Back to Journals » OncoTargets and Therapy » Volume 15
Anlotinib Hydrochloride and PD-1 Blockade as a Salvage Second-Line Treatment in Patients with Progress of Local Advanced Non-Small Cell Lung Cancer in Half a Year After Standard Treatment
Authors Yu C, Jiang L, Yang D, Dong X, Yu R, Yu H
Received 4 July 2022
Accepted for publication 1 October 2022
Published 17 October 2022 Volume 2022:15 Pages 1221—1228
DOI https://doi.org/10.2147/OTT.S380615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Chengqi Yu,1 Leilei Jiang,2 Dan Yang,2 Xin Dong,2 Rong Yu,2 Huiming Yu2
1School of Basic Medical Science, Capital Medical University, Beijing, 100069, People’s Republic of China; 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Radiation Oncology, Peking University Cancer Hospital and Institute, Beijing, People’s Republic of China
Correspondence: Huiming Yu, Department of Radiation Oncology, Peking University Cancer Hospital & Institute, 52# Fucheng Road, Haidian District, Beijing, 100142, People’s Republic of China, Tel +86 13699249320, Fax +86 10-59300192, Email [email protected]
Purpose: As for local advanced non-small cell lung cancer (NSCLC), synchronous radiotherapy and chemotherapy is the standard treatment mode. But for patients with progress in half a year, which means the second-line chemotherapy effect is not ideal for them. We observed the efficacy and safety of anlotinib hydrochloride combined with PD-1 blockade as the second-line treatment for those patients in this trial.
Patients and Methods: From January 2018 to December 2019, 57 patients with the progress of local advanced NSCLC treated with anlotinib plus PD-1 blockade until disease progression or intolerance as a result of adverse events. Patients have been assessed using computed tomography prior to treatment and during follow-up every 2 months until disease progression or death. The primary endpoint was objective response rate (ORR). The secondary endpoints included overall survival (OS), progression-free survival (PFS) and safety. Survival curves were created using the Kaplan–Meier method.
Results: 57 patients were enrolled. The median age was 64 years, and 61.4% of the patients were men. The ORR was 50.9% with a median OS time of 14 months and the 1-year OS rates and PFS rates were 81.8% and 33.3%, respectively. The patients with squamous cell carcinoma, no brain or liver metastases had longer PFS than patients with liver metastasis. When the PFS was calculated from the time of second treatment, the median PFS was 9 months. Most adverse events (AEs) were grade 1– 3, one drug-related death was noted.
Conclusion: The expected outcome of this study is that anlotinib combined with PD-1 blockade has tolerable toxicity and better ORR, OS than second-line chemotherapy. The results may indicate additional treatment options for patients with progress of local advance NSCLC in half a year after standard treatment.
Keywords: anlotinib, PD-1 blockade, local advanced non-small cell lung cancer, angiogenesis, immunotherapy
A Letter to the Editor has been published for this article.
Introduction
Lung cancer is the leading incident cancer and cause of cancer mortality worldwide.1 Non-small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancer cases. About 30% of lung cancer patients are locally advanced lung cancer at the initial diagnosis.2,3 For stage I to III NSCLC treated with surgery or radiotherapy with curative intent, the current literature does not provide evidence that suggests a survival benefit from adding immunotherapy (excluding checkpoint inhibitors) to conventional curative surgery or radiotherapy, but immune checkpoints inhibitors (PD-1/PD-L1) have made significant advances in lung cancer.4,5 For locally advanced NSCLC, synchronous radiotherapy and chemotherapy is the standard of care, but some patients will make progress in half a year after chemoradiotherapy.6 For those patients, docetaxel, pemetrexed, and checkpoint blockade blockers are considered standard second-line therapies based on several randomised controlled trials.7–10 As for those patients, the median PFS of second-line chemotherapy was only 3 months.
Anlotinib hydrochloride is a multitarget tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor, fibroblast growth factor receptor, platelet-derived growth factor receptor, c-Kit, and c-MET, which was approved as a third-line treatment for stage IV NSCLC on May 9, 2018 in China.11,12 However, the objective response rate (ORR) was only 9.2% and the overall survival (OS) time was prolonged only 5.4 months, which was better than the results of other line treatment schemes. Immune checkpoint inhibitors (ICIs) are a novel class of drugs for the treatment of NSCLC.13,14 ICIs activate the host’s immune cells, especially T cells, to target specific tumor cells. Many Phase 3 clinical trials have confirmed anti-PD-1 treatments in advanced NSCLC patients, either as a second-line15–17 or first-line treatment,18 which provided improved efficacy and survival, but not increased the infection risk.19 Compared with second-line docetaxel chemotherapy, treatment with nivolumab or pembrolizumab provided a better ORR and OS.20–22The combination of anlotinib hydrochloride and Immune checkpoint inhibitors is effective and well-tolerated in patients with NSCLC.23 Therefore, we assessed the efficacy and safety of anlotinib combined with immune checkpoint inhibitors as salvage treatment in patients with progress of local advance NSCLC in half a year after standard treatment and investigated the predictors of therapeutic efficacy.
Materials and Methods
Patients and Methods
This clinical study was designed to evaluate the efficacy and safety of anlotinib and immunotherapy in patients with progress of local advanced NSCLC after standard treatment. From January 2018 to December 2019, 57 patients were included in this study in Peking University Cancer Hospital (PUCH). This study was approved by the Research Ethics Committee of PUCH complies with the Declaration of Helsinki. Patients’ demographics and clinical characteristics such as age, sex, smoking history, and performance status were reviewed using a lung cancer cohort. All patients received radiological assessment of tumor response by computed tomography (CT) every 8 weeks. The response to therapy was assessed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The confirmation of treatment results were response to immunotherapy and anlotinib. PFS was defined as the time from the start of treatment to disease progression or death. OS was defined as time from the start of treatment to death from any cause. Liver and kidney function, blood routine test, myocardial enzyme spectrum and thyroid function were reviewed before each immunotherapy. The safety of treatment was assessed using Common Terminology Criteria for Adverse Events version 4.0.
Treatment
All patients received anlotinib (one cycle of 12 mg daily for 14 days, discontinued for 7 days, and repeated every 21 days). Doses were reduced when patients experienced intolerable adverse events (AEs). Patients continued anlotinib until disease progression or intolerance as a result of AEs. Immunotherapy (tislelizumab, 200 mg/3 week; or toripalimab injection, 200 mg/3week) was administered intravenously every 3 weeks until withdrawal of consent, unacceptable toxicity, disease progression at the discretion of the physician, or for 1 year.
Statistical Analysis
Statistical analysis was processed using SPSS version 22.0. The Overall Survival curves and PFS were created using the Kaplan–Meier method using the Log rank test, and a Cox proportional hazards model was used for multivariable survival analysis. Objective response rate and disease control rate was compared using Pearson χ2or Fisher exact test when appropriate. Statistical significance was defined as P < 0.05.
Results
As a result, a total of 57 patients were included in this study. Clinical characteristics of initial treatment are detailed in Table 1, indicating that 30 patients (52.6%) were initially diagnosed in stage IIIA, while the others were stage IIIB. Tislelizumab injection were used in 27 patients. No patient was lost during the follow-up. The median follow-up time was 18 months for the surviving patients and 14 months for the whole group. 40 patients died by the end of the follow-up.
 |
Table 1 Patient Characteristics |
The objective effective rate is shown in Table 2. There was a complete response in 17 (29.8%) patients, a partial response in 12 (21.1%) patients, making an ORR of 50.9%. Table 3 shows the case data after progression. In total, 4 patients had known brain metastases, 30 patients recurred in primary tumor and nodal areas, 14 patients failed in distant areas, 13 patients failed in new nodal areas, and 4 patients failed in liver. The clinical stage of 16 patients was improved compared with the initial treatment.
 |
Table 2 Treatment Response |
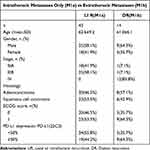 |
Table 3 Stage at Recurrence |
For all patients, the median PFS and OS were 10 and 14 months, respectively. The survival curves were depicted in Figure 1. the 1-year OS rates was 81.8%. As shown in Figure 2, the progression-free survival rate for 1 year was 33.3% and the median time of treatment failure is 9 months.
 |
Figure 1 The overall survival rate for patients. |
 |
Figure 2 The progression-free survival rate for patients. |
The side effects of treatment are given in Table 4, Two patients (3.5%) were off treatment due to toxicity, one patient was grade 5. Drug-related adverse events were reported in 38 patients (66.7%). Typical toxicities included hypertension (36.8%), thyroid-stimulating hormone (26.3%), hand-foot syndrome (35.1%), fatigue (22.8%), impairment of liver function and kidney function (17.5%).
 |
Table 4 Treatment-Related Adverse Events |
Discussion
Lung cancer, which is the most common type of cancer worldwide24 has historically been associated with poor outcomes. At present, a large number of clinical studies have confirmed that immunotherapy alone, combined with chemotherapy in the treatment of lung cancer has good effects.25–28 However, identifying the patients in this population who are most likely to achieve long-term survival with immunotherapy is a key challenge. Associations of baseline characteristics and biomarker status with overall survival in previously treated patients who received PD-1 or PD-L1 inhibitor therapy for unresectable local advanced NSCLC have been explored previously.25–27 The PACIFIC study have proved that durvalumab consolidation after standard therapy showed superior PFS and OS compared to the observation group in a real world setting and randomization clinical trial.29 Therefore, immunotherapy as a second line therapy may be effective. It has been reported that second-line immunotherapy is effective in non-small cell lung cancer, which is better than that of second-line single drug chemotherapy and it’s PFS was 12 months, which is better than single drug chemotherapy.30–32
Anti-angiogenic therapy combined with immunotherapy has been proved to be a good combination therapy mode. Anti-angiogenic drugs can change the immune microenvironment, which is conducive to immunotherapy. As a multi-target and small molecule anti-angiogenic drug, anlotinib has shown good efficacy in the second-line treatment of non-small cell lung cancer.11 The research using first-line treatment of non-small cell lung cancer with anti-angiogenesis drugs show that anti-angiogenesis drugs have a good foundation in changing the immune microenvironment, which is also the theoretical basis of combined therapy. As a domestic approved small molecule multi-target tyrosine kinase inhibitor, anlotinib plays an important role in inhibiting angiogenesis and anti-tumor. Combined with immunotherapy, it can change the immune microenvironment as well as inhibiting angiogenesis in animal trial.33
The results showed that patients with advanced NSCLC who received anlotinib and immunotherapy as second-line had better OS, PFS, and objective response rate compared with patients who received second chemotherapy.34,35 This therapy mode was well tolerated, and the patients maintained a reasonable quality of life. The objective response rate was significantly higher than previous report treated with second line chemotherapy.21,36
To our knowledge, this study is the first Phase 2 trial in the second-line treated anlotinib and immunotherapy to show an OS and PFS benefit. This study has shown that liver metastases might be negative predictors of OS in lung cancer patients. For example, we know that the median PFS and OS of second-line chemotherapy in the treatment of non-small cell lung cancer were only 5.7 and 13.6 months.37 As for the second-line treatment, the previous study in patients with locally advanced NSCLC has evaluated the effects of immunotherapy and show that the median PFS and OS were ranged from 5 to 8.9 months and from 9.3 to 16 months in different cohorts.30,31 For locally advanced non-small cell lung cancer that progresses within half a year after standard radiotherapy and chemotherapy, no matter second-line chemotherapy or immunotherapy, it can not obtain good clinical efficacy. As a small molecule anti-angiogenesis targeted drug, anlotinib is effective in the second-line or third-line treatment of non-small cell lung cancer.11,38,39 In previous studies, anlotinib and immune drugs were used in the first-line treatment of non-small cell lung cancer and found to be effective,39 but this treatment combination model is not the standard treatment model after all. In order to improve the efficacy of second-line treatment of non-small cell lung cancer, the combination of anlotinib and immune drugs was used in this study. Our results showed that the combination of anlotinib and immunotherapy had good efficacy, with median PFS and OS reaching 10 and 14 months respectively, which is significantly higher than that reported in the previous literature for second line chemotherapy.21,34
Regarding toxicity in study, the overall incidence of treatment-related AEs was 66.7%, The most common AEs during treatment were hypertension(36.8%),which is higher than that of previous reported.40,41 Hypertension is a common adverse effect in patients treated with VEGF-targeted agents. The other common AEs were thyroid-stimulating hormone (26.3%), hand-foot syndrome (35.1%), Fatigue (22.8%), Liver function and kidney function (17.5%). Only one patient was found to death due to treatment. In addition, only two patients off treatment due to oral mucositis. Compared to the AEs associated with anlotinib and immunotherapy reported in previous studies,23,42 no new AEs were observed in the present study. Our results show that anlotinib and immunotherapy is well tolerated in patients with the locally advanced NSCLC.
The limitation of this study is the retrospective analysis of small number of enrolled cases, but it is still the first one trying to evaluate the efficacy of arotinib and immunotherapy as the second-line in the treatment of lung cancer. This study has shown that anlotinib combined with PD-1 blockade has tolerable toxicity and better result than second-line chemotherapy. PFS should be chosen as the primary endpoint in the further research which helped to provide a more rigorous and comprehensive conclusion while the protocol also needs to be verified by multicenter prospective clinical studies.
Conclusion
In this study we demonstrated that anlotinib combined with PD-1 blockade as a salvage second-line treatment has good clinical efficacy and high safety than that of second-line chemotherapy in the treatment of patients with the progress of local advanced non-small cell lung cancer in half a year after standard treatment.
Ethics Approval and Informed Consent
This research was approved by the ethics committee of the Ethics Committee of Peking University Cancer Hospital and Institute. All participants are informed consent to the study.
Consent for Publication
All authors confirm that the details of any images, videos, recordings, etc can be published, and that the person(s) providing consent have been shown the article contents to be published.
Acknowledgments
We are thankful to the investigators and patients enrolled in this clinical trial.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81672969, 81472814), a grant from the Health scientific and technological achievements and appropriate technology promotion project of Beijing Municipal Health Commission.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: globocan 2008. Int J Cancer. 2010;127:2893–2917. doi:10.1002/ijc.25516
2. Ahmedin Jemal EM, Ward CJ, Johnson KA, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109(9):djx030.
3. Weinstein L, Ana Stefancic AT, Cummingham KE, Hurley LC, Wender R. Cancer screening, prevention, and treatment in people with mental illness. CA Cancer J Clin. 2016;66(2):133–151. doi:10.3322/caac.21334
4. Zhu J, Li R, Tiselius E, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database System Rev. 2017;12:CD011300. doi:10.1002/14651858.CD011300.pub2
5. Mohsenzadegan M, Peng R-W, Roudi R. Dendritic cell/cytokine-induced killer cell-based immunotherapy in lung cancer: what we know and future landscape. J Cell Physiol. 2020;235(1):74–86. doi:10.1002/jcp.28977
6. Criss SD, Mooradian MJ, Sheehan DF, et al. Cost-effectiveness and budgetary consequence analysis of durvalumab consolidation therapy vs no consolidation therapy after chemoradiotherapy in stage III non–small cell lung cancer in the context of the US health care system. JAMA Oncol. 2019;5(3):358–365. doi:10.1001/jamaoncol.2018.5449
7. Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095–2103. doi:10.1200/JCO.2000.18.10.2095
8. Hanna N, Shepherd FA, Fossella FV, et al. Randomized Phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589–1597. doi:10.1200/JCO.2004.08.163
9. Tassinari D, Drudi F, Lazzari-Agli L, Tombesi P, Sartori S. Second-line treatments of advanced non-small-cell lung cancer: new evidence for clinical practice. Ann Oncol. 2010;21(2):428–429. doi:10.1093/annonc/mdp538
10. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi:10.1056/NEJMoa1507643
11. Han B, Kai L, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 Phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
12. Cheng Y, Han B, Kai L, et al. Effect of anlotinib as a third‐ or further‐line therapy in advanced non‐small cell lung cancer patients with different histologic types: subgroup analysis in the ALTER0303 trial. Cancer Med. 2020;9(8):2621–2630. doi:10.1002/cam4.2913
13. Filippi AR, Di Muzio J, Badellino S, Mantovani C, Ricardi U. Locally-advanced non-small cell lung cancer: shall immunotherapy be a new chance? J Thorac Dis. 2018;10:S1461–S1467. doi:10.21037/jtd.2017.12.53
14. Jabbour SK, Berman AT, Simone CB. Integrating immunotherapy into chemoradiation regimens for medically inoperable locally advanced non-small cell lung cancer. Transl Lung Cancer Res. 2017;6(2):113–118. doi:10.21037/tlcr.2017.04.02
15. Zhang Y, Zhou H, Zhang L. Which is the optimal immunotherapy for advanced squamous non-small-cell lung cancer in combination with chemotherapy: anti-PD-1 or anti-PD-L1? J Immunother Cancer. 2018;6(1):135. doi:10.1186/s40425-018-0427-6
16. Takada K, Toyokawa G, Okamoto T, et al. A comprehensive analysis of programmed cell death ligand-1 expression with the clone SP142 antibody in non-small-cell lung cancer patients. Clin Lung Cancer. 2017;18(5):572–582. doi:10.1016/j.cllc.2017.02.004
17. Paz-Ares L, Kim TM, Vicente D, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a Phase 1 trial. J Thorac Oncol. 2020;15(7):1210–1222. doi:10.1016/j.jtho.2020.03.003
18. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi:10.1056/NEJMoa1917346
19. Petrelli F, Morelli AM, Luciani A, Ghidini A, Solinas C. Risk of infection with immune checkpoint inhibitors: a systematic review and meta-analysis. Target Oncol. 2021;16(5):553–568. doi:10.1007/s11523-021-00824-3
20. Goto K, Ohe Y, Shibata T, et al. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2016;17(8):1147–1157. doi:10.1016/S1470-2045(16)30104-8
21. Yi Y, Liu Z, Fang L, et al. Comparison between single-agent and combination chemotherapy as second-line treatment for advanced non-small cell lung cancer: a multi-institutional retrospective analysis. Cancer Chemother Pharmacol. 2020;86(1):65–74. doi:10.1007/s00280-020-04091-3
22. Lefebvre C, Martin E, Hendriks LEL, et al. Immune checkpoint inhibitors versus second line chemotherapy for patients with lung cancer refractory to first line chemotherapy. Respir Med Res. 2020;78:100788. doi:10.1016/j.resmer.2020.100788
23. Yang S, Zhang W, Chen Q, Qisen G. Clinical Investigation of the efficacy and safety of anlotinib with immunotherapy in advanced non-small cell lung cancer as third-line therapy: a retrospective study. Cancer Manag Res. 2020;12:10333–10340. doi:10.2147/CMAR.S280096
24. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
25. Leora Horn DR, Spigel EE, Vokes EH, et al. Nivolumab versus Docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–3933. doi:10.1200/JCO.2017.74.3062
26. Altorki NK, Markowitz GJ, Dingcheng Gao JL, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19(1):9–31. doi:10.1038/s41568-018-0081-9
27. Herbst RS, Baas P, Perez-Gracia JL, et al. Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial. Ann Oncol. 2019;30(2):281–289. doi:10.1093/annonc/mdy545
28. von Pawel J, Bordoni R, Satouchi M. Long-term survival in patients with advanced nonsmall-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer. 2019;107:124–132. doi:10.1016/j.ejca.2018.11.020
29. Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(12):1670–1680. doi:10.1016/S1470-2045(19)30519-4
30. Chen M, Li Q, Xu Y, et al. Immunotherapy as second-line treatment and beyond for non-small cell lung cancer in a single center of China: outcomes, toxicities, and clinical predictive factors from a real-world retrospective analysis. Thorac Cancer. 2020;11(7):1955–1962. doi:10.1111/1759-7714.13488
31. Geraci E, Chablani L, Geraci E. Immunotherapy as a second-line or later treatment modality for advanced non-small cell lung cancer: a review of safety and efficacy. Crit Rev Oncol Hematol. 2020;152:103009. doi:10.1016/j.critrevonc.2020.103009
32. Vickers AD, Winfree KB, Cuyun Carter G, et al. Relative efficacy of interventions in the treatment of second-line non-small cell lung cancer: a systematic review and network meta-analysis. BMC Cancer. 2019;19(1):353. doi:10.1186/s12885-019-5569-5
33. Gao Q, Tang S, Chen H, et al. Intratumoral injection of anlotinib hydrogel enhances antitumor effects and reduces toxicity in mouse model of lung cancer. Drug Deliv. 2020;27(1):1524–1534. doi:10.1080/10717544.2020.1837292
34. Crequit P, Chaimani A, Yavchitz A, et al. Comparative efficacy and safety of second-line treatments for advanced non-small cell lung cancer with wild-type or unknown status for epidermal growth factor receptor: a systematic review and network meta-analysis. BMC Med. 2017;15(1):193. doi:10.1186/s12916-017-0954-x
35. Barnfield PC, Ellis PM. Second-line treatment of non-small cell lung cancer: new developments for tumours not harbouring targetable oncogenic driver mutations. Drugs. 2016;76(14):1321–1336. doi:10.1007/s40265-016-0628-6
36. Durm G, Hanna N, Durm G. Second-line chemotherapy and beyond for non-small cell lung cancer. Hematol Oncol Clin North Am. 2017;31(1):71–81. doi:10.1016/j.hoc.2016.08.002
37. Wang L, He Z, Yang S, et al. The impact of previous therapy strategy on the efficiency of anlotinib hydrochloride as a third-line treatment on patients with advanced non-small cell lung cancer (NSCLC): a subgroup analysis of ALTER0303 trial. Transl Lung Cancer Res. 2019;8(5):575–583. doi:10.21037/tlcr.2019.09.21
38. Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised Phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654–661. doi:10.1038/bjc.2017.478
39. Zhong Y, Wei Q, Lu Y, et al. Efficacy and safety of anlotinib in patients with advanced non-small cell lung cancer. J Thorac Dis. 2020;12(10):6016–6022. doi:10.21037/jtd-20-2855
40. Tartarone A, Roviello G, Lerose R, Roudi R, Aieta M, Zoppoli P. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol. 2019;15(20):2423–2433. doi:10.2217/fon-2018-0868
41. Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;558:1–13.
42. Xiong Q, Qin B, Xin L, et al. Real-world efficacy and safety of anlotinib with and without immunotherapy in advanced non-small cell lung cancer. Front Oncol. 2021;11:659380. doi:10.3389/fonc.2021.659380
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
