Back to Journals » Infection and Drug Resistance » Volume 12
Antagonistic Effects Of Baicalin On Mycoplasma gallisepticum-Induced Inflammation And Apoptosis By Restoring Energy Metabolism In The Chicken Lungs
Authors Ishfaq M, Zhang W, Hu W, Waqas Ali Shah S, Liu Y, Wang J, Wu Z, Ahmad I, Li J
Received 13 July 2019
Accepted for publication 13 September 2019
Published 1 October 2019 Volume 2019:12 Pages 3075—3089
DOI https://doi.org/10.2147/IDR.S223085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Muhammad Ishfaq,1 Wei Zhang,1 Wanying Hu,1 Syed Waqas Ali Shah,2 Yuhao Liu,1 Jian Wang,1 Zhiyong Wu,1 Ijaz Ahmad,3 Jichang Li1
1Heilongjiang Key Laboratory for Animal Disease Control and Pharmaceutical Development, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, People’s Republic of China; 2College of Animal Science and Technology, Northeast Agricultural University, Harbin 150030, People’s Republic of China; 3The University of Agriculture Peshawar, Peshawar, Khyber Pakhtunkhwa 25130, Pakistan
Correspondence: Jichang Li
Northeast Agricultural University, 600 Changjiang Road, Xiangfang District, Harbin 150030, People’s Republic of China
Tel +86 451 5519 0674
Fax +86 451 5519 1200
Email [email protected]
Background: Baicalin possesses potential anti-inflammatory, anti-tumor and anti-oxidant activities. In the present study, we attempted to investigate the preventive effects of baicalin against Mycoplasma gallisepticum (MG)-induced inflammation, apoptosis and energy metabolism dysfunction in chicken lungs.
Methods: Experimental chickens were randomly divided into 1) control group, 2) MG infection group, 3) MG-infected group treated with baicalin at a dose of 450 mg/kg and 4) baicalin alone treated group (450 mg/kg). After 7 days of post-treatment, serum and lung tissues were collected for different experimental analyses. The hallmarks of inflammation, apoptosis and energy metabolism dysfunction were detected by histological and ultrastructural examination, qRT-PCR, Western blotting and terminal deoxynucleotidyl transferase-mediated dUTP nick endlabeling (TUNEL) assay.
Results: The level of serum inflammatory markers were increased with MG infection. Histological and ultrastructural analysis showed excessive inflammatory cells infiltrates, alveolar wall thickening, hemorrhages, mitochondrial and nuclear damage, including mitochondrial swelling and condensation of DNA in the lungs of chickens infected with MG. TUNEL assay positive-stained nuclei were significantly increased in MG infection group. In addition, the mRNA and protein expression level of energy metabolism-related genes and ATPase activities were significantly reduced. Meanwhile, MG-induced morphological and ultrastructural changes were partially disappeared with baicalin-treatment, and the level of serum inflammatory markers were significantly reduced. It has been noted that baicalin significantly attenuated MG-induced inflammation and apoptosis in the chicken lungs through the suppression of nuclear factor-kappa B and reduced extensive positive-stained apoptotic nuclei. More importantly, ATPase activities and mRNA and protein expression level of energy metabolism-related genes were significantly improved with baicalin-treatment in the lungs of chickens infected with MG.
Conclusion: Conclusively, it has been suggested from these results that baicalin-treatment efficiently prevented MG-induced inflammation, apoptosis and energy metabolism dysfunction in the chicken lungs and provide basis for new therapeutic targets to control MG infection.
Keywords: baicalin, inflammation, Mycoplasma gallisepticum, lungs, energy metabolism
Introduction
Mycoplasma gallisepticum (MG) is the simplest and smallest prokaryotic microorganism, an avian pathogen that causes chronic respiratory disease in chickens, conjunctivitis in house finches and infectious sinusitis in turkeys.1,2 The infection is highly transmissible and results in significant economic losses including reduction in egg production, hatchability, weight gain and low carcass quality.3,4 MG infection induces profound inflammatory responses in chicken respiratory tract, including trachea, air sacs and lungs.5 Researchers reported that MG adheres and colonizes to the surface of ciliated epithelial cells of the respiratory tract.6 This interaction results in various cytopathic changes such as rounding and exfoliation of non-ciliated and ciliated epithelial cells, deciliation followed by lysis, and the release of catarrhal exudate with mucofibrinous plugs and mucus from goblet cells.7–9 Besides cytopathic changes, profound inflammatory responses were produced during MG infection accompanied with the infiltration of lymphocytes and the release of chemokines and cytokines.10,11 Although previous studies reported the chemokines and cytokines involved in MG-induced inflammatory responses,12,13 the complex relationship between these chemokines and cytokines and inflammation-induced damage in chicken’s respiratory tract is still elusive.
Sokolova et al (1998) demonstrated that Mycoplasma infection sensitized host cells to apoptosis, accompanied by morphological features of apoptosis, DNA fragmentation and inhibit proliferation.14 Mycoplasma hypopneumoniae was reported to induce apoptosis in porcine alveolar macrophages via adhesion and stimulating proliferation.15 However, the detail molecular mechanism of MG-induced apoptosis in chicken lungs is still unknown. In the present study, we investigated MG infection-mediated apoptosis accompanied by mitochondrial damage in the chicken lungs. Previously, it has been demonstrated that MG is partially parasitic in nature because it lacks some of the metabolic enzymes for synthesizing nutrients and other metabolic functions, and therefore derive nutrients from host cells for their survival.6,16 Accumulating evidences showed that pathogen infections may affect the energy metabolism resulting in neutral, beneficial or detrimental outcome for both the pathogen and the host cells.17 The normal structure and function of cells could be impaired due to reduced glucose metabolism and energy production.18,19 To investigate the effect of MG infection on energy metabolism in chicken lungs, we analyzed energy metabolism-related enzymes such as succinate dehydrogenase complex iron-sulfur subunit B (SDHB), phosphofructokinase (PFK), pyruvate kinase (PK), aconitase-2 (ACO2), hexokinase-1 (HK1), hexokinase-2 (HK2), lactate dehydrogenase A (LDHA) and lactate dehydrogenase B (LDHB). Nevertheless, these enzymes could be potential therapeutic targets to prevent energy metabolism dysfunction during MG infection.
Baicalin, a flavonoid compound possesses potential therapeutic properties, including anti-microbial, anti-inflammatory, anti-oxidant, anti-hepatotoxic and anti-tumor properties.20,21 Baicalin has the ability to interact with various signaling pathways.21 Researchers demonstrated that baicalin alleviated inflammation through toll-like receptor 2-nuclear factor-kappa B (NF-κB) signaling pathway in several infection models.22,23 Similarly, our previous study explained the preventive effects of baicalin against MG infection-induced oxidative stress and apoptosis via the opposite modulation of nuclear factor erythroid 2-related factor 2 (Nrf2)/Heme oxygenase-1 (HO-1) and NF-κB signaling pathway and restored mitochondrial dynamics imbalance in the spleen of chickens.24 However, the effects of baicalin on MG infection-mediated energy metabolism dysfunction in the chicken lungs is still not reported. The objectives of the present study were to investigate the preventive effects of baicalin against MG-induced energy metabolism dysfunction, inflammation and apoptosis in the lungs of chicken. The study will help in exploring new therapeutic targets to control and treat MG infection.
Materials And Methods
Ethical Statement
The present study was conducted under the approval of Institutional Animal Care and Use Committee of Northeast Agricultural University (Heilongjiang province, China) (SYXK (Hei) 2012–2067) in accordance with Laboratory animal-Guideline for ethical review of animal welfare (GB/T 35,892–2018, National Standards of the People’s Republic of China).
Bacterial Infection
MG strain (Rlow) was provided by Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Heilongjiang, People’s Republic of China). MG were cultured as mentioned in our previous study.25 In brief, 0.1% nicotinamide adenine dinucleotide, 10% freshly prepared yeast extract, and 0.05% thallium acetate, 20% FBS and 0.05% penicillins were supplemented in modified Hayflicks medium. A color change was observed from phenol red to orange at mid-exponential phase of MG. The density of MG was adjusted to 1×109 color change unit per milliliter (CCU/mL) to challenge chickens.
Chickens And Experimental Treatments
A total of 120 one-day-old white leghorn chickens were provided by Chia Chau chicken farm (Heilongjiang, People’s Republic of China). Chickens were adapted to experimental conditions for 1 week, and provided ad libitum feed and freshwater. Ten chickens were randomly assigned into four experimental groups in three replicates including (G1) control group, (G2) MG infection group, (G3) MG-infected group treated with baicalin at a dose of 450 mg/kg and (G4) baicalin alone treated group (450 mg/kg). Chickens were infected with MG Rlow (1×109 CCU/mL) in the bilateral air sacs as mentioned in our previous study.24 Baicalin (purity ≥98.0%, Huifeng Animal Health Co., Ltd. Heilongjiang, People’s Republic of China) was dissolved in distilled water (0.5 mL) and given orally,23 once a day after 3 days of post-infection. After 7 days of baicalin-treatment, chickens were sacrificed by cardiac puncture and blood was collected following euthanasia with sodium pentobarbital as mentioned previously.26 Whole lung tissues including the right and left lobe were collected from each experimental chicken. The left lobe was washed in PBS to remove excess of blood. Then, the lower side of the left lobe was processed for HE staining and the upper side was stored at –80°C for further experimental analyses.
Determination Of Serum Inflammatory Markers
Serum was separated and collected from blood following centrifugation at 1000× g for 10 mins. Tumor necrosis factor-α (TNF-α), IL-6, IL-1β and IL-8 enzyme activities were determined by ELISA on an iMARKTM microplate reader (Bio-Rad Co., Ltd. Shanghai, People’s Republic of China). All the samples were run in duplicate along with a blank control to avoid inter assay variation.
Transmission Electron Microscopy
Lungs ultrastructural examination was carried out as described previously.27 In brief, samples were first fixed in 2.5% glutaraldehyde solution following 1% osmium tetroxide (OsO4) and washed in 0.2 M phosphate buffer (pH=7.2) for 15 mins. Then, the samples were passed through a series of alcohol solution to remove the excess of water, stained with uranyl acetate and 0.2% lead citrate, and examined for ultrastructural analysis under S-3400N transmission electron microscope (HITACHI, Tokyo, Japan).
Histopathological Examination
Histopathological examination was carried out as described earlier,28 with some modifications. Briefly, lungs samples were fixed in 10% formalin overnight, dehydrated in a series of alcohol solution and embedded in wax. After embedding, samples were cut into sections (5 μm), stained with H&E and observed under light microscope (Nikon E100, 40X magnification, Tokyo, Japan).
Detection Of Apoptosis By Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick Endlabeling (TUNEL) Assay
TUNEL assay was performed as described in our previous study.24 In brief, lung tissues were first fixed in formalin and passed through a series of ethanol solution. After embedding in paraffin wax, the sections were cut and mounted on glass slides. Apoptotic cells were detected by a TUNEL assay detection kit (Beyotime biotechnology, Jiangsu, People’s Republic of China) according to the manufacturer’s instructions. Endogenous peroxidase activity was inhibited by hydrogen peroxide, followed by treatment with proteinase K. After the slides were incubated with terminal TdT/nucleotide mixture at 37°C for 1 hr and then rinsed in phosphate buffer solution. Diaminobenzidine and horseradish peroxidase were applied to develop nuclear labelling. Finally, the slides were counterstained with hematoxylin, examined under a fluorescence microscope (Olympus, Tokyo, Japan), and the number of positive cells was counted in three randomly selected sections in each sample.
Determination Of ATPase Activities
ATPase’s activities including Mg++-ATPase, Na+-K+-ATPase, Ca++-Mg++-ATPase and Ca++ATPase activities were detected in lung tissues of all experimental groups using a kit (Cat. no. A016-2, Jiancheng Institute of Biotechnology, Nanjing, People’s Republic of China) according to the manufacturer’s instructions. The activities of the Mg++-ATPase, Na+-K+-ATPase, Ca++-Mg++-ATPase and Ca++ATPase activities were determined by quantifying the level of the inorganic phosphorus (Pi) production from the conversion of ATP to ADP at 660 nm using the molybdenum blue spectrophotometric method as stated previously.29 The inhibitors of all other types ATPase were added to inhibit the hydrolysis of phosphate radicals when testing one type of ATPase activity. The assay was performed twice for each sample to avoid inter assay variation.
Western Blotting
Radioimmunoprecipitation assay and a protease inhibitor phenylmethyl sulfonyl fluoride were employed for the extraction of protein as mentioned earlier.30 Equal amount of proteins were loaded on SDS-PAGE (10–15%) for separation and transferred to nitrocellulose membranes. After blocking with 5% non-fat dry milk in TBST for 1 hr, the membranes were incubated with specific primary antibodies for 12 hrs followed by three times washing in TBST 10 mins each time. The membranes were then incubated with secondary anti-rabbit or anti-mouse IgG peroxidases for 1 hr at ambient temperature. Enhanced chemiluminescence (ECL, Biosharp Life Sciences, Anhui, People’s Republic of China) reagent was used to visualize the bound immune complexes and analyzed by Image J software (National Institute of Health, Bethesda, Maryland).
RNA Isolation And Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted by TRIzol reagent (catalog no. 15596-018, Thermo Fisher Scientific, Carlsbad, CA, USA) from the lungs tissues of all experimental groups. The quality of RNA was verified using a NanoDrop 2000c spectrophotometer (Thermo Scientific, Carlsbad, CA, USA) at 260/280 ratio.31 High-quality RNA was converted into cDNA using Prime Script™ RT reagent Kit with gDNA Eraser (Takara, Dalian, People’s Republic of China). The reaction of qRT-PCR was performed using a kit purchased from Takara, Dalian, People’s Republic of China (catalog no. RR820A), cDNA and the primers (shown in Table 1) for target genes in a Roche LightCycler instrument (Shanghai, People’s Republic of China). The mRNA expression of the target genes was analyzed by 2−△△Ct method,32 following normalization with β-actin gene.
 |
Table 1 Primers Used In qRT-PCR |
Statistical Analysis
The data were obtained from at least three independent experiments (n=3). One way ANOVA was used to determine the statistical significance (p<0.05) among experimental groups, followed by least square difference test through the statistical package for social sciences (SPSS window version 21.0, Chicago, IL, USA). GraphPad prism software (window version 6.01, San Diego, CA, USA) was used to make all the graphs and the data were expressed as mean±SD.
Results
Effect Of MG And Baicalin On Serum Inflammatory Markers
Serum inflammatory markers (Figure 1) were altered by MG infection and baicalin intervention. Compared to control group and baicalin alone group, TNF-α and IL-1β (p<0.05) enzyme activities were significantly increased in MG infection group, while the increase in IL-8 and IL-6 enzyme activities was not statistically significant (p>0.05) in MG infection group compared to control group and baicalin alone group. Meanwhile, baicalin-treatment significantly reduced MG-induced increase in TNF-α and IL-1β (p<0.05) enzyme activities. However, it has been noted that the decrease in IL-8 and IL-6 enzyme activities was not statistically significant (p>0.05) with baicalin-treatment compared to MG infection group.
Histological And Ultrastructural Examination
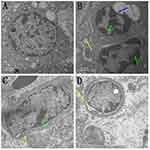
Histological assessment (Figure 2) showed increased inflammatory cells infiltrates and neutrophils, alveolar wall thickening and hemorrhages in MG-infected group (Figure 2B) compared to control group (Figure 2A) and baicalin alone group (Figure 2D). However, baicalin-treatment (Figure 2C) partially alleviated the above abnormal morphological signs compared to MG-infected group (Figure 2B). Additionally, ultrastructural analysis (Figure 3) revealed signs of apoptosis including mitochondrial swelling, chromatin condensation and membrane disruption in MG-infected group (Figure 3B) compared to control (Figure 3A) and baicalin alone group (Figure 3D), while baicalin-treatment (Figure 3C) significantly ameliorated apoptosis-like features in the lungs of chickens compared to model group (Figure 3B).
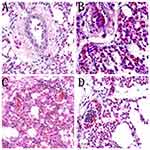 |
Figure 2 The photomicrographs of lung tissues stained with hematoxylin and eosin (40× magnification, scale bar=20 mm) are shown in Figure 2 (n=3). Red arrows show inflammatory cells infiltration and blue arrows show hemorrhagic spots in the lungs. Experimental groups including (A) control group, (B) MG infection group, (C) MG infection group treated with baicalin (450 mg/kg) and (D) baicalin alone treated group (450 mg/kg). |
Baicalin Attenuated Inflammation In Lungs Tissues
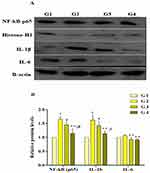
Figures 4 and 5 represent the mRNA and protein expression level of NF-κB and inflammation-related genes, respectively. It has been noted that MG infection causes a significant (p<0.05) increase in the mRNA of NF-κB, IL-6, IL-1β, TNF-α, inducible nitric oxide synthase (iNOS) and cyclooxygenase-1 (Cox-1) mRNA expression compared to control group and baicalin alone group, while the increase in interferon gamma (IFN-γ), prostaglandin E synthase (PTGE) and cyclooxygenase-2 (Cox-2) mRNA expression in MG infection group is not statistically significant (p>0.05) compared to control group and baicalin alone group. In addition, a significant (p<0.05) increase has been noted in NF-κB and IL-1β protein expression level in MG infection group compared to control group, except IL-6 (p>0.05). At the same time, baicalin-treatment significantly (p<0.05) reduced the mRNA expression of NF-κB, IL-6, IL-1β, TNF-α, iNOS and Cox-1 gene compared to MG infection group, except IFN-γ, PTGE and Cox-2 mRNA expression, where the reduction is not statistically significant (p>0.05) compared to MG infection group. The protein expression levels of NF-κB, IL-1β and IL-6 were also significantly (p<0.05) reduced with baicalin-treatment compared to MG infection group.
Suppression Of MG-Induced Apoptosis By Baicalin
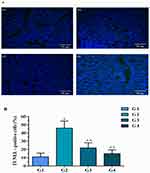
Apoptosis was examined in chicken lungs by TUNEL assay (as shown in Figure 6). The number of positive-stained nuclei was significantly (p<0.05) increased in MG infection group (Figure 6G2) compared to control (Figure 6G1) and baicalin alone group (Figure 6G4) in chicken lungs. Intriguingly, these results are in consistence with ultrastructural analysis that MG infection induced apoptosis in chicken lungs. However, baicalin intervention (Figure 6G3) significantly (p<0.05) reduced positive-stained apoptotic nuclei in chicken lungs compared to MG infection group (Figure 6G2).
Effect Of Baicalin And MG Infection On ATPase Activities
ATPase activities (as shown in Figure 7) were assessed in all experimental groups. It has been noted that Mg++-ATPase (p<0.05), Ca++ATPase (p<0.05) and Ca++-Mg++-ATPase (p<0.05) activities were significantly decreased in MG infection group compared to control group and baicalin alone group. However, the decrease in Na+-K+-ATPase activity was not statistically significant (p>0.05) in MG infection group. Importantly, baicalin-treatment significantly (p<0.05) restored MG-induced decrease in Mg++-ATPase, Ca++ATPase and Ca++-Mg++-ATPase activities compared to MG-infected group, except Na+-K+-ATPase (p>0.05) activity.
Baicalin Restored MG Infection-Mediated Alteration In Energy Metabolism Genes In Chicken Lungs
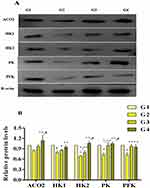
The effect of MG infection and baicalin alone and/or in combination were assessed at both mRNA (Figure 8) and protein expression level (Figure 9). Compared to control group and baicalin alone group, MG infection significantly (p<0.05) downregulated the mRNA expression of PFK, PK, ACO2, HK1, HK2 and LDHA gene, but the decrease in mRNA expression of SDHB and LDHB gene was not statistically significant as compared to control group and baicalin alone group. In addition, the protein expression level of PK, PFK, HK1 and HK2 was significantly (p<0.05) reduced compared to control group, except ACO2 (p>0.05). However, baicalin-treatment significantly (p <0.05) restored the mRNA expression of PFK, PK, ACO2, HK1, HK2 and LDHA compared to MG infection group. It has been noted that MG-induced decrease in mRNA expression of SDHB and LDHB gene was slightly improved (p>0.05) with baicalin-treatment. In addition, baicalin-treatment significantly (p<0.05) alleviated the decrease in protein expression level of PK, PFK, HK1 and HK2 enzyme in chicken lungs compared to MG infection group, except ACO2 (p>0.05) protein level.
Discussion
Studies demonstrated that MG-infected chickens are more susceptible to other infectious agents and leading to decrease production both in meat and eggs.33 The present study aimed to prevent the losses caused by MG infection. Our data indicated that MG infection induced histopathological and ultrastructural changes including increased inflammatory cells infiltrates, cellular and DNA damage in chicken lungs. In addition, the mRNA and protein expression results are in consistence with histopathological results that MG infection induced inflammation in chicken lungs. These findings are in line with previous studies.11,12,16 Researchers demonstrated that increased expression of cytokines was associated with inflammatory responses and gross inflammatory lesions during MG infection.34,35 Majumder et al (2016) explained that lipid-associated membrane proteins in MG is the primary cause of inflammation,16 while other researchers reported that cytoadherence property of MG is responsible to induce inflammation.7,36 The complex relationship among inflammatory chemokines and cytokines associated with lipid-associated membrane proteins and cytoadherence needs to be further investigated. Moreover, our data showed that MG infection induced apoptosis in chicken lungs. Extensive positive stained nuclei were examined in MG infection group. The protein level of apoptosis-related genes was significantly enhanced in the lungs of MG-infected chickens. These results are in agreement with earlier reports that MG infection sensitized host cells to apoptosis14 and impaired the structural integrity of chicken mucosal tissues.7,37,38 Hence, it could be speculated from these results that MG infection associated inflammation and apoptosis could affect energy metabolism in chicken lungs. Therefore, in the current study, we investigated the effect of MG infection on energy metabolism in chicken lungs.
Previous studies demonstrated various cell signaling pathways including immune responses, cell inflammation and cell transformation in connection with nitrogen, carbon and/or energy metabolism by shared enzymes signaling pathways and/or transcriptional regulators.39–42 Earlier studies mainly focused on host inflammatory responses, endosomal vesicle formation, cell survival, apoptosis and autophagy,43,44 while the effect of bacterial infections on energy metabolism and its underlying mechanism is rarely reported,17 which played a pivotal role in elucidating bacterial pathogenesis. Our results showed that MG infection causes a significant decrease in mRNA and protein expression level of energy metabolism-related genes, including HK1, HK2, ACO2, PFK, SDHB and PK. Additionally, ATPase activities were significantly reduced in the lungs of chickens infected with MG. The reduction in ATPase activities and mRNA and protein level of energy metabolism-related genes could possibly be associated with energy metabolism dysfunction in the chicken lungs. These findings showed that MG infection could cause energy metabolism dysfunction. Accumulating evidences showed that bacterial infection impaired/weaken host energy metabolism,45 and caused impairment in the normal homeostasis of the cells leading to the activation of various cellular pathways associated with apoptosis, inflammation and autophagy.46,47 However, it would be enthralling to prevent energy metabolism dysfunction during MG infection by natural or chemical means. In the present study, the effect of baicalin on MG-induced energy metabolism dysfunction was scrutinized.
Our previous study demonstrated that baicalin efficiently inhibited oxidative stress and apoptosis through the opposite modulation of Nrf2 and NF-κB pathway in the spleen of chickens during MG infection.24 Gong et al. (2017) reported that baicalin has excellent pharmacological properties associated with its ability to interact with numerous cell signaling pathways.20 For instance, NF-κB may exacerbate the inflammatory responses through the excessive production of inflammatory mediators and could lead to immune dysregulation,48 while baicalin-treatment suppressed lipopolysaccharide-induced inflammation through the NF-κB signaling pathway.23 Previously, Meng et al. (2013) studied the preventive effects of baicalin against Mycoplasma pneumonia infection.49 In the current study, we reported the effects of baicalin against MG infection-mediated inflammation, apoptosis and energy metabolism dysfunction. Ultrastructural and histopathological examination revealed that MG-induced inflammatory cells infiltrate and abnormal morphological alterations were partially disappeared in the lungs of chicken with baicalin-treatment. Inflammatory markers and NF-κB were significantly suppressed at both mRNA and protein level. In addition, baicalin intervention reduced apoptosis in the chicken lungs. TUNEL assay positive-stained nuclei and apoptosis-related protein expression levels were significantly reduced in MG-infected chickens with baicalin-treatment. More importantly, baicalin improved ATPase enzyme activities in chicken lungs. The level of energy metabolism-related genes was promoted at both mRNA and protein expression level. From these findings, it has been suggested that baicalin confers MG-induced inflammation and apoptosis in the chicken lungs, and could prevent energy metabolism dysfunction. However, the complete molecular mechanism is still unknown.
Conclusion
In conclusion, MG infection induced inflammation and apoptosis in chicken lungs. ATPase activities and the expression of energy metabolism-related genes were significantly reduced in the lungs of MG-infected chickens, while baicalin-treatment significantly ameliorated MG-induced inflammation, apoptosis and energy metabolism dysfunction in chicken lungs. Nevertheless, higher studies are needed to scrutinize the inhibitory mechanisms of baicalin and the complex relationship among energy metabolism dysfunction, inflammatory responses and apoptosis during MG infection.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31772801, 31973005). The first author “Muhammad Ishfaq” gives thanks to the teacher “Chao Fang” of the International College of Cultural Education (Northeast Agricultural University) for his encouragement and support during this study in People’s Republic of China.
Disclosure
All the authors have no potential conflicts of interests regarding the publication of this manuscript.
References
1. Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156.
2. Ley DH, Berkhoff JE, McLaren JM. Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis. 1996;40:480–483. doi:10.2307/1592250
3. Ley DH. Mycoplasma gallisepticum infection. In: Saif YM, editor. Diseases of Poultry.
4. Stipkovits L, Egyed L, Palfi V, et al. Effect of low-pathogenicity influenza virus H3N8 infection on Mycoplasma gallisepticum infection of chickens. Avian Pathol. 2012;41:51–57. doi:10.1080/03079457.2011.635635
5. Pflaum K, Tulman ER, Beaudet J, et al. Global changes in Mycoplasma gallisepticum phase-variable lipoprotein gene vlhA expression during in vivo infection of the natural chicken host. Infect Immun. 2016;84:351–355. doi:10.1128/IAI.01092-15
6. Mohammed J, Frasca S
7. Dykstra MJ, Levisohn S, Fletcher OJ, et al. Evaluation of cytopathologic changes induced in chicken tracheal epithelium by Mycoplasma gallisepticum in vivo and in vitro. Am J Vet Res. 1985;46:116–122.
8. Nunoya T, Tajima M, Yagahashi T, et al. Natural case of salpingitis apparently caused by Mycoplasma gallisepticum in chickens. Avian Pathol. 1997;26:391–398. doi:10.1080/03079459708419221
9. Tajima M, Nunoya T, Yagihashi T. An ultrastructural study on the interaction of Mycoplasma gallisepticum with the chicken tracheal epithelium. Am J Vet. 1979;40:1009–1014.
10. Gaunson JE, Philip CJ, Whithear KG, et al. Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology. 2000;146:1223–1229.
11. Javed MA, Frasca S
12. Rottem S. Interaction of mycoplasmas with host cells. Physiol Rev. 2003;83:417–432. doi:10.1152/physrev.00030.2002
13. Chambaud I, Wroblewski H, Blanchard A. Interactions between mycoplasma lipoproteins and the host immune system. Trends Microbiol. 1999;7:493–499.
14. Sokolova IA, Vaughan AT, Khodarev NN. Mycoplasma infection can sensitize host cells to apoptosis through contribution of apoptotic-like endonuclease(s). Immunol Cell Biol. 1998;76:526–534. doi:10.1046/j.1440-1711.1998.00781.x
15. Liu M, Du G, Liu B, et al. Cholesterol exacerbates Mycoplasma hyopneumoniae-induced apoptosis via stimulating proliferation and adhesion to porcine alveolar macrophages. Vet Microbiol. 2017;211:112–118. doi:10.1016/j.vetmic.2017.10.007
16. Majumder S, Silbart LK. Interaction of Mycoplasma gallisepticum with chicken tracheal epithelial cells contributes to macrophage chemotaxis and activation. Infect Immun. 2016;84:266–274. doi:10.1128/IAI.01113-15
17. Eisenreich W, Heesemann J, Rudel T, et al. Metabolic host responses to infection by intracellular bacterial pathogens. Front Cell Infect Microbiol. 2013;3:24. doi:10.3389/fcimb.2013.00024
18. Cao C, Fan R, Zhao J, et al. Impact of exudative diathesis induced by selenium defciency on LncRNAs and their roles in the oxidative reduction process in broiler chick veins. Oncotarget. 2017;8:20695–20705. doi:10.18632/oncotarget.14971
19. Siemsen DW, Kirpotina LN, Jutila MA, et al. Inhibition of the human neutrophil NADPH oxidase by Coxiella burnetii. Microbes Infect. 2009;11:671–679. doi:10.1016/j.micinf.2009.04.005
20. Gong WY, Zhao ZX, Liu BJ, et al. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur J Med Chem. 2017;126:844–852. doi:10.1016/j.ejmech.2016.11.058
21. de Oliveira MR, Nabavi SF, Habtemariam S, et al. The effects of baicalein and baicalin on mitochondrial function and dynamics: a review. Pharmacol Res. 2015;100:296–308. doi:10.1016/j.phrs.2015.08.021
22. Fu S, Liu H, Xu L, et al. Baicalin modulates NF-κB and NLRP3 inflammasome signaling in porcine aortic vascular endothelial cells infected by Haemophilus parasuis Causing Glässer’s disease. Sci Rep. 2018;8:807. doi:10.1038/s41598-018-19293-2
23. Cheng P, Wang T, Li W, et al. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front Pharmacol. 2017;8:547. doi:10.3389/fphar.2017.00547
24. Ishfaq M, Chen C, Bao J, et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-κB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult Sci. 2019. doi:10.3382/ps/pez406
25. Lu Z, Xie D, Chen Y, et al. TLR2 mediates autophagy through ERK signaling pathway in Mycoplasma gallisepticum-infected RAW264.7 cells. Mol Immunol. 2017;87:161–170. doi:10.1016/j.molimm.2017.04.013
26. Muhammad I, Sun X, Wang H, et al. Curcumin successfully inhibited the computationally identified CYP2A6 enzyme-mediated bioactivation of aflatoxin B1 in arbor acres broiler. Front Pharmacol. 2017;8:143. doi:10.3389/fphar.2017.00143
27. Shi Q, Wang W, Chen M, et al. Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response. Sci Total Environ. 2019;659:354–362. doi:10.1016/j.scitotenv.2018.12.375
28. Wang H, Muhammad I, Li W, et al. Sensitivity of arbor acres broilers and chemoprevention of aflatoxin B1-induced liver injury by curcumin, a natural potent inducer of Phase-II enzymes and Nrf2. Environ Toxicol Pharmacol. 2018;59:94–104. doi:10.1016/j.etap.2018.03.003
29. Wang S, Xu Z, Yin H, et al. Alleviation mechanisms of selenium on cadmium-spiked in chicken ovarian tissue: perspectives from autophagy and energy metabolism. Biol Trace Elem Res. 2018b;186:521–528.
30. Muhammad I, Wang H, Sun X, et al. Dual role of dietary curcumin through attenuating afb1-induced oxidative stress and liver injury via modulating liver phase-I and phase-II enzymes involved in AFB1 bioactivation and detoxification. Front Pharmacol. 2018;9:554.
31. Wang J, Yi M, Chen X, et al. Effects of colistin on amino acid neurotransmitters and blood-brain barrier in the mouse brain. Neurotoxicol Teratol. 2016;55:32–37.
32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 2001;25:402–408. doi:10.1006/meth.2001.1262
33. Pennycott TW, Dare CM, Yavari CA, et al. Mycoplasma sturni and Mycoplasma gallisepticum in wild birds in Scotland. Vet Rec. 2005;156:513–515.
34. Shimizu T, Kida Y, Kuwano K. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through toll-like receptors. Immunology. 2004;113:121–129. doi:10.1111/j.1365-2567.2004.01937.x
35. Shimizu T, Kida Y, Kuwano K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kappa B through TLR1, TLR2, and TLR6. J Immunol. 2005;175:4641–4646. doi:10.4049/jimmunol.175.7.4641
36. Papazisi L, Frasca S
37. Levisohn S. Early stages in the interaction between Mycoplasma gallisepticum and the chick trachea, as related to pathogenicity and immunogenicity. Isr J Med Sci. 1984;20:982–984.
38. Manafi M, Pirany N, Noor Ali M, et al. Experimental pathology of T-2 toxicosis and mycoplasma infection on performance and hepatic functions of broiler chickens. Poult Sci. 2015;94:1483–1492. doi:10.3382/ps/pev115
39. Hsu PP, Sabatini DM. Cancer cell metabolism: warburg and beyond. Cell. 2008;134:703–707. doi:10.1016/j.cell.2008.08.021
40. Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11:81. doi:10.1038/nri2922
41. Andersen JL, Kornbluth S. The tangled circuitry of metabolism and apoptosis. Mol Cell. 2013;49:399–410. doi:10.1016/j.molcel.2012.12.026
42. O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudostarvation. Nature. 2013;493:346–355. doi:10.1038/nature11862
43. Manger ID, Relman DA. How the host ‘sees’ pathogens: global gene expression responses to infection. Curr Opin Immunol. 2000;12:215–218. doi:10.1016/S0952-7915(99)00077-1
44. Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol. 2005;3:281–294. doi:10.1038/nrmicro1126
45. Eisenreich W, Dandekar T, Heesemann J, et al. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Microbiol. 2010;8:401–412. doi:10.1038/nrmicro2351
46. Wang H, Wang L, Zhang Y, et al. Inhibition of glycolytic enzyme hexokinase II (HK2) suppresses lung tumor growth. Cancer Cell Int. 2016;16:9. doi:10.1186/s12935-016-0280-y
47. Liu L, Gong L, Zhang Y, et al. Glycolysis in Panc-1 human pancreatic cancer cells is inhibited by everolimus. Exp Ther Med. 2013;5:338–342. doi:10.3892/etm.2012.787
48. Byun EB, Sung NY, Byun EH, et al. The procyanidin trimer C1 inhibits LPS-induced MAPK and NFkappaB signaling through TLR4 in macrophages. Int Immunopharmacol. 2013;15:450–456. doi:10.1016/j.intimp.2012.11.021
49. Meng Y, Huo J, Lu W, et al. Modulation of P1 and EGF Expression by Baicalin. Int J Mol Sci. 2013;14:146–157. doi:10.3390/ijms14010146
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.