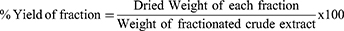Back to Journals » Journal of Experimental Pharmacology » Volume 15
Anti-Diabetic Activities of Hydro-Methanolic Crude Extract and Solvent Fractions of Heteromorpha arborescens (Apiaceae) Leaves in Mice
Authors Zeleke YG, Atnafie SA , Aragaw TJ
Received 22 October 2022
Accepted for publication 28 February 2023
Published 10 March 2023 Volume 2023:15 Pages 107—121
DOI https://doi.org/10.2147/JEP.S392742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Yeshiwas Guadie Zeleke,1 Seyfe Asrade Atnafie,2 Tezera Jemere Aragaw2
1College of Medicine and Health Sciences Comprehensive Specialized Hospital, University of Gondar, Gondar, Ethiopia; 2Department of Pharmacology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Tezera Jemere Aragaw, Tel +251918773670, Fax +2510581141240, Email [email protected]
Background: Heteromorpha arborescens has been used to treat diabetes traditionally. There was no in vivo study to support the claim. This study aimed to confirm anti-diabetic activity of 80% methanol in water extract and solvent fractions of H. arborescens leaves in mice.
Methods: H. arborescens leaves were macerated and extracted in 80% methanol in water. Hydro-methanol extract of H. arborescens leaves were tested in mice models. Overnight fasted mice were randomly divided into five groups for normoglycemic and glucose-loaded models as a negative control, positive control, and three tested groups, whereas, in streptozotocin-induced diabetic models, the mice were grouped into six groups each comprised six mice: diabetic negative control and normal negative control groups treated with 10 mL/kg distilled water, diabetic positive control group treated with Glibenclamide 5 mg/kg and three diabetic tested groups treated with extract at 100, 200, and 400 mg/kg doses. A one-way ANOVA was performed to analyze the data, and the post hoc Tukey’s test was utilized for multiple comparisons. The P-value < 0.05 was considered statistically significant.
Results: Hydro-methanol extract of H. arborescens leaves at 400 mg/kg in normoglycemic mice significantly lowered blood glucose levels (BGLs) (P< 0.01). Mice with oral glucose-loaded test lowered BGLs at dosages of 200 mg/kg (P < 0.05) and 400 mg/kg (P < 0.01) respectively. Single-dose of ethyl acetate, n-hexane fractions and hydro-methanol extract at 100 mg/kg, 400 mg/kg and 200 mg/kg reduced BGLs (P < 0.05, P < 0.001, and P < 0.01) respectively. BGL drops in diabetic mice given daily repeated doses of 200 mg/kg of hydro-methanol extract and 400 mg/kg of ethyl acetate fraction (P < 0.001). Diabetic mice gained weight at a 400 mg/kg hydro-methanol extract and ethyl acetate fraction (P < 0.05 and P < 0.01) respectively. Hydro-methanol extract and ethyl acetate fraction and at 200 mg/kg decreased total cholesterol, triglycerides, and low-density lipoprotein and increased high-density lipoprotein (P < 0.001).
Conclusion: 80% methanol in water extract and solvent fractions of H. arborescens leaves showed anti-diabetic effects and significantly reduced hyperlipidemia in diabetics, this study supported the traditional usage of H. arborescens for treating diabetes; however, species variation could also limit such a straightforward extrapolation of the findings of this study in humans.
Keywords: antidiabetic, Heteromorpha arborescens, in vivo
Introduction
Diabetes mellitus (DM) is a chronic metabolic condition that needs ongoing glucose control and risk-lowering measures.1,2 Around the world, traditional medicines were used for primary healthcare by between 80 and 85% of the population. The pancreatic cell regeneration, antioxidant, cholesterol-lowering, adrenomimetic effect, blocking of pancreatic β-cell potassium channels, stimulation of cyclic adenosyl phosphate, insulin-releasing actions, reduction in insulin resistance, prevention of secondary DM complications, and provision of necessary elements like Ca, Zn, Mg, Mn, and Cu, as well as approximately 1200 plants useful in the treatment of diabetes.3–5 Many Africans believe in spiritual and alternative remedies and prefer to use them as their primary form of healthcare because they are more accessible and affordable than Western drugs.4,6 More than 80% of Ethiopians use traditional medicine to meet their healthcare needs due to cultural acceptance, perceived efficacy against particular ailments, accessibility, and affordability when compared to modern medicines.7 Terpenoids, saponins, glycosides, flavonoids, carotenoids, alkaloids, and other bioactive compounds found in medicinal plants have anti-diabetic activities.8 H. arborescens is a plant that has potential medical benefit in Yemen as well as tropical, southern, and northern Africa.9–12 African tropical plants’ bark, leaves, milky exudate, roots, and root bark are used to make medicines. Treatment of asthma and other respiratory problems, headache, fever, pain, intestinal worms, inflammation, and aphrodisiacs are among the most common medical uses of H. arborescens. H. arborescens leaves and roots have historically been used to cure conditions like diabetes (cooked leaves and roots are drunk), gonorrhea, rabies, and snakebite. The leaves and roots of H. arborescens are used as vegetables by malnourished youngsters.8,9,13,14 Other bioactive substances that are isolated from the leaves, such as 6,7-dimethoxy-2H-chromen-2-one, have anti-inflammatory activities by lowering inflammatory mediators and are used to treat headaches, throbbing headaches, and acute and late phases of inflammation. Two compounds isolated from the leaves of H. arborescens, falcarindiol and sarisan, showed both antifungal and analgesic effects. The presence of biologically active substances in the plant, such as tannins, phenols, alkaloids, saponins, flavonoids, and proanthocyanidin, is thought to contribute to its medicinal potential.12,15,16 Sabinene, δ-3-carene, myrcene, β-phellandrene, and α-pinene are among the principal compounds found in the volatile oil of H. arborescens leaves and blooming sections that have antibacterial and antifungal actions on a variety of microorganisms.9 The total phenolic content of H. arborescens leaves and bark extracts in hexane, ethyl acetate, and methanol was found to be high. Ethyl acetate fraction of leaves (EAL) and methanol extract of bark (MB) had IC50 values for alpha-glucosidase inhibition of 3.12 0.62 and 3.10 1.73 mg/mL, respectively. The volatile oil of H. arborescens leaves and blooming portions contains several primary chemicals that exhibit antibacterial and antifungal effects against a range of microorganisms, including sabinene, δ-3-carene, myrcene, β-phellandrene, and α-pinene.9 H. arborescens leaves and bark extracts in hexane, ethyl acetate, and methanol were found to have a high total phenolic content. The IC50 values for α-glucosidase inhibition in ethyl acetate fraction of leaves (EAL) and methanol extract of bark (MB) were 3.12 ± 0.62 and 3.10 ± 1.73 mg/mL, respectively. The crude extract was shown to be more harmful than solvent fractions in in vitro cytotoxicity tests using monkey kidney cell lines, with an LC50 of 0.04 mg/mL (fractionation decreased toxicity by 35 to 51 fold).18 EAL and MB extracts showed considerable suppression of 2, 2-diphenyl-1-picrylhydrazyl and robust dosage-dependent radical scavenging, β-carotene bleaching, and (DPPH) numerous in vitro studies have shown that H. arborescens has anti-diabetic properties.16,17,19
Rationale of the Study
Five hundred thirty seven million adults (20–79 years) are living with diabetes. This number is predicted to rise to 643 million by 2030 and 783 million by 2045. Over 75% of adults with diabetes live in low and middle-income countries. It is responsible for 6.7 million deaths in 2021 and caused at least USD 966 billion dollars in health expenditure a 316% increase over the last 15 years.20 This may be due to an increase in the proportion of the elderly population, socioeconomic development, urbanization, consumption of processed diets, and decreased physical activity.21,22 Diabetes, which results in lower limb amputation, vision loss, renal dysfunction, absenteeism from work and school, and negative economic repercussions from direct and indirect costs, is the seventh largest cause of death in the world, accounting for 5.2 million (11.3%) fatalities.23–26 Diabetes also shortens one’s life expectancy by 10 years for those with T2DM and by 15 years for those with T1DM, as well as causing early mortality and disability. In Ethiopia, the prevalence of diabetes is 3.2%, according to the 2015 WHO STEPS survey.26–28 The scientific world is looking for effective medicines made from natural sources because the present anti-diabetic pharmaceuticals have severe side effects, are expensive, and are only sometimes available. H. arborescens is used to treat diabetes and has been shown in numerous in vitro experiments to have anti-diabetic properties. The crude extract and solvent fractions of the leaves do not, however, appear to have any in vivo anti-diabetic effects, according to the literature. The aim of this study was to examine the potential acute oral toxicity and in vivo anti-diabetic effects of crude leaf extract and solvent fractions of H. arborescens in Swiss albino mice. The findings of this investigation are anticipated to advance our knowledge of the experimental plant’s acute toxicity, phytochemical make up, and in vivo antidiabetic characteristics. They also act as a starting point for further research.
Materials and Methods
Chemicals
Streptozotocin (Sisco Research Lab. Pvt. Ltd., India), Glibenclamide (Denk, Germany), 40% glucose (Amanta Healthcare Ltd, India), Ketamine HCL injection (Neon Laboratories Limited, India), Diazepam injection (Gland pharm limited, India), and Glucometer (i-QARE DS-W Alliance International CO., Ltd Taiwan) Manual vacuum pump (tw-1A) (Free air displacement 2CFM, Ultimate vacuum 150 micron, voltage 220v- 250v, 5Hz, power 1/4HP, oil capacity 250mL, Zhejiang, China (Mainland). All other chemicals and reagents used are of analytical grade.
Plant Material
Fresh leaves of H. arborescens were harvested on April 6, 2021, from the Tara Gedam Monastery woodland in the Amhara National, Regional State, close to the town of Addis Zemen. The research region is located between 12°8’0” and 12°10’0“N latitude and 37°44’0” and 37°46’0” E longitude along the Addis Ababa-Bahir Dar-Gondar road, with an elevation between 2217 and 2457 meters above sea level.29 It is also 93 kilometers south of Gondar town and 82 kilometers north of Bahir Dar. Mr. Abiyu Eniyew Molla (MSc, Assistant Professor of Botanical Sciences), who works in the Department of Biology at the College of Natural and Computational Sciences at the University of Gondar in Ethiopia, authenticated the specimen and sample was deposited in the Department of Botany’s Herbarium and with certificate number 001/YGZ/2021.
Extraction and Fractionation
Extraction
Fresh H. arborescens leaves were properly washed and cleaned of dirt, and then left to air dry at room temperature in the shade. The dried leaves were manually powdered in a mortar and pestle, and 2219 g of the powder was extracted three times by macerating it in 80% methanol in water in a ratio of 1:10 (w/v) at room temperature for 72 h each time. After that, an air pump was used to filter the extract via gauze and Whatman paper № 1. The filtrates were combined and condensed at 40 °C in a Rotary evaporator under decreased pressure. The final extract was 245 grams in weight with a yield of 11.0%. The dried extract was kept at 4°C until it was used in the actual experiment.26
Fractionation
A dried hydro-methanolic crude extract of H. arborescens leaves was fractionated using polar and non-polar solvents with increasing polarity, including n-hexane, ethyl acetate, and water. A 100 g dry extract was diluted in 500 mL of distilled water, added to a 1000 mL separatory funnel with an equal volume of n-hexane, agitated, and left to stand for two hours until the densities of the n-hexane layer and the aqueous layer could be distinctly separated. After each of the three fractionation stages, the n-hexane fractions were combined. The water layer was separated three times using the same procedure for the ethyl acetate fraction after the n-hexane fraction had been collected. The n-hexane and ethyl acetate fractions were condensed and dried in a drying oven set at 40°C. The leftover water layer was condensed at 40 °C using a hot air oven and rotary evaporator. The condensed water layer was frozen for a whole night in a cold freezer then lyophilized to remove the water. The dried fractions were stored in absolutely airtight bottles that were kept in a refrigerator at 4°C for later usage.19 The formula below was used to determine the dried fractions’ % yield (w/w).
Experimental Animals
In the animal house of the Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, female mice weighing 18–22 g, aged 6–8 weeks used in acute toxicity test and male mice weighing 20–30 g and aged 6–8 weeks were reared for the actual experiment. The mice were maintained in plastic cages with sawdust (regularly changed every 3 days). Six mice were kept in a single cage with a standard pellet diet, a 12-hour light/dark cycle, and enough clean tap water. The animals kept for seven days to acclimatize to the laboratory setting before the actual trials began. Considerate techniques of handling and caring for the animals were employed throughout the experiment in accordance with international recommendations for the use and care of laboratory animals.19,30
Phytochemical Screening
Using common chemicals and techniques, it was possible to qualitatively identify secondary bioactive components in solvent fractions and crude hydro-methanol extract from H. arborescens leaves.31,32
Acute Toxicity Study
The crude extract’s acute oral toxicity was assessed in healthy female Swiss albino mice weighing 18–22 g and aged 6–8 weeks using the Organization for Economic Cooperation and Development (OECD) № 425 guideline limit test procedure. Prior to testing, five female mice were housed for seven days to help them get acquainted to the laboratory environment. After a 4-hour fast and unlimited access to water, one mouse was chosen at random to participate in the oral acute toxicity trial. After determining the body weight at fasting, a 2000 mg/kg hydro-methanolic crude extract of H. arborescens leaves was administered orally. A firm inspection took thirty minutes and was repeated every twenty-four hours. Changes were seen in somatomotor activity, behavioral patterns, skin and fur, eyes, mucous membranes, respiratory and circulatory systems, as well as the CNS. Tremors, convulsions, salivation, diarrhea, lethargy, drowsiness, and coma were all taken into account. Throughout the initial toxicity examination, the mouse did not exhibit any toxicity symptoms for four days. The remaining four Swiss albino female mice were given comparable doses and were monitored for 14 days.19,33,34
Grouping and Dosing of Animals
The normoglycemic, oral glucose-loaded and streptozotocin-induced diabetes mouse models all used male mice. Mice were divided into 5 groups of 6 mice each under normoglycemic and oral glucose-loaded settings. Group I, the negative control group, received water with 8% Tween 80. Glibenclamide 5 mg/kg was administered to a positive control group (group II), whereas H. arborescens leaves extract doses of 100, 200, and 400 mg/kg were administered to the other three test groups (groups III–V). Mice were randomized into 14 groups at random, each receiving a single dosage of the therapy (6 mice per group). Glibenclamide 5 mg/kg was given to group II mice as a positive control, and 10 mL/kg of 8% Tween 80 in water was given to group I animals orally. H. arborescens leaves extract (HALE), n-hexane (n-HF), ethyl acetate (EAF), and water (DWF) fractions were administered at dosages of 100, 200, and 400 mg/kg, respectively, to groups III through XIV. To simulate more common applications, the trial was conducted orally.17,19,35 The Ethyl acetate fraction of H. arborescens leaves was more effective in a single-dose treated diabetic mice was used in the daily repeated dose-treated diabetic mice model. Nine groups of diabetic mice were randomly assigned in this investigation (8 groups of diabetic and 1 group of normal mice, 6 mice per group). Group I & II; non-diabetic mice and diabetic negative control mice were treated with vehicle (0.1% DMSO in 10 mL/kg distilled water). Group III; positive diabetic control mice were treated with Glibenclamide 5 mg/kg. The test groups from group IV–IX were treated with 100, 200, and 400 mg/kg of the hydro-methanolic crude extract and similar doses of ethyl acetate fraction of H. arborescens leaves, respectively. 198 mice were used in this experimental study, and each group got doses every day for two weeks.
Determination of Anti-Diabetic Activity
Effects of Hydro-Methanolic Extract of H. arborescens Leaves on Blood Glucose Levels of Normoglycemic Mice
Non-diabetic male mice were fasted for six hours with free access of clean tap water. They were randomized into five groups (six mice per group). Group I mice were treated with 8% TW80 10 lm/kg, group II treated with Glibenclamide 5 mg/kg and groups III–V were treated with 100, 200, and 400 mg/kg 80% methanol in water extract of H. arborescens leaves. Blood glucose levels (BGL) were measured using the aseptic tail-tip amputation procedure before therapy as a baseline (0 hr) and at 1, 2, 4, and 6 hours after therapy. Test strip kits (Auto Cod, Taiwan) were used to check BGLs, and the average of the results was collected three times.19,36,37
Effects 80% Methanol in Water Extract of H. arborescens Leaves on Oral Glucose- Loaded Mice
Overnight (12- to 14-hour) fasted mice were randomized into five groups (six mice per group). Group I mice were treated with 8% TW80 10 lm/kg, group II were treated with Glibenclamide 5 mg/kg and groups III–V were treated with 100, 200, and 400 mg/kg 80% methanol in water extract of H. arborescens leaves respectively and tested for oral glucose tolerance which is suitable for evaluating the anti-diabetic activity of plant extracts as overnight fasting increases insulin sensitivity and, consequently, insulin-stimulated glucose intake.19,30,38–40 Each mouse got a dosage of 2 g/kg of a 40% glucose solution after receiving 8% Tween 80, Glibenclamide, and hydro-methanolic crude extract for a half-hour. A vein section of mouse tail vein was created and duplicate readings were taken using the Glucometer and test strips, and the average values of the three measurements were calculated. BGLs in each mouse were determined before oral glucose-loading (0 hour) and 30, 60, and 120 minutes following glucose delivery.19,36
Induction of Experimental Diabetes
Experimental case of diabetes mellitus was created in 12–14 hours of fasting mice using streptozotocin (STZ) 150 mg/kg solution intraperitoneally (IP) in 0.1 M fresh cold citrate buffer (PH 4.5).41 To prevent hypoglycemia-related death, mice were given unrestricted access to glucose 10% solution 24 hours after receiving a STZ injection. The mice were subsequently given permission to eat. Those mice with overnight fasting blood glucose levels ≥ 200 mg/kg were classified as diabetic and used in this investigation.30,36,37 Mice were evaluated for diabetes 72 hours after STZ injection. Streptozotocin [2-deoxy-2-(3-methyl-3-nitrsourea)-1-D-glucopyrazone](C8H15N3O7) causes diabetes in mice.42
Due to its better stability in aqueous solution before and after injection in animals, STZ is a better diabetogenic agent than alloxan, with greater reproducibility and wider species effectiveness, and DNA methylation, nitric oxide, and reactive oxygen species production are the major mechanisms associated with pancreatic β-cell death secondary to STZ exposure.43
Evaluation of Single-Dose Hydro-Methanolic Crude Extract and Solvent Fractions of H. arborescens Leaves on Blood Glucose Level in STZ-Induced Diabetic Mice
Diabetic mice Fasted for 12–14 hours randomized into 14 groups (six mice per group). Group I mice were treated with 10 mL/kg of 0.1% dimethyl sulphoxide (DSMO) in water, group II were treated with Glibenclamide 5 mg/kg and groups III–IV were treated with 100, 200, and 400 mg/kg 80% methanol in water extract, n-hexane, ethyl acetate, and water fractions of H. arborescens leaves respectively. Before starting therapy (at 0 hours), BGLs were assessed. Subsequent measurements were taken at 2, 4, 6, and 8 hours.36
Effect of Daily Repeated Doses of Hydro-Methanolic Crude Extract and Ethyl Acetate Fraction of H. arborescens Leaves on Blood Glucose, Body Weight, and Serum Lipid Levels of STZ-Induced Diabetic Mice
Nine groups of mice were randomly divided (8 groups of diabetic and 1 group of normal mice, 6 mice per group). Group I–II (non-diabetic and diabetic mice negative controls) received 10 mL/kg of 0.1% dimethyl sulphoxide (DSMO) in water, group III (positive control) mice received 5 mg/kg of Glibenclamide, and groups IV–IX received 100, 200, and 400 mg/kg of 80% methanol in water extract and 100, 200, and 400 mg/kg of ethyl acetate fraction of H. arborescens leaves respectively for 14 days. 72 hours after the injection of STZ and Citrate buffer, each mouse’s fasting BGLs and body weight were measured as a baseline (day 0), and assessments were then carried out on the 7th and 14th days of treatment following an overnight (12–14 hours) fast. Mice were executed on the fifteenth day with an IP injection of ketamine 150 mg/kg and diazepam 5 mg/kg. Visual examination (to make sure there was no sign of respiration) and heartbeat tests were done to confirm total euthanasia (by feeling the chest between the thumb and fingers). A sterile test tube was used to collect blood samples from diabetic mice, which were then centrifuged for 10 minutes at 2000 rpm after being left at room temperature for two hours to allow for coagulation. In order to determine the concentrations of triglycerides (TG), total cholesterol (TC), high and low-density lipoproteins (HDL & LDL), and other serum constituents, the supernatant of centrifuged blood was promptly decanted into sterile test tubes.19,36,44 All euthanized mice carcasses were then interred in order to prevent contamination and environmental risk.
Statistical Analysis
Statistical analysis was performed by IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, N.Y., USA) to do numerous comparisons both within and between groups, with one-way ANOVA followed by a Tukey’s HSD post hoc test. When the findings were presented as mean ± SEM, P<0.05 was deemed statistically significant.
Results
Percentage Yields of Extract and Fractions of H. arborescens Leaves
The % (w/w) yields of H. arborescens leaves in n-hexane, ethyl acetate, water, and hydromethanolic crude extract. The extract solidified when chilled; it was a brownish semisolid at room temperature. (See Table 1)
 |
Table 1 Summary of Hydromethanolic Crude Extract and Solvent Fractions of H.arborescens Leaves |
Phytochemical Screening
Table 2: summarizes the results of qualitative phytochemical screening of hydro-methanolic extract and solvent fractions of H. arborescens leaves.
 |
Table 2 Summary of Qualitative Phytochemical Screening of Crude Extract and Solvent Fractions of H.arborescens Leaves |
Acute Oral Toxicity Test
The safety of a crude extract of H. arborescens leaves 2000 mg/kg in 80% methanol in water was demonstrated. For 24 hours and 14 days of observation, none of the five female mice showed any signs of acute toxicity or mortality. H. arborescens leaves crude extract in 80% methanol in water revealed an LD50 greater than 2000 mg/kg. This prompted the choice of dosages of 80% methanol in water crude extract of H. arborescens leaves at 100, 200, and 400 mg/kg for further in vivo research.
Antidiabetic Effects of Hydromethanolic Extract of H. arborescens Leaves in Normoglycemic Mice
The BGLs of the groups did not significantly differ at the beginning of the experiment. Glibenclamide significantly (p < 0.05) decreased BGLs at 1 hour, (p < 0.01) at 2 and 4 hours, and (p < 0.001) at 6 hours post-treatment when compared to the untreated group. The hydro-methanolic crude extract of H. arborescens leaves at doses of 100 mg/kg and 200 mg/kg failed to cause hypoglycemia in normal mice at any time point, and it significantly (p < 0.05) decreased BGLs at 1 and 2 hours, (p < 0.01) at 4 hours, and (p < 0.001) at 6 hours when compared to groups that had received 100 mg/kg of crude extract. The 400 mg/kg dose of the crude extract, however, significantly (p < 0.05) and (p < 0.01) lowered BGLs in comparison to the negative control and (p < 0.05) at 6 hours following treatment in comparison to the groups which had received the 100 mg/kg dose of the crude extract. During the shortest time periods, there were no statistically significant differences in mean BGLs between the Glibenclamide 5 mg/kg and crude extract at 400 mg/kg dosage treated groups. (See Table 3)
 |
Table 3 Effect of H.arborescens Leaves Extract on BGLs of Normoglycemic Mice |
Effect of Hydro-Methanolic Crude Extract of H. arborescens Leaves on Blood Glucose Levels of Oral Glucose-Loaded Mice
For all groups, variations in baseline BGLs were found to be statistically significant. After oral glucose loading, all mice’s BGLs peaked 0.5 hours later, indicating the induction of hyperglycemia. In comparison to the negative control group, Glibenclamide reduced BGLs at 1 hour, 2 hours, and 1 hour after oral glucose-loading (p < 0.001, p < 0.01, and p < 0.05, respectively). At 400 mg/kg at 1 hour (p < 0.05) and 2 hours (p < 0.01) after oral glucose loading, BGLs similarly decreased. Hydro-methanolic crude extract of H. arborescens leaves at 200 mg/kg (p < 0.05) decreased BGLs at 2 hours after oral glucose-loading. (See Table 4)
 |
Table 4 Effect of H.arborescens Leaves Extract on BGLs of Oral Glucose-Loaded Diabetic Mice |
Effect of Single-Dose Hydro-Methanolic Extract and Solvent Fractions of H. arborescens Leaves on BGLs of STZ-Induced Diabetic Mice
Post hoc analysis showed that Glibenclamide significantly (p < 0.05) lowered BGLs 4 hours after therapy in all groups as compared to the negative control group. When compared to the 400 mg/kg and 200 mg/kg water fraction treated groups at 6 and 8 hours after treatment, it had significant effects (p < 0.05 and p < 0.01, respectively), and when compared to the negative control dose of both the hydro-methanolic crude extract and water fraction of H. arborescens leaves, it had significant effects (p < 0.001 and p < 0.001). BGLs were lowered by 200 mg/kg hydro-methanolic crude extract at 6 and 8 hours after treatment compared to the negative control and at 8 hours after treatment compared to the 200 and 400 mg/kg distilled water fraction treated groups (p < 0.05). BGLs were lower at 6 hours when comparing hydro-methanolic crude extract at 100 and 400 mg/kg to the negative control and 100 mg/kg of distilled water fraction (p < 0.01). (p < 0.05) when compared to doses of distilled water fraction of 200 and 400 mg/kg, (p < 0.01) when compared to dose of hydro-methanolic crude extract, and (p < 0.001) when compared to the negative control and dose of distilled water fraction of 100 mg/kg treated groups after 8 hours after treatment. The n-hexane fraction at 200 mg/kg dose significantly decreased blood glucose levels compared to the negative control, 100 mg/kg doses of both the hydro-methanolic crude extract and the distilled water fraction treated groups at 6 hours after treatment, and (P < 0.05) compared to the negative control, 100 mg/kg doses of both the hydro-methanolic crude extract and the distilled water fraction treated groups at 8 hours after treatment. The 400 mg/kg dose of the n-hexane fraction, the 400 mg/kg dose of the distilled water fraction, and the 200 mg/kg doses of the crude extract and distilled water fraction all treated groups were all compared to the negative control group, the 100 mg/kg doses of both the crude extract and distilled water fraction treated groups, the 400 mg/kg dose of the n-hexane fraction, and the 200 mg/kg doses of the crude extract and distilled water The ethyl acetate fraction significantly (p < 0.05) lowered BGLs as compared to the negative control group, the treated groups’ 100 mg/kg dosages, and the hydro-methanolic crude extract and distilled water fractions at 6 and 8 hours after treatment, respectively. Groups that received 200 mg/kg doses in comparison to the negative control and 100 mg/kg doses of both crude extract and distilled water six and eight hours after treatment were treated. The ethyl acetate fraction at the 400 mg/kg dose resulted in statistically significant (p < 0.01) BGL reductions six hours after treatment when compared to the negative control, the crude extract 100 mg/kg dose, the distilled water fraction 100, 200, and 400 mg/kg dosed groups, the crude extract 100 mg/kg dose, the distilled water fraction 100, 200, and 400 mg/kg dosed groups, and the crude extract 100 mg/kg dose. At the list bit dose ranges, the n-hexane fraction had a similar effect to the ethyl acetate fraction at the lower and higher dose levels, with a substantial (p < 0.05) decrease in BGLs. When compared to the negative control group at 6 and 8 hours after treatment, the antihyperglycemic impact of crude extract and the n-hexane fraction was dose-dependent; however, the distilled water fraction did not significantly lower BGLs across all dose ranges and time points. Accordingly, the crude extract and ethyl acetate fraction were used in daily repeated dose studies on STZ-induced diabetic mice. (See Table 5)
 |
Table 5 Effects of Single-Dose Crude Extract and Solvent Fractions of H.arborescens Leaves on BGLs of STZ-Induced Diabetic Mice |
Effect of Daily Repeated Doses of Hydro-Methanolic Extract and Ethyl Acetate Fraction of H. arborescens Leaves on Blood Glucose Levels of STZ-Induced Diabetic Mice
On STZ-induced diabetic mice for 14 days, daily repeated dosages of the hydro-methanolic crude extract and the ethyl acetate fraction of H. arborescens leaves were tested. After STZ and citrate buffer injections into each group of mice, baseline BGLs were measured 72 hours later, and it was found that they were all significantly (p < 0.01) higher than normal control groups when STZ was given. After seven days of therapy, Glibenclamide 5 mg/kg significantly reduced mean BGLs in comparison to the diabetic control (DC) and 100 mg/kg doses of both crude extract and ethyl acetate fraction treated groups (p < 0.001 and p < 0.05, respectively). In contrast to the diabetic control group, the hydro-methanolic crude extract of H. arborescens leaves significantly reduced blood glucose levels at doses of 200 and 400 mg/kg (p < 0.01 and p < 0.001, respectively). In comparison to the diabetic control group, the ethyl acetate fraction of H. arborescens leaves significantly reduced BGLs (p < 0.01 at 100 mg/kg and p < 0.001 at 200 and 400 mg/kg dosages). When compared to the Glibenclamide 5 mg/kg treatment group, there were no statistically significant differences between the effects of the H. arborescens leaf ethyl acetate and hydro-methanolic crude extract at doses of 200 and 400 mg/kg. Mean BGLs were considerably lower in the Glibenclamide 5 mg/kg, crude extract, and ethyl acetate fraction treatment groups at 200 and 400 mg/kg doses compared to the diabetic control group (p < 0.001). In comparison to the group receiving Glibenclamide, the BGL of the diabetic control group is considerably (p < 0.001) greater. Between the healthy control group and the Glibenclamide 5 mg/kg, 200 mg/kg, and 400 mg/kg doses of both crude extract and ethyl acetate fraction treatment groups, there were no discernible differences in BGLs. The doses of 400 mg/kg of ethyl acetate fraction, 5 mg/kg of Glibenclamide, 400 mg/kg of crude extract, and 39.77% of ethyl acetate fraction showed the greatest percentage reductions in BGL. (See Table 6)
 |
Table 6 Effect of Daily Repeated Dose Crude Extract and Ethyl Acetate Fraction of H.arborescens Leaves on BGLs of STZ-Induced Diabetic Mice |
Effect of Daily Repeated-Doses of Hydro-Methanolic Extract and Ethyl Acetate Fraction of H. arborescens Leaves on Body Weights of STZ-Induced Diabetic Mice
Every week, the effects of hydro-methanolic crude extract and ethyl acetate fraction from H. arborescens leaves were examined on the body weights of STZ-induced diabetic mice. No statistically significant differences in baseline body weight were found between any group and the diabetic control group, mean body weight in normal control mice. Glibenclamide 5 mg/kg, the hydro-methanolic crude extract, and the ethyl acetate fraction of H. arborescens leaves at a dose of 400 mg/kg were substantially (p < 0.05) greater after days of treatment in comparison to the diabetic control group. At dosages of 100 and 200 mg/kg, the crude extract and ethyl acetate fraction, however, exhibited no appreciable impact on body weight. After 14 days of therapy, the weight of the diabetic control group was considerably decreased by the crude extract and ethyl acetate fraction (p < 0.001 at 400 mg/kg and (p < 0.05 and p < 0.01, respectively) at 200 mg/kg dosages). Compared to the crude extract 100 mg/kg dose treated group, the ethyl acetate fraction at 400 mg/kg dose demonstrated a significant (p < 0.05) weight increase. In comparison to the diabetic control group, Glibenclamide 5 mg/kg generated significant (p < 0.05 and p < 0.001) body weight gains on days 7 and 14. Last but not least, 100 mg/kg of crude extract and ethyl acetate fraction dosages were required to produce appreciable weight gains at all time periods when compared to diabetic control. Between the Glibenclamide 5 mg/kg normal control, hydromethanolic crude extract, or ethyl acetate fraction at 400 mg/kg dose treatment groups, there were no statistically significant differences in body weight. (See Table 7)
 |
Table 7 Effect of Crude Extract and Ethyl Acetate Fraction of H. Arborescens Leaves on Body Weight of STZ- Induced Diabetic Mice |
Effect of Daily Repeated Doses of Hydro-Methanolic Extract and Ethyl Acetate Fraction of H. arborescens Leaves on Serum Lipid Profile of STZ-Induced Diabetic Mice
An automatic chemistry analyzer was used to determine each mouse’s blood lipid profile after daily dose administration of hydro-methanolic crude extract and ethyl acetate fraction of H. arborescens leaves to STZ-induced diabetic mice for two weeks (BT2000, Italy). When compared to diabetic control and the crude extract and ethyl acetate fraction 100 mg/kg dose treated groups, serum total cholesterol levels in the Glibenclamide 5 mg/kg and both hydro-methanolic crude extract and ethyl acetate fraction at 200 and 400 mg/kg dose treated groups were significantly higher (p < 0.001). When compared to diabetic control and 100 mg/kg doses of both crude extract and ethyl acetate fraction treated group, TG and cholesterol levels were significantly (p < 0.001) lower in Glibenclamide 5 mg/kg, 200 and 400 mg/kg doses of crude extract, and 400 mg/kg dose ethyl acetate fraction treated groups, whereas (p < 0.01) in 100 mg/kg doses of both crude extract and ethyl acetate fraction. While cholesterol levels were significantly (p < 0.001) higher in the Glibenclamide 5 mg/kg, both crude extract and ethyl acetate fraction treated groups at the 200 and 400 mg/kg dose levels, and significantly (p < 0.001) lower in the crude extract and ethyl acetate fraction treated groups at the 100 mg/kg dose level, they were significantly (p < 0.001) lower in the crude extract and ethyl acetate fraction treated groups at the 200 mg/kg. (See Table 8)
 |
Table 8 Effect of Daily Repeated Dose Crude Extract and Ethyl Acetate Fraction of H. Arborescens Leaves on Serum Lipid Profiles of STZ-Induced Diabetic Mice |
Discussion
In the current study, the anti-diabetic and anti-hyperlipidemic properties of an extract from H. arborescens leaves were investigated in STZ-induced diabetic mouse models. To assess peripheral glucose utilization (peripheral insulin action) and/or pancreatic beta-cell insulin production parameters, oral glucose-tolerance and normoglycemic tests are crucial.45,46 In normoglycemic mice, 400 mg/kg of the crude extract of H. arborescens leaves caused hypoglycemia, whereas 200 and 400 mg/kg dosages reduced hyperglycemia in mice given oral glucose. Reduced glucose absorption, increased peripheral glucose consumption, or a combination of the two can cause this. The presence of flavonoids, tannins, and polyphenols in crude extract increase insulin sensitivity and inhibit glucagon release, improving glucose absorption into peripheral cells, and slowing the progression of glucose intolerance. Ethyl acetate fraction was effective in three doses but crude extract and the n-hexane fraction had dose-dependent effects on BGLs in STZ-induced diabetic mice. The ethyl acetate fraction was more efficient at lowering BGLs across all dose ranges due to the availability of flavonoids and phenols by adsorbing, degrading, neutralizing, and terminating free radicals through their redox potentials.17 The ethyl acetate fraction of the acetone extract of Ficus lutea leaves stimulated the highest levels of glucose uptake in C2C12 muscle cells, the lowest levels of extracellular glucose in H-4-II-E liver cells, and the highest levels of insulin secretion in RIN-m5F pancreatic beta-cells, according to other studies.47 Adenanthera pavonina leaf methanolic extract was found to have the highest total phenolic content and DPPH scavenging activity in the ethyl acetate fraction.48 Numerous solvent extracts of H. arborescens leaves posses phenols, flavonoids, proanthocyanidin, alkaloids, and saponins.16 Polyphenols and flavonoids inhibit α-amylase and α-glucosidase activity.49,50 Phenols inhibit acetylcholinesterase, α-amylase, α-glucosidase, butyrylcholinesterase, and Fe2+ chelating effects, OH, ABTS, DPPH, and NO radical scavenging capacities.51 Alkaloids, phenolic acids, anthocyanins, saponins, carotenoids, terpenes, sugars, proteins, capsaicinoids, and fatty acids were all the subject of more research.52 They were found to inhibit alpha-glucosidase and alpha-amylase. Our qualitative phytochemical study of H. arborescens leaves in hydro-methanolic extract, n-hexane, ethyl acetate, and distilled water fractions revealed the presence of the majority of these beneficial phytochemicals. In both healthy and STZ-induced diabetic mice, these substances will be in charge of the antidiabetic and antihyperglycemic actions. Some of these essential oils exhibit anti-hyperglycemic, anti-dyslipidemic, and the capacity to reduce oxidative stress associated with diabetes mellitus.53,54 A GC-MS analysis of the essential oils extracted from H. arborescens leaves previously revealed fifty-two chemical elements overall, including fundamental additives such α-pinene, α-elemene, β-pinene, β-myrcene, β-ocimene, and D-limonene.51 A second GC-MS investigation of the essential oils from H. arborescens leaves and blooming parts revealed about 60 components. Sabinene, germacrene-D, β-pinene, and β-myristicin were the most important of these.55 In our study, methanolic crude extract and crude extract in oral glucose-loaded normal mice both induced hypoglycemia in the normal mice. In contrast, ethyl acetate and n-hexane fractions in single-dose treated with STZ-induced diabetic mice showed antihyperglycemic effects. Citrus aurantifolia leaves essential oil administered intraperitoneally for 14 days to alloxan-induced hyperglycemic rats resulted in significant decreases in fasting blood and hepatic glucose levels, serum TC, TG, and LDL concentrations despite significant increases in hepatic glycogen and serum HDL levels.53,56 Serum concentrations of NO, malondialdehyde (MDA), and pro-inflammatory cytokines were much lower in diabetic rats administered essential oils (EOs) than in diabetic rats given the typical antidiabetic drug. In comparison to diabetic control rats, diabetic rats treated with EOs had improved lipid profiles, higher insulin sensitivity in peripheral tissues, and increased insulin production from β-cells of pancreatic islets.57 These results are totally in line with our previous research, which indicated that the presence of these essential oils in our plant may have contributed to the BGL, body weight, and blood lipid profile changes that were observed in diabetic mice. When administered in a single, large dosage, the selective pancreatic islet cytotoxic agent streptozotocin results in full β-cell necrosis, insulin insufficiency, and hyperglycemia.58 Hyperglycemia increases the production of pro-inflammatory cytokines, lipid peroxidation, and reactive oxygen and nitrogen species and cause oxidative stress in tissues and pancreatic islet cells which results in diabetes. Oxidative stress reduces glucose absorption, insulin sensitivity, and generation of insulin, which causes β-cell dysfunction.57 Vitamins, terpenoids, phenolics, lignins, stilbenes, tannins, flavonoids, quinones, coumarins, alkaloids, amines, betalains found in Apiaceae species showed free radical scavenging and antioxidant properties.59–62 Hydro-alcoholic extracts of Eryngium billardieri given in diabetic rats increased insulin secretion and serum insulin levels, decreased blood sugar and glucose tolerance by reducing oxidative stress.63,64 Another in vitro study using an acetone extract of H. arborescens leaves found promising antioxidant, anti-inflammatory, and anti-arthritic properties through the inhibition of nitric oxide release in lipopolysaccharide-stimulated RAW 264.7 macrophages, FRAP assay, and the scavenging of DPPH and ABTS radicals.65 The presence of proteins, fibers, calcium, glucose, vitamins A, E, and C, low-fat in H. arborescens leaves can aid in weight management and protect against disorders associated with obesity and antioxidant activity and Phosphorus provides the structural framework for DNA and RNA. Minerals (Ca, Mg, K, P, Na, Zn, Mn, Cu, and Fe) are required proper bodily growth and maintenance.66 Both normal and diabetic mice showed anti-diabetic, and anti-dyslipidemic effects, and the weight gain seen in diabetic mice as revealed by hydro-methanolic crude extract and ethyl acetate fraction most likely results from an effect similar to insulin or from stimulation of insulin secretion from either regenerated or survived pancreatic beta-cells. Diabetic control mice routinely lost weight, leads us to the conclusion that our plant may have had insulin-like effects. The research indicates that leaf extracts of H. arborescens stimulate peripheral glucose absorption, possess antioxidant and free radical scavenging capabilities, and inhibit pancreatic lipase, pancreatic α-amylase, and intestinal glucosidase enzymes. By inhibiting lipid and carbohydrate metabolizing enzymes, H. arborescens hydro-methanolic extract and different solvent fractions have antioxidant and free radical scavenging effects, inhibit carbohydrate metabolizing enzymes and thereby inhibit glucose absorption, stimulate peripheral glucose utilization, and lower serum lipid levels. Last but not least, H. arborescens leaf hydro-methanolic extract and beneficial solvent fractions may prevent further damage to the pancreatic β-cell from both the STZ given and oxidative stressors caused by hyperglycemia. Additionally, it might help partially injured islet cells heal, and insulin secretion may have contributed to any results seen.
Conclusion
H. arborescens leaves’ hydro-methanolic crude extract and solvent fractions showed antidiabetic and significantly reduced hyperlipidemia in diabetic mice. This study supported the traditional usage of H. arborescens for treating diabetes; however, species variation could also limit such a straightforward extrapolation of the findings of this study in humans. Hereafter, further studies should be conducted on the identification of the exact antidiabetic and anti-hyperlipidemia mechanism(s) of action of the 80% methanolic extract as well as the solvent fractions. Isolation and identification of pharmacologically active compounds from the active fractions should also be done.
Abbreviations
AACE, American Association of Clinical Endocrinologists; ADA, American Diabetes Associations; ANOVA, Analysis Of Variance; BGL, Blood Glucose Level; DKA, Diabetic Keto Acidosis; DM, Diabetes Mellitus; DMSO, Dimethyl Sulfoxide; DR, Diabetic Retinopathy; FPG, Fasting Plasma Glucose; HDL, High density lipoprotein cholesterol; HHS, Hyperosmolar Hyperglycemic State; IGT, Impaired Glucose Tolerance; LDL, Low Density Lipoprotein cholesterol; OECD, Organization for Economic Cooperation and Development; OGTT, Oral Glucose Tolerance Test; TC, Total cholesterol; TG, Triglyceride; WHO, World Health Organization.
Data Sharing Statement
The corresponding author can be contacted for access to the datasets that were used and/or analyzed for this study.
Ethical Approval
The Basel Declaration on Laboratory Animal Welfare, the ICLAS Ethical Guidelines, and the care and use of research-based designs were all followed during the execution of the experiments.67,68 The Department of Pharmacology Ethical Review Committee at the University of Gondar’s School of Pharmacy granted authorization for the research under the reference number SoP4/102/13. The research was finished, and the animals received the appropriate care.
Acknowledgments
The University of Gondar’s approval of the ethical clearance, aid with the laboratory facilities, and identification of experimental plant are all greatly appreciated by the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Della Manna T, Setian N, Savoldelli RD, et al. Diabetes mellitus in childhood: an emerging condition in the 21st century. Revista da Assoc Méd Br. 2016;62:594–601.
2. American Diabetes Association. Standards of medical care in diabetes—2018 abridged for primary care providers. Am Diabetes Assoc. 2018;36(1):14.
3. Jeeva S, Anlin Sheebha Y. A review of antidiabetic potential of ethnomedicinal plants. Med Aromat Plants. 2014;3(4):1–8.
4. Skalli S, Hassikou R, Arahou M. An ethnobotanical survey of medicinal plants used for diabetes treatment in Rabat, Morocco. Heliyon. 2019;5(3):e01421. doi:10.1016/j.heliyon.2019.e01421
5. Jarald E, Joshi SB, Jain D. Diabetes and herbal medicines. Iranian Journal of Pharmacology & Therapeutics. 2008;7:97–106.
6. Kasole R, Martin HD, Kimiywe J. Traditional medicine and its role in the management of diabetes mellitus:“patients’ and herbalists’ perspectives”. Evid Based Complementary Alternative Med. 2019;2019:548.
7. Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Alternative Med Rev. 2002;7(1):45–58.
8. Odeyemi S, Bradley G. Medicinal plants used for the traditional management of diabetes in the Eastern Cape, South Africa: pharmacology and toxicology. Molecules. 2018;23(11):2759. doi:10.3390/molecules23112759
9. Maroyi AL. Heteromorpha arborescens: a review of its botany, medicinal uses, and pharmacological properties. Asian J Pharmaceutical Clin Res. 2018;11(11):75–82. doi:10.22159/ajpcr.2018.v11i11.29108
10. Setshogo MP, Mbereki CM. Floristic diversity and uses of medicinal plants sold by street vendors in Gaborone, Botswana. The African Journal of plant Science and Biotechnology. 2011;5(1):69–74.
11. Salehi B, Ata A. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9(10):551. doi:10.3390/biom9100551
12. Nkomo M, Nkeh-Chungag BN, Kambizi L, Ndebia EJ, Sewani-Rusike C, Iputo JE. Investigation of the antinociceptive and anti-inflammatory properties of Heteromorpha arborescens (Apiaceae). Af J Traditional. 2011;8(4):658.
13. Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):1–38. doi:10.1186/1746-4269-11-4
14. Moshi MJ, Mbwambo ZH. Experience of Tanzanian traditional healers in the management of non-insulin dependent diabetes mellitus. Pharm Biol. 2002;40(7):552–560. doi:10.1076/phbi.40.7.552.14691
15. Villegas M, Vargas D, Msonthi JD, Marston A, Hostettmann K. Isolation of the antifungal compounds falcarindiol and sarisan from. Heteromorpha trifoliata Planta med. 1988;54(01):36–37. doi:10.1055/s-2006-962326
16. Abifarin TO, Otunola GA, Afolayan AJ. Assessment of the phytochemical, antioxidant and antibacterial activities of Heteromorpha arborescens (Spreng.) Cham & Schltdl. leaf extracts. F1000Research. 2020;9:1079. doi:10.12688/f1000research.25197.1
17. Sunil C, Kumar V, Van Staden J. In vitro alpha-glucosidase inhibitory, total phenolic composition, antiradical and antioxidant potential of Heteromorpha arborescens (Spreng.) Cham. & Schltdl. leaf and bark extracts. South Af J Botany. 2019;1(124):380–386. doi:10.1016/j.sajb.2019.05.017
18. Adamu M, Bagla VP, Eloff JN. Fractionation of Heteromorpha arborescens var abyssinica (Apiaceae) leaf extracts based on polarity leads to a marked change in cytotoxicity that may yield a commercially useful product. South Af J Botany. 2016;1(103):36–40. doi:10.1016/j.sajb.2015.08.009
19. Abifarin TO, Otunola GA, Afolayan AJ. Cytotoxicity, Anti-Obesity and Anti-Diabetic Activities of Heteromorpha arborescens (Spreng.) Cham Leaves. Processes. 2021;9(9):1671. doi:10.3390/pr9091671
20. Sun H, Saeedi P, Karuranga S, et al. Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
21. Glovaci D, Fan W. Wong, Epidemiology of diabetes mellitus and cardiovascular disease. Int J Med. 2019;21(4):1–8.
22. Jamali N, Soureshjani EH, Mobini GR, Samare-Najaf M, Clark CC, Saffari-Chaleshtori J. Medicinal plant compounds as promising inhibitors of coronavirus (COVID-19) main protease: an in silico study. J Biomol Struct Dyn. 2021;24:1–2.
23. Unwin N. Volume 3: The Practice of Public Health, the Epidemiology and Prevention of Diabetes Mellitus. Public Health: 2009:1068–1080.
24. Saeedi P, Salpea P, Karuranga S, et al. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2020;1(162):108086. doi:10.1016/j.diabres.2020.108086
25. Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2020;1(162):108072. doi:10.1016/j.diabres.2020.108072
26. Bishu KG, Jenkins C, Yebyo HG, Atsbha M, Wubayehu T, Gebregziabher M. Diabetes in Ethiopia: a systematic review of prevalence, risk factors, complications, and cost. Obesity Med. 2019;1(15):100132. doi:10.1016/j.obmed.2019.100132
27. Dereje N, Earsido A, Temam L, Abebe A. Prevalence and associated factors of diabetes mellitus in Hosanna Town, Southern Ethiopia. Ann Global Health. 2020;86:1. doi:10.5334/aogh.2663
28. Sun H, Saeedi, P, Karuranga, S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. J Diabetes Res. 2022;183:109119.
29. Tessfa E, Ejigu D, Degife G, Tassie N. Diversity, relative abundance, and habitat association of avian species in Tara Gedam Monastery forest and adjacent habitats, Northwestern Ethiopia. Ethiopian J Sci Technol. 2020;13(1):65–80. doi:10.4314/ejst.v13i1.5
30. Singh MP, Pathak K. Animal models for biological screening of anti-diabetic drugs: an overview. Eur J Exp Biol. 2015;5(5):37–48.
31. Beena P, Rajesh KJ, Arul B. Preliminary phytochemical screening of Cicer arietinum in folklore medicine for hepatoprotection. J Innov Pharm Biol Sci. 2016;3:153–159.
32. Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42. doi:10.3390/plants6040042
33. Saeed F, Ahmad M. Anti-diabetic and acute toxicity studies of Annona squamosa L. ethanolic leaves extract. Int J Phytomed. 2017;9:6. doi:10.5138/09750185.2182
34. Kumar S, Singh R, Vasudeva N, Sharma S. Acute and chronic animal models for the evaluation of anti-diabetic agents. Cardiovasc Diabetol. 2012;11(1):1–3. doi:10.1186/1475-2840-11-9
35. Vermeire TG, Baars AJ, Bessems JG, Blaauboer BJ, Slob W, Muller JJ. Toxicity testing for human health risk assessment. InRisk Assessment Chem. 2007;2:227–280.
36. Belayneh YM, Birhanu Z, Birru EM, Getenet G. Evaluation of in vivo antidiabetic, antidyslipidemic, and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L. (Solanaceae). J Exp Pharmacol. 2019;11:29. doi:10.2147/JEP.S192264
37. Shewamene Z, Abdelwuhab M, Birhanu Z. Methanolic leaf exctract of Otostegia integrifolia Benth reduces blood glucose levels in diabetic, glucose loaded and normal rodents. BMC Complement Altern Med. 2015;15(1):1–7. doi:10.1186/s12906-015-0535-5
38. Togashi Y, Shirakawa J, Okuyama T, et al. Evaluation of the appropriateness of using glucometers for measuring the blood glucose levels in mice. Sci Rep. 2016;6(1):1–9. doi:10.1038/srep25465
39. Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–32. doi:10.1152/ajpendo.90617.2008
40. Alene M, Abdelwuhab M, Belay A, Yazie TS. Evaluation of Antidiabetic Activity of Ajuga integrifolia (Lamiaceae) Root Extract and Solvent Fractions in Mice. Evid Based Complementary Alternative Med. 2020;2020:1–11. doi:10.1155/2020/6642588
41. Induced type 1 diabetes model Mouse Metabolic Phenotyping Centers; 2013. Available from: https://www.mmpc.org/shared/document.aspx?id=152&docType=ProtocolMMPC_U_Mass-STZ-.
42. Arora S, Ojha SK, Vohora D. Characterisation of streptozotocin induced diabetes mellitus in Swiss albino mice. Global J Pharmacol. 2009;3(2):81–84.
43. Eleazu CO, Eleazu KC, Chukwuma S, Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013;12(1):1–7. doi:10.1186/2251-6581-12-60
44. Euthanasia Policy. Available from: https://research.uoregon.edu/manage/integrity-compliance/animal-research/euthanasia-policy.
45. Hammeso WW, Emiru YK, Ayalew Getahun K, Kahaliw W. Antidiabetic and antihyperlipidemic activities of the leaf latex extract of Aloe megalacantha baker (Aloaceae) in streptozotocin-induced diabetic model. Evid Based Complementary Alternative Med. 2019;23:5224.
46. Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care. 2003;26(4):1026–1033. doi:10.2337/diacare.26.4.1026
47. Olaokun OO, McGaw LJ, van Rensburg IJ, Eloff JN, Naidoo V. Antidiabetic activity of the ethyl acetate fraction of Ficus lutea (Moraceae) leaf extract: comparison of an in vitro assay with an in vivo obese mouse model. BMC Complement Altern Med. 2016;16(1):1–2. doi:10.1186/s12906-016-1087-z
48. Wickramaratne MN, Punchihewa JC, Wickramaratne DB. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement Altern Med. 2016;16(1):1–5. doi:10.1186/s12906-016-1452-y
49. Olaokun OO, McGaw LJ, Eloff JN, Naidoo V. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. BMC Complement Altern Med. 2013;13(1):1. doi:10.1186/1472-6882-13-94
50. Suprayogi A, Rahminiwati M, Satyaningtijas A, et al. Identification Of Compounds Flavonoids Namnam Leaf Extract (Cynometra Cauliflora) As Inhibiting A-Glucosidase. J Phys. 2020;1594:12005.
51. Ajiboye BO, Ojo OA, Okesola MA, et al. vitro antioxidant activities and inhibitory effects of phenolic extract of Senecio biafrae (Oliv and Hiern) against key enzymes linked with type II diabetes mellitus and Alzheimer’s disease. Food Sci Nutrition. 2018;6(7):1803–1810. doi:10.1002/fsn3.749
52. Papoutsis K, Zhang J, Bowyer MC, Brunton N, Gibney ER, Lyng J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties. A Rev Food Chemistry. 2021;15(338):128119. doi:10.1016/j.foodchem.2020.128119
53. Ibrahim FA, Usman LA, Akolade JO, Idowu OA, Abdulazeez AT, Amuzat AO. Antidiabetic potentials of Citrus aurantifolia leaf essential oil. Drug Res. 2019;69(04):201–206. doi:10.1055/a-0662-5607
54. Abifarin TO, Otunola GA, Afolayan AJ. Chemical Composition of Essential Oils Obtained from Heteromorpha arborescens (Spreng.) Cham. and Schltdl Leaves Using Two Extraction Methods. Sci World J. 2020;2020:1–6. doi:10.1155/2020/9232810
55. Mwangi JW, Achola KJ, Lwande W, Hassanali A, Laurent R. Volatile components of heteromorpha trifoliata (wendl.). Flavour Fragrance j. 1994;Sep(5):241–243. doi:10.1002/ffj.2730090508
56. Abdollahi M, et al. Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja Khuzestanica in rat in vivo: a toxicopharmacological study. Med Sci Monitor. 2003;9(9):BR331–BR335.
57. Akash MS, Rehman K, Mahmood MH. Essential Oils Downregulate Pro-Inflammatory Cytokines and Nitric Oxide-mediated Oxidative Stress in Alloxan-induced Diabetogenic Rats. Endocr Metab Immune Disord Drug Targets. 2020;2:65.
58. Furman BL. Streptozotocin‐Induced Diabetic Models in Mice and Rats. Current Protocols. 2021;1(4):e78. doi:10.1002/cpz1.78
59. Umar MB, Kabiru AY, Mann A, Ogbadoyi EO. In vivo antihyperglycaemic activity of crude and partitioned fractions of selected medicinal plants. BIOMED Natural and Applied Science. 2021;01:43–56. doi:10.53858/bnas01014356
60. Ahmed SS, Fahim J, Abdelmohsen UR. Chemical and biological potential of Ammi visnaga (L.) Lam. and Apium graveolens L.: a review (1963-2020). J Adv Biomed Pharmaceutical Sci. 2021;4(3):160–176. doi:10.21608/jabps.2021.55949.1115
61. Christova-Bagdassarian VL, Bagdassarian KS, Atanassova MS. Phenolic profile: antioxidant and antibacterial activities from the Apiaceae family (dry seeds). Mintage J Pharmaceutical Med Sci. 2013;2:26–31.
62. Mottaghipisheh J, Boveiri Dehsheikh A, Mahmoodi Sourestani M, Kiss T, Hohmann J, Csupor D. Ducrosia spp., Rare Plants with Promising Phytochemical and Pharmacological Characteristics: an Updated Review. Pharmaceuticals. 2020;13(8):175. doi:10.3390/ph13080175
63. Furman BL. Streptozotocin‐induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70(1):5–47.
64. Saleem F, Sarkar D, Ankolekar C, Shetty K. Phenolic bioactives and associated antioxidant and anti-hyperglycemic functions of select species of Apiaceae family targeting for type 2 diabetes relevant nutraceuticals. Ind Crops Prod. 2017;15(107):518–525. doi:10.1016/j.indcrop.2017.06.023
65. Elisha IL, Dzoyem JP, McGaw LJ, Botha FS, Eloff JN. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Complement Altern Med. 2016;16(1):1. doi:10.1186/s12906-016-1301-z
66. Abifarin TO, Otunola GA, Afolayan AJ. Nutritional composition and antinutrient content of Heteromorpha arborescens (Spreng.) Cham. & Schltdl. leaves: an underutilized wild vegetable. Food Sci Nutrition. 2021;9(1):172–179. doi:10.1002/fsn3.1978
67. National Research Council. Guide for the Care and Use of Laboratory Animals. 2015
68. Mohr BJ, Fakoya FA, Hau J, Souilem O, Anestidou L. The governance of animal care and use for scientific purposes in Africa and the Middle East. ILAR J. 2016;57(3):333–346. doi:10.1093/ilar/ilw035
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.