Back to Journals » OncoTargets and Therapy » Volume 13
Anti-Angiogenic Therapy in the Treatment of Non-Small Cell Lung Cancer
Authors Tian W , Cao C, Shu L, Wu F
Received 9 August 2020
Accepted for publication 14 October 2020
Published 24 November 2020 Volume 2020:13 Pages 12113—12129
DOI https://doi.org/10.2147/OTT.S276150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Wentao Tian,1,2 Chenghui Cao,1,2 Long Shu,1 Fang Wu1
1Department of Oncology, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan, People’s Republic of China; 2Xiangya School of Medicine, Central South University, Changsha 410013, Hunan, People’s Republic of China
Correspondence: Fang Wu
Department of Oncology, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan, People’s Republic of China
Tel +86 13574858332
Email [email protected]
Abstract: Angiogenesis plays an essential role in the development of most solid tumors by delivering nutrients and oxygen to the tumor. Therefore, anti-angiogenic therapy, particularly anti-VEGF and anti-VEGF receptor (VEGFR) therapy, has been a popular strategy to treat cancer. However, anti-angiogenic therapy does not significantly improve patients’ outcomes when used alone because the cutdown of the vessels transforms tumor cells to a hypoxia-tolerant phenotype. While combining anti-angiogenic therapy with other therapies, including chemotherapy, radiotherapy, immunotherapy, and anti-epidermal growth factor receptor (EGFR) therapy, has a promising efficacy due to the vessel normalization effect induced by anti-angiogenic agents. Here, we review the characteristics of tumor angiogenesis, the mechanisms, clinical applications, and prospects of combining anti-angiogenic therapy with other therapies in the treatment of non-small cell lung cancer.
Keywords: angiogenesis, non-small cell lung cancer, anti-angiogenesis therapy, combination therapy, immunotherapy
Introduction
Lung cancer is the most common cancer and the leading cause of cancer-related mortality globally.1 Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer, and 75% of NSCLC patients present with late-stage disease at the time of diagnosis, which leads to a poor prognosis with low 5-year overall survival (OS). Although progress has been achieved in immunotherapy and molecular-targeted therapy, the patients’ survival remains poor. Nevertheless, since bevacizumab, the first anti-angiogenic drug, was approved for the treatment of NSCLC in 2004, anti-angiogenic therapy has been proven to be an effective strategy in the treatment of NSCLC, which has been a popular strategy to treat advanced NSCLC.
The walls of blood vessels are composed of endothelial cells (ECs) and mural cells, which are embedded in an extracellular matrix. Angiogenesis, the process of new blood vessels forming from pre-existing ones, is crucial not only in various physiological processes such as embryogenesis and wound healing but also in the growth, proliferation, and metastases of NSCLC.2 When the tumors grow, new vessels start to form around and inside of tumors to provide nutrients and oxygen.3 However, the vessels are typically immature and characterized by disorganization, high heterogeneity, and high permeability, which cause various drug resistances.4 Therefore, anti-angiogenesis has become a key target for cancer treatment.5,6 Combining anti-angiogenic therapy with other therapies, especially immunotherapy, has a hopeful prospect.
This review summarizes the mechanisms and methods of anti-angiogenic therapy, and highlights the prospect of anti-angiogenic therapy to combine with other therapies in the treatment of NSCLC.
Tumor Angiogenesis in NSCLC
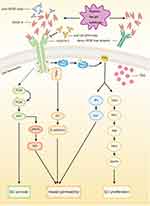
Numerous growth factors are involved in angiogenesis, among which the vascular endothelial growth factor (VEGF) family, consisting of VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF), play a critical role.7 In the VEGF family, VEGF-A (often referred to as VEGF) is the dominant mediator of angiogenesis and closely related to angiogenesis in NSCLC. VEGF receptor (VEGFR) 1 and VEGFR-2 are the receptors for VEGF-A.3,8 Although the binding affinity of VEGF-A to VEGFR-1 is much higher than that to VEGFR-2, VEGFR-2 plays a decisive role in angiogenesis in NSCLC.9 In contrast, VEGFR-1 is not relevant in adults’ physiological angiogenesis, while it assists tumor angiogenesis, as the overexpression of VEGFR-1 and PlGF can improve the cell invasiveness and drug resistance of NSCLC.8,10 There is evidence that high VEGFR-1 expression associates with low survival rate in patients with NSCLC.11 Besides, the binding of VEGF-C/D to VEGFR-3 contributes to lymphangiogenesis, and neuropilins (NRPs) can act as co-receptors and interact with all VEGF family.12
VEGFR-2 is a transmembrane receptor tyrosine kinase, containing a ligand-binding domain, a transmembrane domain, and a tyrosine kinase domain. The binding of VEGF-A to VEGFR-2 initiates the dimerization and phosphorylation of VEGFR-2, followed by the activation of several signaling pathways. First, phospholipase Cγ (PLCγ) is activated directly and then hydrolyzes phosphatidylinositol 4,5 bisphosphate (PIP2), the latter of which decomposes into inositol 3,4,5 trisphosphate (IP3) and 1,2-diacylglycerol (DAG). IP3 leads to the influx of Ca2+, which increases vessel permeability. DAG activates protein kinase C (PKC), followed by the activation of mitogen‐activated protein kinase (MAPK) through the PKC-Raf-MEK-MAPK pathway, which strengthens the proliferation of EC. Second, Src is activated indirectly, and then phosphorylates endothelial cadherin (E-cadherin), leading to looser EC-EC junctions. Finally, the phosphoinositide 3 kinase (PI3K)/Akt signaling pathway is activated via multiple pathways. The activation of Akt is associated with EC survival and the upregulation of endothelial nitric oxide synthase (eNOS) which further increases vessel permeability9,13,14 (Figure 1).
In contrast, there are also endogenous anti-angiogenic forces. The activation of VEGFR-2 upregulates the expression of delta-like ligand (Dll) 4, which is located on the membranes of ECs.15 Contrarily, the binding of Dll-4 to Notch receptors 1 and 4 expressed on the membranes of other ECs downregulates the expression of VEGFR-2, and blocking Dll-4 induces the formation of unfunctional vessels and stop tumor growth.16 In addition, there are several endogenous anti-angiogenic molecules, such as platelet factor 4 and Interferon-γ (IFN-γ).17 The endogenous anti-angiogenic regulation contributes to prevention of over-angiogenesis and maturation of existing vessels.18
Most tumors must develop new blood vessels from pre-existing ones in order to grow beyond a minimum size of 2–3 mm3, and the same is true for NSCLC.19 It is commonly held that VEGF-A is over-expressed in NSCLC, and the progression of NSCLC is heavily relied on angiogenesis.17 Li and co-workers revealed that the larger or more advanced the tumors of NSCLC are, the more likely the tumors present excessive angiogenesis. Their result also showed that angiogenesis in squamous cell lung carcinoma is more abundant than that in lung adenocarcinoma.20 Moreover, a high level of circulating VEGF-A is correlated with poor OS in NSCLC, which may be valuable in predicting patients’ prognosis.11,21
Although ECs are the main target cells for VEGF-A, most VEGF-A in tumor tissues is secreted by tumor cells.22 Hypoxia, acidosis, and cytokines are major stimuli of the secretion of VEGF-A, among which hypoxia is the strongest stimulus23–25 (Figure 1). Hypoxia is a hallmark of cancer and hypoxia-inducible factors (HIFs) are over-expressed in NSCLC.26 Hypoxia induces the over-expression of VEGF-A directly through HIFs, which can bind to the promoter element of VEGF-A and initiate the transcription of VEGF-A.14
The activation of epidermal growth factor receptor (EGFR) is also a pro-angiogenic factor, as it can upregulate the production and secretion of VEGF.27 Although there are no valid predictive biomarkers of response to treatment with anti-angiogenetic inhibitors, it was reported that the expression of VEGF-A was increased in EGFR-mutant NSCLC compared with EGFR-wild type.28,29 In addition, patients with EGFR mutation benefitted more from the treatment of anti-angiogenic therapy + chemotherapy than wild-type patients [median progression-free survival (PFS) 10.5 vs 6.6 months; P=0.0278].29 These findings indicate a potential of anti-angiogenic therapy as an important part of treatment strategy in EGFR-mutant NSCLC patients.
In physiological state, newly formed vessels go through a maturing process to form regular spatial pattern and enable normal function, and the process requires a low level of VEGF.30 The hypersecretion of VEGF in tumors disturbs the pre-existing balance between the pro- and anti-angiogenic forces, accelerates vessel formation, and evades the newly formed vessels from the maturing process.18 Therefore, tumor vessels are typically abnormal and unfunctional, characterized by less pericyte coverage, heterogenous vessel diameter and increased permeability.31
Mechanisms of Anti-Angiogenesis Therapy
The heterogenous diameter and increased permeability of tumor vessels can be obstructions in the treatment of cancer. First, the heterogeneity in vessel diameter results in heterogeneity in blood flow velocity, with some blood even staying static.18,32 This condition impairs gas exchange in the tumor tissue, and fosters hypoxia and acidosis.23,25,33 Second, elevated permeability increases the interstitial fluid pressure (IFP) inside of the tissue, which creates a resistance force against drug infiltration, forcing therapeutic components to gather around tumors rather than inside of them.18 Both the situations impair drug efficacy and assist multiple drug resistances34 (Figure 2).
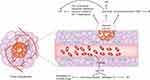 |
Figure 2 Tumor angiogenesis induces drug resistances through multiple mechanisms including inducing hypoxia, acidosis, and high IFP. |
However, anti-angiogenic therapy can normalize tumor vessels by interfering the interaction between VEGF and VEGFR or disturbing the angiogenic signaling.18 So far, numerous anti-angiogenic drugs have been developed to treat various cancers, all of which can be classified into 4 types: (1) anti-VEGF monoclonal antibody (mAb); (2) anti-VEGFR mAb; (3) decoy VEGF-trap receptor; (4) VEGFR tyrosine kinase inhibitors (TKIs).35 (Table 1) Besides, endostatin (endostar) can also inhibit the proliferation of EC by inhibiting a wide range of angiogenic factors. Anti-VEGF mAb (bevacizumab) and anti-VEGFR mAb (ramucirumab) interfere the interaction between VEGF and VEGFR, which have shown promising efficacy in clinical practice.36,37 Decoy VEGF-trap receptor (aflibercept) competitively binds with VEGF and reduces the chance of the binding of VEGF and VEGFR, which has promising efficacy in metastatic colorectal cancer, but has not shown distinguished efficacy in NSCLC.38 VEGFR-TKIs block the intracellular signaling of VEGF/VEGFR (Figure 1). A meta-analysis reported that VEGFR-TKIs generally had advantage in terms of PFS but not OS in advanced NSCLC patients compared with placebo, chemotherapy, or anti-EGFR therapy, and the TKIs increased the risks of adverse events (AEs) of the patients as well.39
 |
Table 1 Four Types of Anti-Angiogenic Agents Approved for the Treatment of Malignant Tumors |
It is noteworthy that the anti-angiogenic agents can only normalize vessels when used at a low dose. In contrast, when they are used at a high dose, with too many vessels pruned, the condition of hypoxia gets worse.18 What is more, anti-angiogenic therapy does not significantly improve patients’ outcomes when used alone, because the cutdown of the vessels transforms tumor cells to a hypoxia-tolerant phenotype, which enhances the revascularization and the invasion of the tumor.14
The normalized vessels are embodied in normal shape, orderly distribution, and decreased permeability with more compact pericyte coverage and EC-EC conjunctions.31 The normalization can reverse multiple drug resistances and benefit other therapies through alleviating hypoxia and decreasing IFP. The vessel normalization theory indicates the potential synergistic effect of anti-angiogenic therapy in combination with other therapies.
Anti-Angiogenic Therapy Combined with Other Therapies
Combined with Radiotherapy
Radiotherapy induces cell cycle arrest or cell death by exposing tumor cells to ionizing radiation. In an aerobic microenvironment, tumor cells generate enough reactive oxygen species (ROS) which cause lethal DNA damage.40 As oxygen is involved in the radiotherapy-induced DNA damage, hypoxia can be a limitation for the efficacy of radiotherapy and a factor for radiotherapy resistance.5 Many studies have focused on increasing tumor oxygenation through anti-angiogenic therapy to improve the therapeutic effect of ionizing radiation.41
In 2012, a Phase I study, which enrolled 6 patients with inoperable Stage III NSCLC, assessed the pulmonary toxicity after bevacizumab + concurrent thoracic radiotherapy. Unfortunately, the study was terminated because 4 patients developed grade 2–3 pneumonitis.42 The Phase II HELPER study, which enrolled 73 patients with unresectable stage III NSCLC, evaluated the efficacy and safety of endostar + chemoradiotherapy. The result showed a preferable OS (median 34.7 months), while 58.2% of patients had grade≥3 AEs.43 Another similar study showed similar outcomes of a preferable OS (estimated median 24.0 months) with higher risk of grade≥3 AEs in the combination group.44 In addition, in a phase II study, endostatin can prevent tumor tissue edema when combined with radiotherapy in the treatment of brain metastases of NSCLC compared with radiotherapy alone.45 In another phase II study, sunitinib + radiotherapy in the treatment of brain metastases of NSCLC showed a promising safety but no survival benefit.46
In general, although anti-angiogenic therapy is mechanically logical to improve the efficacy of radiotherapy, the clinical outcomes showed poor survival improvements and unfavorable safety. Whereas this combination may be suitable for patients with brain metastases to prevent edema.
Combined with Chemotherapy
Hypoxia and acidosis contribute heavily to chemotherapy resistance.47 Hypoxia induces chemotherapy resistance through multiple mechanisms, such as cell cycle arrest in G1/G2/S stage and suppression of DNA repair.33 And as most of cytotoxic agents are weak bases which can be neutralized immediately when entering a low-PH environment, acidosis can impair the efficacy of these agents as well23 (Figure 2). Anti-angiogenic therapy can alleviate hypoxia and acidosis via vessel normalization, and cooperate with chemotherapy.48 Thus, many trials aimed to assess the efficacy of anti-angiogenic therapy + chemotherapy in patients with NSCLC, among which we list the landmark Phase III clinical trials and their results in Table 2.
 |
Table 2 Previous Reported Stage III Clinical Trials of Anti-Angiogenic Therapy Combined with Chemotherapy in the Treatment of NSCLC |
Bevacizumab is a completely humanized mAb which binds to VEGF-A and interferes the interaction between VEGF-A and VEGFR-2.49,50 In 2006, the US Food and Drug Administration (FDA) approved bevacizumab for patients with unresectable, locally advanced, recurrent, or metastatic nonsquamous NSCLC.51 The phase III ECOG4599 trial compared the efficacy and safety of carboplatin + paclitaxel with that of carboplatin + paclitaxel + bevacizumab in the patients with recurrent or advanced NSCLC. The result showed that carboplatin + paclitaxel + bevacizumab had a distinguished advantage in prolonging patients’ PFS (median 6.2 months vs 4.5 months; hazard ratio (HR)=0.66; 95% confidence interval (CI) 0.57–0.77; P<0.001) and overall survival (OS) (median 12.3 months vs 10.3 months; HR=0.79; 95% CI 0.67–0.92; P=0.003), while the rates of clinically significant bleeding, neutropenia, and other 7 AEs increased in the carboplatin + paclitaxel + bevacizumab group (P<0.05).52 BEYOND, a similar phase III trial conducted among Chinese NSCLC patients, confirmed the advantage of bevacizumab + carboplatin + paclitaxel as first-line treatment in prolonging the OS (median 9.2 months vs 6.5 months; HR=0.40; 95% CI 0.29–0.54; P<0.001) and PFS (median 24.3 months vs 17.7 months; HR=0.68; 95% CI 0.50–0.93; P=0.0154) of the patients, and the safety results were similar to the ECOG4599 study.53 The phase III AVAPERL trial reported a favorable PFS prolongation (median 7.4 months vs 3.7 months; HR=0.57; 95% CI 0.44–0.75; P<0.0001) but no significant OS extension (median 17.1 months vs 13.3 months; HR=0.87; 95% CI 0.63–1.21;P=0.29) in the maintenance bevacizumab + pemetrexed group compared with maintenance bevacizumab, while grade ≥3 AEs, such as neutropenia, hypertension, and anemia, occurred more often in the combination group.54 The phase III POINTBREAK trial reported that pemetrexed + carboplatin + bevacizumab had similar efficacy with paclitaxel + carboplatin + bevacizumab (median OS 12.6 months vs 13.4 months; HR=1.0; 95% CI 0.86–1.16; P=0.949).55 In general, it is commonly reported that bevacizumab + chemotherapy is effective in prolonging patients’ survival compared with chemotherapy alone or bevacizumab alone. However, the incidences of bleeding, neutropenia, and many other hematological AEs increase in the combination therapy group compared with chemotherapy or bevacizumab alone.
Ramucirumab is another completely humanized mAb, which targets on VEGFR-2.56 The phase III REVEL trial compared the efficacy and safety of ramucirumab + docetaxel with those of docetaxel alone in patients with stage IV NSCLC after platinum-based therapy. The results showed that the combination therapy prolonged the PFS (median 4.5 months vs 3.0 months; HR=0.76; 95% CI 0.68–0.86; P<0.0001) and OS (median 10.5 months vs 9.1 months; HR=0.86; 95% CI 0.75–0.98; P=0.023) of the patients compared with docetaxel alone. No significantly increased incidence of grade≥3 AE (79% vs 71%) occurred, and the toxicities can be reduced with appropriate dose reductions and supportive care.37 The study indicates ramucirumab as a reliable agent to treat advanced NSCLC. Based on this delightful result, the FDA approved the combined use of ramucirumab with docetaxel for metastatic NSCLC patients with disease progression on or after platinum-based chemotherapy in 2014.
Nintedanib is an orally available angiogenic inhibitor which binds to not only VEGFR 1–3 but also platelet-derived growth factor receptors (PDGFR) α/β and fibroblast growth factor receptors (FGFR) 1–3.57 The phase III LUME-Lung 1 trial compared the efficacy of docetaxel + nintedanib with docetaxel alone as second-line therapy in patients with stage IIIB/IV NSCLC. The results showed docetaxel + nintedanib extended the PFS in the total population (median 3.4 months vs 2.7 months; HR=0.79; 95% CI 0.68–0.92; P=0.0019) the OS in the adenocarcinoma population (median 12.6 months vs 10.3 months; HR=0.83; 95% CI 0.70–0.99; P=0.0359) compared with docetaxel alone.58 Another phase III trial (LUME-Lung 2) evaluated the efficacy and safety of pemetrexed + nintedanib in pretreated NSCLC patients. The result showed an improvement in the PFS (median 4.4 months vs 3.6 months; HR=0.83, 95% CI 0.70–0.99, p=0.00435) with no significant difference in the OS (median 12.0 months vs 12.7 months; HR=1.01, 95% CI 0.85–1.21, p=0.8940) in the pemetrexed + nintedanib group compared with pemetrexed group.59 In the two studies, grade≥3 AEs both occurred more often in the combination group, but the incidences of neutropenia and bleeding were similar in the experimental and control group, respectively. These two studies indicated that nintedanib + chemotherapy is an effective second-line option for patients with advanced NSCLC.58,59 In 2014, the European Medicines Agency (EMA) approved nintedanib + docetaxel in the treatment of advanced lung adenocarcinoma with disease progression on or after chemotherapy.
Anlotinib is a multi-targeting TKI which targets on VEGF receptors 1–3, c-kit, FGFR 1–4, and PDGFR α/β.60 The phase III ALTER 0303 trial assessed the efficacy and safety of anlotinib in Chinese patients with advanced NSCLC. The results showed significantly longer PFS (median 5.4 months vs 1.4 months; HR=0.25, 95% CI 0.19–0.31, p<0.001) and OS (median 9.6 months vs 6.3 months; HR=0.68, 95% CI 0.54–0.87, p=0.002) in the anlotinib group compared with the placebo group, even though anlotinib was not combined with chemotherapy, and the treatment was tolerable as well.61 In 2018, anlotinib was approved for third-line treatment of advanced NSCLC by China Food and Drug Administration (CFDA).
In general, all the above studies have stressed that bevacizumab, ramucirumab, nintedanib can prolong patients’ survival generally when combined with chemotherapy and provide a new approach in treating advanced NSCLC, but meanwhile AEs caused by this combination therapy should be taken seriously. However, in some other clinical trials (Table 2) where chemotherapy was combined with other anti-angiogenic agents (eg, vandetanib, sorafenib, motesanib, aflibercept, cediranib), no significant survival advantage was observed in the combination group.62–69
Combined with Immunotherapy
Immunotherapy is a kind of treatment that assists the immune system in fighting cancer. It has been proven to be effective to treat various cancers with slighter side effects.70 Immune checkpoint inhibitors (ICIs) are critical agents of immunotherapy.71 There are various types of ICIs, such as cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitors (eg, ipilimumab), programmed cell death protein 1 (PD-1) inhibitors (eg, pembrolizumab, nivolumab), and programmed cell death 1 ligand 1 (PD-L1) inhibitors (eg, atezolizumab).72
Immunosupportive tumor microenvironment (TME) is essential for better performance of immunotherapy. Nevertheless, pathological vessels create immunosuppressive TME through various mechanisms. Firstly, the high IFP hinders immune cells from infiltrating into tumors. And the loose EC-EC adhesions impede the extravasation of immune cells.73 Secondly, hypoxia and acidosis reprogram the macrophages into the immunosuppressive M2 phenotype.74 Hypoxia also induces the production of chemokines that recruit immunosuppressive regulatory T cells (Tregs).75,76 Besides, the high level of VEGF decreases the abundance of mature dendritic cell (DC) and thus interfere the antigen presentation.77 Finally, hypoxia up-regulates PD-L1, 2, 3-dioxygenase (IDO), interleukin-6 (IL-6) and interleukin-10 (IL-10), which inhibit anti-tumor immune response.78,79 In a word, pathological angiogenesis induces immunosuppressive TME and therefore develops immunotherapy resistance.
However, anti-angiogenic therapy reverses tumor microenvironment into an immunosupportive type by decreasing IFP and alleviating hypoxia.35 Decreased IFP promotes immune cell infiltration and inhibits the recruitment of Tregs and myeloid-derived suppressor cells (MDSCs). Alleviated hypoxia downregulates PD-L1 and transduces macrophages into immunosupportive M1 phenotype. Anti-VEGF agents also block the inhibitory signal for DC differentiation and decrease overall MDSC pool.80 Besides, immune checkpoint inhibitors (anti-PD-L1 agents and anti-CTLA-4 agents) have a synergistic effect with anti-angiogenic therapy, as they activate T cells and the activated T cells secret IFN-γ which induces vessel normalization as well.81
These findings indicate the advantages of anti-angiogenic therapy + ICIs. There are several ongoing clinical studies assessing the efficacy and safety of this combination therapy. We list them in Table 3.
 |
Table 3 Clinical Trials of Anti-Angiogenic Therapy Combined with Immunotherapy in the Treatment of NSCLC |
The phase I JVDF trial evaluated the safety and tolerance of ramucirumab + pembrolizumab. This study enrolled 92 patients with three types of malignant tumors, including 27 participants with nonsquamous NSCLC. During the treatment with ramucirumab + pembrolizumab, only 7% of nonsquamous NSCLC patients had serious treatment-related AE, such as asthenia and myocardial infarction. The objective response rate (ORR) was 30% (95% CI 13.8–50.2).82 A similar phase I trial (ChechMate 012) also revealed a tolerable safety and a high ORR (57%).83 These results demonstrated that ramucirumab + pembrolizumab is manageably safe.
Another phase II trial (NCT04379739), which enrolled 92 patients with advanced nonsquamous NSCLC, evaluated the efficacy and safety of apatinib + camrelizumab as second or further line therapy. The results showed that apatinib + camrelizumab had promising efficacy (ORR=30.8%) and manageable safety, and the patients with high blood tumor mutation burden (bTMB) had better results than those with low bTMB (median PFS 7.8 months vs 5.6 months).84
The phase III Impower150 trial assessed the efficacy and safety of bevacizumab + atezolizumab + chemotherapy as first-line therapy in patients with stage IV or recurrent metastatic nonsquamous NSCLC. The patients received carboplatin + paclitaxel + atezolizumab (ACP), atezolizumab + bevacizumab + carboplatin + paclitaxel (ABCP), or bevacizumab + carboplatin + paclitaxel (BCP). As the previous studies had revealed that the patients with EGFR/ALK alterations hardly benefit from immunotherapy, patients were detected with EGFR/ALK alterations and effector T cell (Teff) level. And the Teff gene signature has proven to be a more precise immunological marker than PD-L1 level. The results showed that the PFS was drastically prolonged in the ABCP group than the BCP group among the wild type (WT) population (median 8.3 months vs 6.8 months; HR=0.62; 95% CI 0.52 to 0.74; P<0.001). Particularly, in the Teff-high WT population, the PFS prolongation was even more significant (median 11.3 months vs 6.8 months; HR=0.51; 95% CI 0.38 to 0.68; P<0.01). In the WT population, the OS was longer when treated with ABCP than treated with BCP (median 19.2 months vs 14.7 months; HR=0.78; 95% CI 0.64 to 0.96; P=0.02). The incidences of treatment-related AE were similar among all three groups, and neutropenia and hypertension are the common AEs.85 In the subgroup analysis of Impower150, the ABCP therapy showed advantages in every subgroup. In addition, in the patients with PD-L1 expression level >50%, the PFS difference is more significant (median 12.6 months vs 6.8 months; HR=0.39; 95% CI 0.25 to 0.60). The PFS is also prolonged in patients with low PD-L1 level and those with low Teff expression. This study provides a prospect of atezolizumab + bevacizumab in treating a large range of patients with late-stage nonsquamous NSCLC, especially those with a high PD-L1 expression level.85
In general, the combination of angiogenic inhibitors and ICIs shows a manageable safety and an out-standing efficacy, which can benefit a larger group of patients with NSCLC. Yet, our understanding of this combination therapy is very limited at present. There are still a bunch of queries and challenges when it comes to its wide-range application. A series of related clinical trials are underway (Table 3), so more results of the follow-up studies are expected.
Combined with Anti-EGFR Therapy
EGFR, which is generally the receptor for epidermal growth factor (EGF) and transforming growth factor (TGF)-α, is a member of the HER/erbB family. EGFR is over-expressed in NSCLC cells and takes a critical part in numerous tumorigenic processes, such as the survival, proliferation, adhesion, differentiation, migration, transformation, and motility of tumor cells.86 Anti-EGFR agents (such as gefitinib, erlotinib) have advantages in prolonging patients’ OS and PFS.87 International guidelines recommend EGFR-TKIs as the first-line treatment for advanced NSCLC patients who are positive for EGFR-sensitive mutations. Different EGFR-TKIs have different pharmacological mechanisms. First-generation EGFR-TKIs (such as gefitinib, erlotinib and icotinib) bind with EGFR reversibly. Most of second-generation EGFR-TKIs (such as afatinib, dacomitinib) are multi-target drugs that form irreversible covalent bonds with EGFR tyrosine and inhibit the activities of other members of the ErbB family (such as ErbB-2, ErbB-4).88 The majority of patients receiving the first- or second-generation EGFR-TKIs eventually develop resistance, and the T790M mutation is associated with the resistance in most cases. Third-generation EGFR-TKIs (represented by osimertinib) not only selectively inhibit EGFR-sensitive mutations but also overcome T790M-mutation-mediated resistance.89
As mentioned above, EGFR-mutation in NSCLC is generally accompanied by the over-expression of VEGF-A. Contrarily, anti-EGFR therapy can down-regulate VEGF level and decrease microvascular density.90 However, anti-EGFR therapy alone cannot inhibit tumor angiogenesis, but when combined with anti-VEGF/VEGFR therapy, a greater vessel normalization effect can be achieved.91 Moreover, anti-EGFR resistance is regularly accompanied by high VEGF expression.92 Using anti-VEGF/VEGFR agents is likely to reverse this resistance. So far, many studies have focused on the combination of anti-VEGF/VEGFR therapy and anti-EGFR therapy. We list the phase III clinical trials which evaluated the combination of anti-VEGF/VEGFR therapy and anti-EGFR therapy in Table 4.
 |
Table 4 Stage III Clinical Trials of Anti-Angiogenic Therapy Combined with Anti-EGFR Agents in the Treatment of NSCLC |
The phase II JO25567 trial compared the safety and efficacy of erlotinib + bevacizumab (EB) with that of erlotinib alone (E) in the treatment of EGFR-mutation-positive advanced or recurrent NSCLC patients. The result showed a significant prolongation (6.3 months) in patients’ PFS in the EB group.93 Meanwhile, more grade≥3 AEs occurred in the EB group (90.7% vs 53.2%) but most of them are manageable.93
In 2011, the result of the phase III Be Ta study, which compared EB with E, was reported. The study did not require the participants to be EGFR-mutation positive. Although the PFS was prolonged in the EB group (median 3.4 months vs 1.7 months, HR=0.62, 95% CI 0.52 to 0.75), the OS did not show significant difference (median 9.3 months vs 9.2 months, HR=0.97, 95% CI 0.80 to 1.18, P=0.7583), probably because the study did not take EGFR mutation into key account.94 NEJ026 was a follow-up phase III trial comparing EB with E in patients with advanced or recurrent EGFR-mutation-positive NSCLC. It is reported that the PFS is significantly prolonged in the EB group (median 16.9 months vs 13.3 months, HR=0.605, 95% CI 0.417 to 0.877, P=0.016); however, EB provided no further benefit to the OS of the patients (median 50.7 months vs 46.2 months, HR=1.007, 95% CI 0.681 to 1.490, P=0.973).95,96 In 2017, Wang et al conducted a phase III study, which evaluated the efficacy and safety of EB + panitumumab in patients with stage II–IV NSCLC. The OS (median 10.4 months vs 8.9 months, P=0.003) and PFS (median 4.6 months vs 1.9 months, P=0.031) are significantly prolonged in the combination group compared with erlotinib alone.97 In 2019, the result of a similar phase III study (CTONG 1509) conducted among Chinese EGFR-mutation-positive patients also showed a significant prolongation in PFS in the EB group (median 18.0 months vs 11.3 months, HR=0.55, 95% CI 0.41 to 0.75, P<0.001).98 These findings indicate that the combination of EB may benefit EGFR-mutation-positive patients. Further clinical data are needed to verdict the hypothesis.
The phase III ATLAS trial validated the advantage of EB in patients with stage IIIB/IV, or recurrent NSCLC. The result showed that EB significantly prolonged the PFS (median 4.8 months vs 3.7 months, HR=0.71, 95% CI 0.58 to 0.86, P<0.001) but did not extend the OS (median 14.4 months vs 13.3 months, HR=0.92, 95% CI 0.70 to 1.21, P=0.5341) compared with bevacizumab alone. Both groups (B group and EB group) showed a similar incidence of AEs.99 Another phase III study evaluated efficacy and safety of sunitinib + erlotinib treatment in patients with refractory NSCLC. The findings showed significant improvements in patients’ PFS (median 3.6 months vs 2.0 months, HR=0.807, 95% CI 0.695 to 0.937, P=0.0023) and ORR (10.6% vs 6.9%) but not in OS (median 9.0 months vs 8.5 months, HR=0.922, 95% CI 0.797 to 1.067, P=0.1388).100 All these studies suggest that anti-angiogenic therapy + anti-EGFR therapy can enhance anti-tumor activity and is effective in prolonging survival. More follow-up results are expected.
Discussion
Angiogenesis is essential in the development and drug resistance of several solid tumors. But unlike physiological angiogenesis, tumors tend to form not only excessive but also spatially chaotic and unfunctional vessels, characterized by increased permeability and varying diameter and blood flow velocity, which results in high IFP, hypoxia, and acidosis in the TME. All these conditions impair drug filtration and contribute to the resistances of several therapies, including radiotherapy, chemotherapy, immunotherapy, and anti-EGFR therapy. VEGF, especially VEGF-A, is critical in tumor angiogenesis, whose level is elevated in most solid tumor types. Anti-VEGF/VEGFR agents have become the cardinal anti-angiogenic agents because of its benefit to vessel normalization when used at low dose. Although anti-angiogenic therapy alone cannot achieve satisfactory outcomes, the effect of vessel normalization can alleviate hypoxia, and transform tumor TME into an immunosupportive type. So, anti-angiogenic therapy has great potential in combined use with chemotherapy, anti-EGFR therapy, and especially immunotherapy.
However, there are still difficulties encountered in anti-angiogenesis therapy. First, although it seems that EGFR-mutant NSCLC patients are more likely to benefit from anti-angiogenic therapy, researchers have not found any valid predictive biomarkers of response to anti-angiogenetic treatment to filter out potential non-responders. Second, the combination therapies with anti-angiogenetic inhibitors increase the risk of infrequent serious AEs, such as bleeding and neutropenia. Efforts should be made to reduce these adverse reactions. What is more, there are many questions are yet to be answered when it comes to the combination therapies with anti-angiogenetic inhibitors, such as, the proper timing of anti-angiogenic combination therapy, and the suggestive dose and proportion of the drugs.
Besides the classic anti-angiogenesis drugs listed in Table 1, there are also newly developed anti-angiogenic drugs targeting on VEGFR-1, PDGFR, and angiopoietin-1/2, which are still under evaluation in pre-clinical or clinical models.8,19 Chinnasamy et al developed anti-VEGFR2 chimeric antigen receptor (CAR) T cell in mice model as an effective strategy for tumor regression, which still needs further evaluation of efficacy and safety in humans.101 We believe with more discoveries reported in the future, combination therapy with anti-angiogenic agents will be a promising strategy to treat NSCLC and bring benefits to more patients.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81902351); Chinese Thoracic Oncology Group/Guangdong Provincial Key Lab of Transitional Medicine in Lung Cancer [No.2017B030314120]; Beijing Xisike Clinical Oncology Research Foundation [Y-BMS-2019-100]; Changsha Science and Technology Plan Project [No.kq1907077].
Disclosure
The authors report no conflicts of interest for this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
2. Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi:10.1038/nature04478
3. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi:10.1056/NEJMra0706596
4. Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi:10.1038/nm0603-685
5. Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426.
6. Nowak-Sliwinska P, Alitalo K, Allen E, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018;21(3):425–532.
7. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–1770.
8. Lacal PM, Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res. 2018;136:97–107. doi:10.1016/j.phrs.2018.08.023
9. Staels W, Heremans Y, Heimberg H, De Leu N. VEGF-A and blood vessels: a beta cell perspective. Diabetologia. 2019;62(11):1961–1968. doi:10.1007/s00125-019-4969-z
10. Sun K, Liao Q, Chen Z, Chen T, Zhang J. Expression of Livin and PlGF in human osteosarcoma is associated with tumor progression and clinical outcome. Oncol Lett. 2018;16(4):4953–4960.
11. Zheng CL, Qiu C, Shen MX, et al. Prognostic impact of elevation of vascular endothelial growth factor family expression in patients with non-small cell lung cancer: an updated meta-analysis. Asian Pac J Cancer Prev. 2015;16(5):1881–1895. doi:10.7314/APJCP.2015.16.5.1881
12. Mercurio AM. VEGF/neuropilin signaling in cancer stem cells. Int J Mol Sci. 2019;20:3. doi:10.3390/ijms20030490
13. Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):114–127. doi:10.1111/joim.12019
14. Mahdi A, Darvishi B, Majidzadeh AK, Salehi M, Farahmand L. Challenges facing antiangiogenesis therapy: the significant role of hypoxia-inducible factor and MET in development of resistance to anti-vascular endothelial growth factor-targeted therapies. J Cell Physiol. 2019;234(5):5655–5663. doi:10.1002/jcp.27414
15. Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444(7122):1083–1087. doi:10.1038/nature05313
16. Baharlou R, Tajik N, Behdani M, et al. An antibody fragment against human delta-like ligand-4 for inhibition of cell proliferation and neovascularization. Immunopharmacol Immunotoxicol. 2018;40(5):368–374. doi:10.1080/08923973.2018.1505907
17. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi:10.1038/35025220
18. Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi:10.1200/JCO.2012.46.3653
19. Coelho AL, Gomes MP, Catarino RJ, et al. Angiogenesis in NSCLC: is vessel co-option the trunk that sustains the branches? Oncotarget. 2017;8(24):39795–39804. doi:10.18632/oncotarget.7794
20. Li L, Zhao W, Sun X, et al. 18F-RGD PET/CT imaging reveals characteristics of angiogenesis in non-small cell lung cancer. Transl Lung Cancer Res. 2020;9(4):1324–1332. doi:10.21037/tlcr-20-187
21. Hu P, Liu W, Wang L, Yang M, Du J. High circulating VEGF level predicts poor overall survival in lung cancer. J Cancer Res Clin Oncol. 2013;139(7):1157–1167.
22. Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97(7):978–985. doi:10.1038/sj.bjc.6603923
23. Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–777. doi:10.1038/nrd3554
24. Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci U S A. 2005;102(50):18111–18116.
25. Rey S, Schito L, Wouters BG, Eliasof S, Kerbel RS. Targeting hypoxia-inducible factors for antiangiogenic cancer therapy. Trends Cancer. 2017;3(7):529–541. doi:10.1016/j.trecan.2017.05.002
26. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi:10.1016/j.cell.2012.01.021
27. Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Can Res. 1999;5(2):257–265.
28. Reinmuth N, Jauch A, Xu EC, et al. Correlation of EGFR mutations with chromosomal alterations and expression of EGFR, ErbB3 and VEGF in tumor samples of lung adenocarcinoma patients. Lung Cancer. 2008;62(2):193–201. doi:10.1016/j.lungcan.2008.03.011
29. Tanaka I, Morise M, Miyazawa A, et al. Potential benefits of bevacizumab combined with platinum-based chemotherapy in advanced non-small-cell lung cancer patients with EGFR mutation. Clin Lung Cancer. 2020;21(3):273–280. doi:10.1016/j.cllc.2020.01.011
30. Sun X, Evren S, Nunes SS. Blood vessel maturation in health and disease and its implications for vascularization of engineered tissues. Crit Rev Biomed Eng. 2015;43(5–6):433–454. doi:10.1615/CritRevBiomedEng.2016016063
31. Liu Z, Wang Y, Huang Y, et al. Tumor vasculatures: a new target for cancer immunotherapy. Trends Pharmacol Sci. 2019;40(9):613–623. doi:10.1016/j.tips.2019.07.001
32. Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60(15):4251–4255.
33. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi:10.1038/nrc3064
34. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi:10.1016/j.ccell.2014.10.006
35. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60.
36. Tassinari D, Sartori S, Papi M, et al. Bevacizumab in the treatment of advanced, non-squamous non-small cell lung cancer: an evidence-based approach. Oncology. 2011;80(5–6):350–358. doi:10.1159/000328781
37. Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi:10.1016/S0140-6736(14)60845-X
38. Neal JW, Wakelee HA. Aflibercept in lung cancer. Expert Opin Biol Ther. 2013;13(1):115–120. doi:10.1517/14712598.2013.745847
39. Wang S, Yang Z, Wang Z. Are VEGFR-TKIs effective or safe for patients with advanced non-small cell lung cancer? Oncotarget. 2015;6(20):18206–18223. doi:10.18632/oncotarget.4524
40. Orth M, Lauber K, Niyazi M, et al. Current concepts in clinical radiation oncology. Radiat Environ Biophys. 2014;53(1):1–29.
41. Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Can Res. 2003;9(6):1957–1971.
42. Lind JS, Senan S, Smit EF. Pulmonary toxicity after bevacizumab and concurrent thoracic radiotherapy observed in a phase I study for inoperable stage III non-small-cell lung cancer. J Clin Oncol. 2012;30(8):e104–108. doi:10.1200/JCO.2011.38.4552
43. Zhai Y, Ma H, Hui Z, et al. HELPER study: a phase II trial of continuous infusion of endostar combined with concurrent etoposide plus cisplatin and radiotherapy for treatment of unresectable stage III non-small-cell lung cancer. Radiother Oncol. 2019;131:27–34. doi:10.1016/j.radonc.2018.10.032
44. Bao Y, Peng F, Zhou QC, et al. Phase II trial of recombinant human endostatin in combination with concurrent chemoradiotherapy in patients with stage III non-small-cell lung cancer. Radiother Oncol. 2015;114(2):161–166. doi:10.1016/j.radonc.2014.11.039
45. Jiang X, Ding M, Qiao Y, Liu Y, Liu L. Recombinant human endostatin combined with radiotherapy in the treatment of brain metastases of non-small cell lung cancer. Clin Transl Oncol. 2014;16(7):630–636. doi:10.1007/s12094-013-1129-7
46. Novello S, Camps C, Grossi F, et al. Phase II study of sunitinib in patients with non-small cell lung cancer and irradiated brain metastases. J Thorac Oncol. 2011;6(7):1260–1266. doi:10.1097/JTO.0b013e318219a973
47. Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther. 2008;7(12):3670–3684. doi:10.1158/1535-7163.MCT-08-0715
48. Kim JY, Kim YM. Tumor endothelial cells as a potential target of metronomic chemotherapy. Arch Pharm Res. 2019;42(1):1–13. doi:10.1007/s12272-018-01102-z
49. de Goeje PL, Poncin M, Bezemer K, et al. Induction of peripheral effector CD8 T-cell proliferation by combination of paclitaxel, carboplatin, and bevacizumab in non-small cell lung cancer patients. Clin Can Res. 2019;25(7):2219–2227. doi:10.1158/1078-0432.CCR-18-2243
50. Hakozaki T, Okuma Y, Hashimoto K, Hosomi Y. Correlation between the qualification for bevacizumab use and the survival of patients with non-small cell lung cancer harboring the epidermal growth factor receptor mutation: a retrospective analysis. J Cancer Res Clin Oncol. 2019;145(10):2555–2564. doi:10.1007/s00432-019-02985-1
51. Gentzler RD, Yentz SE, Patel JD. Bevacizumab in advanced NSCLC: chemotherapy partners and duration of use. Curr Treat Options Oncol. 2013;14(4):595–609. doi:10.1007/s11864-013-0255-3
52. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi:10.1056/NEJMoa061884
53. Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase iii study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(19):2197–2204. doi:10.1200/JCO.2014.59.4424
54. Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25(5):1044–1052. doi:10.1093/annonc/mdu098
55. Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(34):4349–4357.
56. Takeda K, Daga H. Ramucirumab for the treatment of advanced or metastatic non-small cell lung cancer. Expert Opin Biol Ther. 2016;16(12):1541–1547. doi:10.1080/14712598.2016.1248397
57. Valenzuela C, Torrisi SE, Kahn N, Quaresma M, Stowasser S, Kreuter M. Ongoing challenges in pulmonary fibrosis and insights from the nintedanib clinical programme. Respir Res. 2020;21(1):7.
58. Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–155. doi:10.1016/S1470-2045(13)70586-2
59. Hanna NH, Kaiser R, Sullivan RN, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): a randomized, double-blind, phase III trial. Lung Cancer. 2016;102:65–73. doi:10.1016/j.lungcan.2016.10.011
60. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi:10.1186/s13045-018-0664-7
61. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA oncol. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
62. Laurie SA, Solomon BJ, Seymour L, et al. Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC clinical trials group study BR29. Eur J Cancer. 2014;50(4):706–712. doi:10.1016/j.ejca.2013.11.032
63. Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol. 2012;30(29):3640–3647. doi:10.1200/JCO.2012.42.6932
64. Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol. 2012;30(23):2829–2836. doi:10.1200/JCO.2011.41.4987
65. Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol. 2012;30(25):3084–3092. doi:10.1200/JCO.2011.39.7646
66. Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(11):1835–1842. doi:10.1200/JCO.2009.26.1321
67. de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29(8):1067–1074. doi:10.1200/JCO.2010.29.5717
68. Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619–626. doi:10.1016/S1470-2045(10)70132-7
69. Kubota K, Yoshioka H, Oshita F, et al. Phase III, randomized, placebo-controlled, double-blind trial of motesanib (AMG-706) in combination with paclitaxel and carboplatin in East Asian patients with advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2017;35(32):3662–3670. doi:10.1200/JCO.2017.72.7297
70. Fuca G, de Braud F, Di Nicola M. Immunotherapy-based combinations: an update. Curr Opin Oncol. 2018;30(5):345–351. doi:10.1097/CCO.0000000000000466
71. Hendriks L, Besse B. New windows open for immunotherapy in lung cancer. Nature. 2018;558(7710):376–377. doi:10.1038/d41586-018-05312-9
72. Dafni U, Tsourti Z, Vervita K, Peters S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer. 2019;134:127–140. doi:10.1016/j.lungcan.2019.05.029
73. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi:10.1158/0008-5472.CAN-15-0255
74. Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. doi:10.1158/0008-5472.CAN-09-4672
75. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi:10.1038/nm1093
76. Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226–230. doi:10.1038/nature10169
77. Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–1103. doi:10.1038/nm1096-1096
78. Liu M, Wang X, Wang L, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. 2018;11(1):100. doi:10.1186/s13045-018-0644-y
79. Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204.
80. Perdrizet K, Leighl NB. The role of angiogenesis inhibitors in the era of immune checkpoint inhibitors and targeted therapy in metastatic non-small cell lung cancer. Curr Treat Options Oncol. 2019;20(3):21. doi:10.1007/s11864-019-0617-6
81. Arce Vargas F, Furness AJS, Solomon I, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T Cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46(4):577–586. doi:10.1016/j.immuni.2017.03.013
82. Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, Phase 1a/b trial. Lancet Oncol. 2019;20(8):1109–1123. doi:10.1016/S1470-2045(19)30458-9
83. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi:10.1016/S1470-2045(16)30624-6
84. Zhou C Efficacy of PD-1 monoclonal antibody SHR-1210 plus apatinib in patients with advanced non-squamous NSCLC with wild‐type EGFR and ALK. 2019; ASCO Annual Meeting.
85. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948
86. Santaniello A, Napolitano F, Servetto A, et al. Tumour microenvironment and immune evasion in EGFR addicted NSCLC: hurdles and possibilities. Cancers (Basel). 2019;11:10. doi:10.3390/cancers11101419
87. Sukrithan V, Deng L, Barbaro A, Cheng H. Emerging drugs for EGFR-mutated non-small cell lung cancer. Expert Opin Emerg Drugs. 2019;24(1):5–16. doi:10.1080/14728214.2018.1558203
88. Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–350. doi:10.1124/jpet.112.197756
89. Akkermans R. Third-generation EGFR-TKIs-a new hope for NSCLC. Lancet Respir Med. 2014;2(7):520. doi:10.1016/S2213-2600(14)70095-5
90. Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18(5):1007–1021, viii. doi:10.1016/j.hoc.2004.06.002
91. Gerber HP, Kowalski J, Sherman D, Eberhard DA, Ferrara N. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res. 2000;60(22):6253–6258.
92. Vallbohmer D, Zhang W, Gordon M, et al. Molecular determinants of cetuximab efficacy. J Clin Oncol. 2005;23(15):3536–3544. doi:10.1200/JCO.2005.09.100
93. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non-small-cell lung cancer (JO25567): updated safety results. Drug Safety. 2018;41(2):229–237. doi:10.1007/s40264-017-0596-0
94. Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377(9780):1846–1854. doi:10.1016/S0140-6736(11)60545-X
95. Maemondo M, Fukuhara T, Saito Het al. NEJ026 Final overall survival analysis of bevacizumab plus erlotinib treatment for NSCLC patients harboring activating EGFR-mutations. ASCO Virtual Scientific Program; 2020.
96. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi:10.1016/S1470-2045(19)30035-X
97. Wang Y, Wang H, Jiang Y, Zhang Y, Wang X. A randomized phase III study of combining erlotinib with bevacizumab and panitumumab versus erlotinib alone as second-line therapy for Chinese patients with non-small-cell lung cancer. Biomed Pharmacother. 2017;89:875–879. doi:10.1016/j.biopha.2017.02.097
98. Zhou Q, Wu YL, Cheng Y, et al. CTONG 1509: phase III study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR-mutated NSCLC. Ann Oncol. 2019;30:v603.
99. Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(31):3926–3934. doi:10.1200/JCO.2012.47.3983
100. Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol. 2012;30(17):2070–2078. doi:10.1200/JCO.2011.39.2993
101. Chinnasamy D, Yu Z, Kerkar SP, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Can Res. 2012;18(6):1672–1683. doi:10.1158/1078-0432.CCR-11-3050
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.