal localization in sites less accessible to biological drugs.16 Therefore, homeostatic eosinophils can basically represent a reservoir of eosinophils, capable of balancing the homeostasis of the inflammatory processes they mediate.
The importance of interaction between IL-5 and iEos derives from the production by bronchial epithelial cells (BEC);17 passing through the bloodstream, IL-5 reaches the bone marrow, where it stimulates the progenitors of eosinophils who begin their migration to the pulmonary parenchyma thanks to the effect of IL-5 itself and chemokines.18 It has become evident that the expansion of hematopoietic compartments in the bone marrow (BM) promotes differentiation and trafficking of mature eosinophils to the airways. Hematopoietic progenitor cells egress the BM and home to the lungs, where in situ differentiation within the tissue provides an ongoing source of proinflammatory cells. In addition to this, hematopoietic progenitor cells in the airways can respond to locally derived alarmins to produce several cytokines, thereby themselves acting as effector proinflammatory cells that potentiate type 2 responses in eosinophilic asthma.18 Understanding in depth the eosinophilopoietic processes and correctly applying the new pharmacological strategies can determine a profound modulation of these processes and allow to often obtain very positive clinical responses.
Mepolizumab
Mepolizumab is a humanized N-glycosylated IgG1 kappa monoclonal antibody (mAb) able to inhibit IL-5 with high specificity (half maximum inhibitory concentration, 1 nM) and affinity (kilodalton = 4.2 pM), thus preventing its binding with the alpha chain of the receptor present on the surface of eosinophils (Figure 1).19 Mepolizumab is made up of two light chains and two heavy chains connected by a disulfide bridge.20
Randomized clinical trials (RCTs) DREAM, MENSA and SIRIUS have shown a significant clinical benefit of mepolizumab in terms of reduction of exacerbations and saving effect of steroids in patients with peripheral PBE ≥ 300 cells/μL.21–23 Moreover, the MUSCA study demonstrated an important and significant improvement in quality of life (QoL) and in pre-bronchodilator forced expiratory volume in 1 second (FEV1) values throughout the duration of the study (24 weeks).24 A post hoc analysis of the DREAM and MENSA studies later confirmed that higher baseline blood eosinophil count (>500 cells/μL) had a certain predictive value of clinical efficacy.25
The COSMOS and COLUMBA extension studies have shown an excellent safety and tolerability profile, with stable and lasting clinical efficacy up to 4.5 years of follow-up.26,27 However, a decrease in lung function was noted during the COLUMBA study, probably due to a natural progression of asthma or a reduction in the dose of OCS following the benefit obtained with mepolizumab.27
An indirect comparison study based on literature data and on a Cochrane review showed that in patients with severe refractory eosinophilic asthma and similar levels of blood eosinophilia, mepolizumab is more effective in reducing clinically significant exacerbations and in terms of controlling asthma compared to the competitors reslizumab and benralizumab.28 It should be remembered that the indirect comparison design involves methodological limitations, so it will be necessary to carry out head-to-head comparative studies in order to establish the real superiority of a drug compared to the others.
Some recent real-life studies have been published, which confirmed that mepolizumab was safe, resulted in significant reduction in the annual rate of exacerbation, reduction of interruption of the required dose of OCS, and improvements in asthma control and lung function.29 Furthermore, mepolizumab was also effective in patients with mixed allergic and eosinophilic phenotypes and after switching to omalizumab.30,31
The approved dose of mepolizumab for the treatment of eosinophilic refractory asthma is 100 mg subcutaneously (SC).32 In selected cases, this dose was considered inadequate, such as in overweight or obese patients. Some authors made a switch with reslizumab, IV administered with weight-adjusted dosage and it appeared to be more effective than fixed-dose mepolizumab in terms of reduction of airway eosinophilia and exacerbations in patients with steroid-dependent eosinophilic asthma.33 However, very recent data show that the 100 mg dose of mepolizumab is effective as well in patients with eosinophilic granulomatosis with polyangiitis (EGPA).34 In this study, the authors demonstrated that low doses of mepolizumab (unlike the approved indication for EGPA – 300 mg as 3 separate 100-mg injections administered SC once every 4 weeks) allow clinically relevant benefits on asthma exacerbation rates, symptoms, oral corticosteroid (OCS) sparing effect and immunosuppressive use in EGPA patients. These effects occurred without any relapse of EGPA for extrapulmonary manifestations.
Benralizumab
Benralizumab is a fully humanized IgG1k afucosylated mAb which binds to the α-chain of the IL-5 receptor (IL-5R), with consequent inhibition of receptor activation mediated by IL-5 (Figure 1).35 Afucosylation improves the interaction of benralizumab with its binding site and strongly induces the main feature of this drug, cell-mediated antibody-dependent cytotoxicity (ADCC) by natural killer (NK)-cells. Through ADCC, benralizumab can markedly reduce eosinophils and other IL-5R + cells, such as progenitors of eosinophils, basophils and ILC2s.36
A peculiar feature is that benralizumab is insensitive to circulating levels of IL-5, which can increase during exacerbations of asthma (an aspect that limits the effectiveness of competitors).37 Through tissue depletion of eosinophils, benralizumab is also insensitive to the effects of IL-3 and GM-CSF, a detail that can make the response to this drug even more effective.38
The SIROCCO and CALIMA Phase 3 studies have shown that benralizumab is effective in significantly reducing the rate of exacerbations and improving the symptoms of asthma and the quality of life in patients with severe eosinophilic asthma.39,40 In addition to this, the treatment with benralizumab resulted in significant reduction in the use of chronic oral corticosteroids, as demonstrated by the ZONDA phase 3 trial. In this study, benralizumab therapy resulted in a 75% reduction in the OCS dose compared to 25% in the placebo group (p = 0.001). About 50% of patients exposed to benralizumab were able to completely stop OCS compared to 19% in the placebo group.41
In phase 3 extension trial BORA after 2 years of treatment there were no side-effects related to long-term eosinophil depletion and the incidence of other adverse events, including opportunistic infections, were similar to placebo.42
An indirect comparison between mepolizumab and benralizumab also placed benralizumab at a higher level in terms of OCS-sparing effect and asthma control.43
The administration of benralizumab at a dosage of 30 mg administered by SC injection each 4 weeks (Q4W) for the first 3 doses and every 8 weeks (Q8W) thereafter is approved for the treatment of patients with severe eosinophilic asthma aged 12 years or older.44
In a pooled analysis from the SIROCCO and CALIMA RCTs, the effect of treatment with benralizumab Q8W was more evident in patients who had baseline PBE ≥300 cells/μL, OCS therapy, chronic rhinosinusitis with nasal polyposis (CRSwNP) and forced vital capacity (FVC) < 65%. These parameters can help identify patients potentially responsive to benralizumab.45 As found for mepolizumab, another pooled analysis of phase 3 SIROCCO and CALIMA studies showed that treatment with benralizumab reduced exacerbations and improved lung function regardless of serum IgE levels and the presence of atopy.46 These results confirmed that anti-IL-5 and anti-eosinophilic mAbs are indicated not only for refractory eosinophilic asthma but also in patients with a mixed allergic/eosinophilic phenotype.
Some retrospective real-life studies have been published on benralizumab which reaffirm its effectiveness in terms of improved asthma control, lung function, and a steroid-sparing effect as well as reduction in emergency department visits and hospitalizations.47 As with mepolizumab, benralizumab was also effective in patients with a combined allergic-eosinophilic phenotype.48
Reslizumab
Reslizumab is a humanized recombinant mAb IgG4 kappa designed to block IL-5 (Figure 1). Reslizumab is an antagonist of IL-5 binding and interaction with its receptor. It was approved for eosinophilic asthma in 2016, in patients aged 18 years or older. Unlike mepolizumab and benralizumab, it is administered intravenously (IV) once every 4 weeks with a dosage based on body weight (3 mg/kg via IV infusion over 20–50 minutes) for patients with a PBE level >400 cells/μL.49
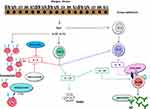 |
Figure 1 T2 high inflammatory pathways. |
Phase 3 studies showed a significant reduction in sputum eosinophil counts, a modest but significant improvement in QoL, FEV1 and asthma control in terms of a reduction of the exacerbation rate up to 50%.50,51 In a post hoc analysis of pooled data from phase 3 BREATH RCTs, add-on reslizumab treatment reduced the frequency of clinical exacerbations of asthma by 83% compared to placebo among patients with CRSwNP. These results confirm that this subpopulation is more sensitive to the effects of reslizumab than patients with eosinophilic refractory asthma without CRSwNP.52
To date, only one real life study has been published on reslizumab, involving 26 patients.53 In this retrospective study, reslizumab was well tolerated and significantly reduced the rate of exacerbations and to a lesser extent the OCS dosage. These data confirm the usefulness of anti-IL5 therapy in a carefully selected phenotype of severe asthma with evidence of eosinophilic airway inflammation.
Dupilumab
IL-4 and IL-13 are pleiotropic Th2 cytokines, which share the IL-4Rα receptor and a common regulatory pathway.54 Dupilumab is a fully humanized IgG4 monoclonal antibody directed against the α subunit of the IL-4 receptor, capable of blocking the signaling of IL-4 and IL-13 (Figure 1).55
Phase 3 RCT Liberty Asthma VENTURE demonstrated that the maximum efficacy of dupilumab was observed in type 2 phenotype (baseline eosinophil blood count ≥ 150 cell/μL and basal fractional exhaled nitric oxide (FeNO)) ≥ 25 parts per billion (ppb). A 65.8% reduction in the severe exacerbation rate was observed in these patients compared to the placebo group.56 In another phase 3 study (Liberty Asthma QUEST), the reduction in average dose of OCS was 70.1% in the dupilumab group, compared to 41.9% in the placebo group.57 Treatment with this mAb resulted in a 59% reduction in severe exacerbation rate with an increase in FEV1 of 0.22 L. It is very important to note that in this study patients were recruited regardless of the presence of high levels of type 2 biomarkers, such as basal eosinophil count in the blood or sputum, FeNO or IgE. Dupilumab is therefore a promising therapeutic option also in allergic asthma or in the case of a combined allergic and eosinophilic phenotype, as confirmed by a recent post hoc analysis of the QUEST study.58
In a exploratory post hoc analysis of dupilumab 300 mg every two weeks (Q2W), there appeared to be discrepancies in patients with PBE ≥ 150/μL and FeNO < 25 ppb in terms of a significant reduction in exacerbations but no improvement in FEV1 compared to placebo. Conversely, in patients with PBE < 150/μL and FeNO ≥ 25 ppb there was agreement on both the improvement of exacerbations and FEV1.56
In a phase 3 study in patients with asthma and with or without chronic rhinosinusitis (CRS), dupilumab 200mg/300mg administered SC every two weeks reduced annualized severe exacerbation rates by 63%/61% respectively, in patients with CRS, and by 42%/40% in patients without CRS (all P <0.001 vs placebo).59 This result confirms that also in this case, as seen, for example, for benralizumab and reslizumab, the presence of sinusitis with or without CRSwNP, is a “clinical biomarker” capable of predicting a better response towards dupilumab. By evaluating the available data, the tolerability profile of dupilumab was generally similar to placebo as regards to the incidence of adverse events (AE). In a pooled analysis of data from the QUEST and VENTURE studies, the adverse reactions that occurred most commonly with dupilumab with a numerically higher incidence than placebo included injection site reactions, oropharyngeal pain and blood eosinophilia ≥3000/μL.60 This event occurred in 1.2% with dupilumab compared to 0.3% with placebo (combined data) in the QUEST study48 and 13% with dupilumab compared to 1% with placebo in the VENTURE trial.57
In the first and only real-life cohort study of predominantly steroid-dependent severe asthma, dupilumab significantly improved asthma control and lung function and reduced OCS use and the rate of exacerbations. Despite the limitations due to the nature of the study, these results are consistent with the efficacy data of the controlled studies.61
Omalizumab
Omalizumab is a humanized monoclonal antibody with specificity for the IgE molecule at the binding site for the high-affinity IgE receptor (FcεRI) (Figure 1). Omalizumab is approved as add-on treatment therapy for moderate-to-severe allergic asthma in adults, adolescents and children (6 to <12 years of age).62 Suitable patients for omalizumab are those with an IgE level of ≥30–1500 IU/mL and bodyweight of 20–150 kg with doses up to 600 mg Q2W. Omalizumab blocks the interaction of free IgE with FcεRI on mast cells, antigen-presenting cells and basophils. Omalizumab indirectly regulates the expression of FcεRI on basophils, mast cells and dendritic cells, influencing the production of type 2 cytokines and inhibiting T2 high inflammation.63–65
Over many years of use, the efficacy of omalizumab in patients with severe uncontrolled allergic asthma has been confirmed by several randomized and real-life studies. These studies highlighted its efficacy and safety, with a significant reduction in frequency of asthma exacerbations (up to 50%), improved QoL, and reduced use of OCS.66 This treatment has shown good efficacy even in patients with non-allergic asthma, most often treated for longer periods,67 giving credit to the hypothesis of a IgE production even without systemic sensitization.68 Other studies have confirmed its efficacy also in prevention of seasonal exacerbations, of interferon-α production in response to rhinoviruses infection.69
Recent studies confirmed that a long-term treatment with omalizumab reduces type 2 inflammation by acting on different types of cells that play a fundamental role in the pathogenesis of allergic asthma but also thanks to the ability to detach IgE from its receptor.70 In the EXTRA study, the subpopulation with atopy with PBE ≥ 260 cells/μL and FeNO ≥ 19.5 ppb is associated with an increased probability of response to omalizumab.71 Based on available evidence, the combination of presence of total serum IgE within the dosage range, a history of frequent exacerbations, persistent asthma symptoms, decrease in FEV1, allergen specific IgE for perennial inhalants, increase in FeNO and increase in PBE is associated with an increased likelihood of response to omalizumab.
The availability of omalizumab in real-life treatment of allergic asthma represented a great advance for the therapeutic control of severe asthma in more severe phenotypes. These important therapeutic actions of omalizumab have been documented by several RCTs, and more recently by numerous real life studies with an experience of over 10 years in daily clinical practice. These studies demonstrated the therapeutic efficacy of omalizumab to significantly reduce asthma exacerbations, as well as improve symptoms and quality of life.72
How to Choose the Right Therapeutic Option in the Current Landscape
The availability of a growing number of options among biological drugs makes it necessary to accurately define which one to choose (Table 1). In the absence of real predictive biomarkers, it is not always a simple task, especially in the case of mixed or combined inflammatory phenotypes.
 |
Table 1 Therapeutic Options for Type 2 Asthma |
Among the available biomarkers, PBE are a parameter that correlates effectively with the response to anti-IL-5 and anti-eosinophils mAbs mepolizumab and benralizumab, in particular in patients with >300 cells/μL or better yet 500 cells/μL.21 The reliability data about FeNO are still partially controversial in determining effectiveness of these mAbs.73 However, the EXTRA study showed that high atopy-related blood eosinophilia and FeNO values can positively predict the response to omalizumab,71 and patients with these phenotypic characteristics loose the benefits of treatment more quickly after withdrawal, as demonstrated by the XPORT study.65
These data increase the difficulty of choosing between the various biological drugs in case of overlap, although long-term efficacy and safety data are in favour of omalizumab,74 which remains mainly indicated for patients with early-onset refractory allergic asthma, with sensitization to perennial inhalant allergens, with or without blood eosinophilia. Currently, there are no direct comparison data between anti-IgE and anti-IL-5; therefore, considering a possible partial overlap between omalizumab and patients unsuitable for mepolizumab, comparative studies will be needed to evaluate the effectiveness of the two classes of mAbs. A meta-analysis revealed a certain heterogeneity between the patients treated and different selection criteria for the use of the two classes of mAbs, that do not allow definitive recommendations for the preferential use of omalizumab against mepolizumab, even if no significant differences in efficacy emerged.75
An international cross-sectional observational study showed that the overlap of eligibility for treatment with anti-IL-5 or anti-IgE therapies varied between 27% and 37% of patients.76 It is therefore necessary to pay utmost attention in choosing the most suitable therapy, as phenotypic and eligibility overlap for biologics is frequent. In this regard, it must be remembered that the presence of atopy and the total level of IgE at baseline do not influence mepolizumab, as demonstrated by a subanalysis of the DREAM trial.22 A recent post hoc analysis of patients enrolled in the DREAM, MENSA and SIRIUS studies and treated with mepolizumab after ineffective treatment with omalizumab, confirmed results from the previous studies, showing that these patients responded positively to mepolizumab, regardless of previous use of omalizumab.77
The need to identify biomarkers able to predict the effectiveness of biological drugs is high at the moment. For this purpose, there are international projects such as the “Unbiased BIOmarkers for the Prediction of Respiratory Disease Outcomes” consortium. The U-BIOPRED cohort is characterized by poor symptoms control, increased comorbidities and airways inflammation, despite high levels of treatment. Proteomics datasets will be available in the near future; the basis of systems medicine approach and will be probably able to give a real turning point in the identification of asthma phenotypes.78
Is Eosinophilic Asthma Really Only for Anti-Eosinophilic/Anti-IL-5 Biologics?
To evaluate the indication for one of the biological therapies for severe asthma, it is crucial a phenotype-based approach and a careful classification of comorbidities such as CRSwNP, allergic rhinitis and atopic dermatitis. The importance of the phenotype is testified by what happened with the first studies on mepolizumab, which had failed their objectives due to an improper selection of the study population. Leckie et al had performed the first randomized, double-blind, placebo-controlled study of this biologic. There were no significant improvements in respiratory function despite decreased airway and blood eosinophilia after 4 and 16 weeks.79 A subsequent RCT of 362 patients with uncontrolled asthma despite inhaled corticosteroid therapy was aimed at evaluating the effect of three intravenous infusions of mepolizumab (250 or 750 mg every month) on clinical outcome measures.80 This study also failed clinical endpoints despite a significant reduction in blood and sputum eosinophils in both treatment groups.80 After better identifying the patients most likely to respond, the history of this drug for the treatment of patients with severe eosinophilic asthma has changed dramatically, demonstrating great efficacy in patients with the correct characteristics.21–23
As for now, for severe refractory eosinophilic asthma there are targeted therapies available against IL-5 and eosinophils (Table 1). The anti-IL-4/IL-13 option dupilumab is already available for the treatment of asthma in many countries and will soon be available in other countries where it is not currently marketed. These mAbs have proven to be very effective in reducing exacerbations of asthma, improving lung function in some cases and controlling asthma. A significant steroid-sparing effect has been confirmed in many randomized clinical trials.81 This will likely lead to remove the systemic steroid option in the next few years as the last alternative to GINA step 5.
The first choice and most important drugs are those that target cells and one of the most important cytokine in eosinophilic asthma, IL-5. These drugs have been specifically designed and studied for eosinophilic asthma, but it is not always easy to choose between biological products available because, as already mentioned, some patients have overlapping characteristics and they can theoretically benefit from different treatments. Therefore, clinicians find it difficult to establish the optimal therapy for a specific patient. Moreover, for patients who meet the prescription criteria for different mAbs, present atopy and high levels of PBE, there are not clear indications on how to make a certain choice.
In order to help clinicians in choosing the most suitable add-on treatment for these patients, some authors proposed to consider omalizumab as first-line therapy due to its efficacy and safety assessed by a large body of real-life data and over a decade of surveillance post-marketing.82 Confirming that the choice remains complex, not all patients taking omalizumab seem to respond well to treatment even if selected correctly. In this regard, according to real-life data from the Italian NEONet group, 32% of patients treated with omalizumab dropped out mainly due to lack of efficacy.83
As of literature data, even in the case of omalizumab, it appears that higher values of PBE (≥300/μL) as well as FeNO cut-off >30 ppb are predictive of greater efficacy (Figure 2).84 To date, eosinophils have been considered the main clinical marker of type 2 inflammation in respiratory diseases, although their relevance in identifying severity of asthma is still a matter of debate.85–87
Blood eosinophilia has been identified as a risk factor for exacerbations of asthma, regardless of symptom control.86,87 The blood count of eosinophils, however, seems more useful and reliable as a predictive biomarker of response to biological treatments aimed at eosinophils compared to a distinctive sign of the severity of asthma. Some authors demonstrated, in patients with severe asthma being treated with mepolizumab, that reduction in the exacerbation rate was significantly greater in patients with PBE ≥ 150/μL compared to those with PBE < 150/μL.88 Data from phase 3 studies on mepolizumab also confirmed the predictive role of blood eosinophil levels in response to treatment in patients with severe eosinophilic asthma. Likewise, 2 RCTs on reslizumab demonstrated the important role of baseline PBE in patient selection.89,90 Elevated PBE have also been shown to be an essential condition for the efficacy of benralizumab in patients with severe uncontrolled asthma.39,40
FeNO appears to be a good indicator of asthma severity and control, perhaps better than PBE level. This is more evident in patients with allergic asthma, but the correlation between sputum eosinophil counts, PBE counts and FeNO is not supported by reliable evidence.91,92
Patients with elevated FeNO, blood eosinophilia with or without atopy are those most suitable for dupilumab (Figure 2), as shown by some studies.57
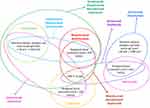 |
Figure 2 Overlapping of biological drugs in the context of inflammatory phenotypes. |
Another possibility for selecting the right patient for the right pharmacological option is the use of clinical phenotypes. Patients with fixed airway obstruction, for example, can benefit more from mAbs such as benralizumab, reslizumab or dupilumab,39,40,50 which have shown consistent improvements in respiratory function compared to omalizumab and mepolizumab.22,93
The subpopulation of patients with persistent symptoms, tissue inflammation and blood and sputum eosinophilia despite prolonged use of OCS are termed “steroid resistant” or “steroid refractory” asthma patients.94
Many of the recent biological studies for refractory asthma have shown significant benefits in weaning patients with chronic OCS use or at least in a significant reduction of the average OCS dose. Data on omalizumab ability to afford the weaning of the OCS are not certain, since no specific RCTs have been conducted. Reslizumab has also not been specifically studied for this indication.95,96 Mepolizumab, benralizumab and dupilumab instead have been studied specifically for OCS dependent patients and have been found to have a consistent efficacy in reducing the use of OCS.23,41,56 When choosing a biological drug for patients with chronic need for OCS and with evidence of eosinophilia (150–300 cells/mL), mepolizumab, benralizumab or dupilumab are the most correct choices (Figure 2). If the patient has no evidence of eosinophilia, but high FENO, dupilumab may be the best option.
The real-life studies showed not only the effectiveness of all biologics, but also that in the case of combined phenotypes they can be interchangeable or therapeutic switches can be made in case of ineffectiveness of one or the other. The choice must therefore be guided by the combined evaluation of the biomarkers currently available, by the age of onset of asthma (earlier, for example, in the case of pure allergic asthma), by comorbidities such as nasal polyposis, atopic dermatitis and obesity and finally by the experience and confidence of the clinician, which remains fundamental.
Conclusions
As the number of therapeutic options increases, the choice of biological drugs can be made only after careful considerations of the particular endotype, patients comorbidities and clinical data. In patients with severe refractory eosinophilic asthma, anti-IL5 and anti-eosinophilic mAbs remain the first choice, regardless of the FeNO values and the presence of atopy, provided the presence of an eosinophil level ≥300 cells/μL. There are real-life data that suggested that in patients with sub-optimal response to mepolizumab, a switch to benralizumab may be associated with better outcomes. This can allow us to increase the possibility of obtaining therapeutic success, even if these are choices to be made with great attention and in a thoughtful way.
Regarding dupilumab, the evidence is that it is more effective in patients with a high FeNO endotype, although it has also been effective in patients with high PBE and low FeNO, albeit to a lesser extent. Therefore, the studies published so far indicate this drug as a possible therapeutic option in severe eosinophilic asthma, however carefully evaluating the levels of these biomarkers.
Finally, omalizumab can also be effective in eosinophilic asthma, but in combination with the presence of total serum IgE in the dosage range, specific IgE allergens for perennial inhalants and possibly an increase in FeNO.
The biological drugs for severe asthma are therefore all effective and safe on condition of a careful choice, that can be a difficult task because head-to-head comparison studies do not yet exist and the published studies are limited in some cases by a certain heterogeneity, especially as regards the type of enrolled patients. In this regard, an example is the first head-to-head RCT PREDICTUMAB (NCT03476109), comparing mepolizumab and omalizumab. The selection of the correct therapeutic option can therefore be guided after a careful evaluation of the particular endotype and phenotype, from the combined evaluation of inflammatory biomarkers, clinical picture and comorbidities. The careful evaluation of all these parameters can therefore help the physician in the optimal management of these complex patients, for whom it is often possible to achieve exceptional results by managing the available options in the best possible way.
Abbreviations
SRA, severe refractory asthma; QoL, quality of life; ED, emergency department; OCS, oral corticosteroids; GM-CSF, granulocyte-macrophage colonies stimulating factor; ILC2, group 2 innate lymphoid cells; PBE blood eosinophils; FE, familial eosinophilia; rEos, tissue resident eosinophils; iEos, inflammatory eosinophils; BEC, bronchial epithelial cells; BM, bone marrow; mAb, monoclonal antibody; RCTs, randomized clinical trials; FEV1, forced expiratory volume in 1 second; SC, subcutaneously; EGPA, eosinophilic granulomatosis with polyangiitis; IL-5R, IL-5 receptor; ADCC, cell-mediated antibody-dependent cytotoxicity; NK, natural killer-cells; Q4W, every 4 weeks; Q8W, every 8 weeks; IV, intravenously; Q2W, every two weeks; CRSwNP, chronic rhinosinusitis with nasal polyposis; FVC forced vital capacity; FeNO, fractional exhaled nitric oxide; IFN, interferon; pDCs, plasmacytoid dendritic cells; FcεRI, high affinity IgE receptor; pDCs plasmacytoid dendritic cells; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RV16, rhinovirus 16.
Disclosure
Francesco Menzella has received research grants from AstraZeneca, Novartis and Sanofi; lecture fees and advisory board fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, and Sanofi. The other authors declare no conflicts of interest.
References
1. Trevor JL, Chipps BE. Severe asthma in primary care: identification and management. Am J Med. 2018;131(5):484. (). doi:10.1016/j.amjmed.2017.12.034
2. Luskin AT, Chipps BE, Rasouliyan L, et al. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2(5):
3. Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201(3):276–293. doi:10.1164/rccm.201904-0903SO
4. Uhm TG, Kim BS, Chung IY. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2012;4(2):68–79. doi:10.4168/aair.2012.4.2.68
5. Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–294. doi:10.1016/j.coi.2008.04.001
6. Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–1309. doi:10.1093/intimm/dxp102
7. Licona-Limon P, Kim LK, Palm NW, et al. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi:10.1038/ni.2617
8. Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e78. doi:10.1016/j.jaci.2015.05.037
9. Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425e31. doi:10.1084/jem.172.5.1425
10. Prakash Babu S, Chen YK, Bonne-Annee S, et al. Dysregulation of interleukin 5 expression in familial eosinophilia. Allergy. 2017;72(9):1338e45. doi:10.1111/all.13146
11. Adachi T, Alam R. The mechanism of IL-5 signal transduction. Am J Physiol. 1998;275:C623e33. doi:10.1152/ajpcell.1998.275.3.C623
12. Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi:10.1146/annurev.immunol.24.021605.090720
13. Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8(3):464–475. doi:10.1038/mi.2015.2
14. Chu VT, Beller A, Rausch S, et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40(4):582–593. doi:10.1016/j.immuni.2014.02.014
15. Jung Y, Wen T, Mingler MK, et al. IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8(4):930–942. doi:10.1038/mi.2014.123
16. Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126(9):3279–3295. doi:10.1172/JCI85664
17. Salter BM, Sehmi R. Hematopoietic processes in eosinophilic asthma. Chest. 2017;152(2):410–416. doi:10.1016/j.chest.2017.01.021
18. Wu CA, Peluso JJ, Zhu L, et al. Bronchial epithelial cells produce IL-5: implications for local immune responses in the airways. Cell Immunol. 2010;264(1):32–41. doi:10.1016/j.cellimm.2010.04.008
19. Zia-Amirhosseini P, Minthorn E, Benincosa LJ, et al. Pharmacokinetics and pharmacodynamics of SB-240563, a humanized monoclonal antibody directed to human interleukin-5, in monkeys. J Pharmacol Exp Ther. 1999;291(3):1060–1067.
20. Hart TK, Cook RM, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol. 2001;108(2):250–257. doi:10.1067/mai.2001.116576
21. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
22. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:10.1016/S0140-6736(12)60988-X
23. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291
24. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi:10.1016/S2213-2600(17)30125-X
25. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–556. doi:10.1016/S2213-2600(16)30031-5
26. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38(9):2058–2070.e1. doi:10.1016/j.clinthera.2016.07.010
27. Khatri S, Moore W, Gibson PG, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143(5):1742–1751.e7. doi:10.1016/j.jaci.2018.09.033
28. Busse W, Chupp G, Nagase H, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190–200.e20. doi:10.1016/j.jaci.2018.08.031
29. Kallieri M, Zervas E, Katsoulis K, et al. Mepolizumab in severe eosinophilic asthma: a 2-year follow-up in specialized asthma clinics in Greece: an interim analysis. Int Arch Allergy Immunol. 2020;181(8):613–617. doi:10.1159/000508559
30. Carpagnano GE, Pelaia C, D’Amato M, et al. Switching from omalizumab to mepolizumab: real-life experience from Southern Italy. Ther Adv Respir Dis. 2020;14:1753466620929231. doi:10.1177/1753466620929231
31. Pelaia C, Crimi C, Pelaia G, et al. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy. 2020;50(7):780–788. doi:10.1111/cea.13613
32. European Medicines Agency - European Public Assessment Report (EPAR) for Nucala (mepolizumab). https://www.ema.europa.eu/en/medicines/human/EPAR/nucala.
33. Mukherjee M, Aleman Paramo F, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197(1):38–46. doi:10.1164/rccm.201707-1323OC
34. Vultaggio A, Nencini F, Bormioli S, et al. Low-dose mepolizumab effectiveness in patients suffering from eosinophilic granulomatosis with polyangiitis. Allergy Asthma Immunol Res. 2020;12(5):885–893. doi:10.4168/aair.2020.12.5.885
35. Menzella F, Lusuardi M, Galeone C, et al. The clinical profile of benralizumab in the management of severe eosinophilic asthma. Ther Adv Respir Dis. 2016;10(6):534–548. doi:10.1177/1753465816667659
36. Pelaia C, Calabrese C, Vatrella A, et al. Benralizumab: from the basic mechanism of action to the potential use in the biological therapy of severe eosinophilic asthma. Biomed Res Int. 2018;2018:4839230. doi:10.1155/2018/4839230
37. Tai PC, Sun L, Spry CJ. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin Exp Immunol. 1991;85(2):312–316. doi:10.1111/j.1365-2249.1991.tb05725.x
38. Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–1096.e5. doi:10.1016/j.jaci.2013.05.020
39. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi:10.1016/S0140-6736(16)31324-1
40. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi:10.1016/S0140-6736(16)31322-8
41. Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi:10.1056/NEJMoa1703501
42. Busse WW, Bleecker ER, FitzGerald JM, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7(1):46–59. doi:10.1016/S2213-2600(18)30406-5
43. Menzella F, Latorre M, Ruggiero P, Bagnasco D, Heffler E. Reduction of oral corticosteroids in patients with severe eosinophilic asthma treated with Benralizumab: could it represent a marker of treatment efficacy? Expert Opin Biol Ther. 2019;19(7):601–606. doi:10.1080/14712598.2019.1613367
44. Caminati M, Menzella F, Guidolin L, et al. Targeting eosinophils: severe asthma and beyond. Drugs Context. 2019;8:212587. doi:10.7573/dic.212587
45. Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52(4):1800936. doi:10.1183/13993003.00936-2018
46. Chipps BE, Newbold P, Hirsch I. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120(5):504–511.e4. doi:10.1016/j.anai.2018.01.030
47. Padilla-Galo A, Levy-Abitbol R, Olveira C, et al. Real-life experience with benralizumab during 6 months. BMC Pulm Med. 2020;20(1):184. doi:10.1186/s12890-020-01220-9
48. Pelaia C, Busceti MT, Crimi C, et al. Real-Life effects of benralizumab on exacerbation number and lung hyperinflation in atopic patients with severe eosinophilic asthma. Biomed Pharmacother. 2020;129:110444. doi:10.1016/j.biopha.2020.110444
49. Mavissakalian M, Brady S. The current state of biologic therapies for treatment of refractory asthma. Clin Rev Allergy Immunol. 2020. doi:10.1007/s12016-020-08776-8
50. Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125–1132. doi:10.1164/rccm.201103-0396OC
51. Sahota J, Robinson DS. Update on new biologics for intractable eosinophilic asthma: impact of reslizumab. Drug Des Devel Ther. 2018;12:1173–1181. doi:10.2147/DDDT.S109489
52. Weinstein SF, Katial RK, Bardin P, et al. Effects of reslizumab on asthma outcomes in a subgroup of eosinophilic asthma patients with self-reported chronic rhinosinusitis with nasal polyps. j allergy clin immunol pract. 2019;7(2):589–596.e3. doi:10.1016/j.jaip.2018.08.021
53. Ibrahim H, O’Sullivan R, Casey D, et al. The effectiveness of Reslizumab in severe asthma treatment: a real-world experience. Respir Res. 2019;20(1):289. doi:10.1186/s12931-019-1251-3
54. Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015;75(1):25–37. doi:10.1016/j.cyto.2015.05.008
55. Menzella F, Bertolini F, Biava M, et al. Severe refractory asthma: current treatment options and ongoing research. Drugs Context. 2018;7:212561. doi:10.7573/dic.212561
56. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092
57. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi:10.1056/NEJMoa1804093
58. Corren J, Castro M, O’Riordan T, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. 2020;8(2):516–526. doi:10.1016/j.jaip.2019.08.050
59. Maspero JF, Katelaris CH, Busse WW, et al. Dupilumab efficacy in uncontrolled, moderate-to-severe asthma with self-reported chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(2):527–539.e9. doi:10.1016/j.jaip.2019.07.016
60. Sanofi-Aventis U.S. LLC and Regeneron Pharmaceuticals Inc. Dupixent (dupilumab) injection: US prescribing information. 2019. Available from: http://www.accessdata.fda.gov.
61. Dupin C, Belhadi D, Guilleminault L, et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin Exp Allergy. 2020;50(7):789–798. doi:10.1111/cea.13614
62. Katsaounou P, Buhl R, Brusselle G, et al. Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma. Respir Med. 2019;150:51–62. doi:10.1016/j.rmed.2019.02.003
63. MacGlashan DW
64. Mitchell PD, El-Gammal AI, O’Byrne PM. Anti-IgE and biologic approaches for the treatment of asthma. Handb Exp Pharmacol. 2017;237:131–152.
65. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140(1):162–169.e2. doi:10.1016/j.jaci.2016.08.054
66. Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139(1):28–35. doi:10.1378/chest.10-1194
67. Menzella F, Piro R, Facciolongo N, et al. Long-term benefits of omalizumab in a patient with severe non-allergic asthma. Allergy Asthma Clin Immunol. 2011;7(1):9. doi:10.1186/1710-1492-7-9
68. Forester JP, Calabria CW. Local production of IgE in the respiratory mucosa and the concept of entopy: does allergy exist in nonallergic rhinitis? Ann Allergy Asthma Immunol. 2010;105(4):249–255. doi:10.1016/j.anai.2010.02.001
69. Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–1485. doi:10.1016/j.jaci.2015.09.008
70. Maggi L, Rossettini B, Montaini G, et al. Omalizumab dampens type 2 inflammation in a group of long-term treated asthma patients and detaches IgE from FcεRI. Eur J Immunol. 2018;48(12):2005–2014. doi:10.1002/eji.201847668
71. Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi:10.1164/rccm.201208-1414OC
72. Pelaia C, Calabrese C, Terracciano R, de Blasio F, Vatrella A, Pelaia G. Omalizumab, the first available antibody for biological treatment of severe asthma: more than a decade of real-life effectiveness. Ther Adv Respir Dis. 2018;12:1753466618810192. doi:10.1177/1753466618810192
73. Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. doi:10.1136/thoraxjnl-2014-205634
74. Menzella F, Galeone C, Formisano D, et al. Real-life efficacy of omalizumab after 9 years of follow-up. Allergy Asthma Immunol Res. 2017;9(4):368–372. doi:10.4168/aair.2017.9.4.368
75. Nachef Z, Krishnan A, Mashtare T, Zhuang T, Mador MJ. Omalizumab versus Mepolizumab as add-on therapy in asthma patients not well controlled on at least an inhaled corticosteroid: a network meta-analysis. J Asthma. 2017;1–12.
76. Albers FC, Müllerová H, Gunsoy NB, et al. Biologic treatment eligibility for real-world patients with severe asthma: the IDEAL study. J Asthma. 2018;55(2):152–160. doi:10.1080/02770903.2017.1322611
77. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335–1344. doi:10.1111/all.12914
78. Shaw DE, Sousa AR, Fowler SJ, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46(5):1308–1321. doi:10.1183/13993003.00779-2015
79. Leckie MJ, Ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–2148. doi:10.1016/S0140-6736(00)03496-6
80. Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–1071. doi:10.1164/rccm.200701-085OC
81. Bakakos A, Loukides S, Bakakos P. Severe Eosinophilic Asthma. J Clin Med. 2019;8(9):1375. doi:10.3390/jcm8091375
82. Bousquet J, Brusselle G, Buhl R, et al. Care pathways for the selection of a biologic in severe asthma. Eur Respir J. 2017;50:1701782. doi:10.1183/13993003.01782-2017
83. Caminati M, Senna G, Stefanizzi G, et al. Drop-out rate among patients treated with omalizumab for severe asthma: literature review and real-life experience. BMC Pulm Med. 2016;16(1):128. doi:10.1186/s12890-016-0290-5
84. Tran TN, Khatry DB, Ke X, Ward CK, Gossage D. High blood eosinophil count is associated with more frequent asthma attacks in asthma patients. Ann Allergy Asthma Immunol. 2014;113:19–24. doi:10.1016/j.anai.2014.04.011
85. Yancey SW, Keene ON, Albers FC, et al. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol. 2017;140:1509–1518. doi:10.1016/j.jaci.2017.10.005
86. Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3:849–858. doi:10.1016/S2213-2600(15)00367-7
87. Zeiger RS, Schatz M, Li Q, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2:741–750. doi:10.1016/j.jaip.2014.06.005
88. Katz LE, Gleich GJ, Hartley BF, et al. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11:531–536. doi:10.1513/AnnalsATS.201310-354OC
89. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789–798. doi:10.1016/j.chest.2016.03.032
90. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799–810. doi:10.1016/j.chest.2016.03.018
91. Crespo A, Giner J, Torrejón M, et al. Clinical and inflammatory features of asthma with dissociation between fractional exhaled nitric oxide and eosinophils in induced sputum. J Asthma. 2016;53:459–464. doi:10.3109/02770903.2015.1116086
92. Calciano L, Portas L, Corsico AG, et al. Biomarkers related to respiratory symptoms and lung function in adults with asthma. J Breath Res. 2018;12:026012. doi:10.1088/1752-7163/aa9c86
93. McNicholl DM, Heaney LG. Omalizumab: the evidence for its place in the treatment of allergic asthma. Core Evid. 2008;3:55–66. doi:10.3355/ce.2008.012
94. Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2006;117:522–543. doi:10.1016/j.jaci.2006.01.032
95. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;CD003559.
96. Soler M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi:10.1183/09031936.01.00092101
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
