Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Anlotinib Enhances the Therapeutic Effect of Bladder Cancer with GSDMB Expression: Analyzed from TCGA Bladder Cancer Database & Mouse Bladder Cancer Cell Line
Authors Wang C , Cao Q, Zhang S, Liu H, Duan H, Xia W, Shen H , Wang C
Received 30 November 2022
Accepted for publication 6 March 2023
Published 17 March 2023 Volume 2023:16 Pages 219—228
DOI https://doi.org/10.2147/PGPM.S398451
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Chen Wang,1,2 Qifeng Cao,2,* Shun Zhang,2,* Hailong Liu,2 Huangqi Duan,2 Weimin Xia,2 Haibo Shen,2 Cheng Wang1
1Department of Urology, The People’s Hospital of SND, Suzhou, People’s Republic of China; 2Department of Urology, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cheng Wang, Department of Urology, The People’s Hospital of SND, Suzhou, People’s Republic of China, Tel +86-15050163288, Email [email protected] Haibo Shen, Department of Urology, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China, Tel +86-18601712802, Email [email protected]
Introduction and Objective: The mitogen-activated protein kinase (MAPK) pathway is inhibited by the pan-target inhibitor Anlotinib, which induces tumor cell death. In addition to the common apoptosis and necrosis, there is also a pyroptosis mode of cancer cell death in recent years, which is mainly manifested by the cleavage of gasdermin proteins (GSDMs). Gasdermin B (GSDMB) participates in the progression and outcome of bladder cancer. The efficacy and mechanism of Anlotinib in the treatment of GSDMB-positive bladder tumors have not been studied to date.
Methods: The relationship between GSDMB expression and tumor stage, overall survival rate, immunotherapy response, tumor recurrence and progression rate was analyzed from the TCGA bladder cancer database. Anlotinib was used to treat GSDMB-positive bladder cancer in mice followed by flow analysis of the secretion of inflammatory factors related to pyroptosis and the level of anti-tumor factors. Western blot analysis detected which MAPK and MEK signal transduction pathways.
Results: TCGA data analysis showed that the overall survival rate of bladder cancer patients with high GSDMB expression was better than that of patients with low GSDMB expression. In vivo experiments showed that Anlotinib was more effective in the treatment of GSDMB-positive bladder cancer than GSDMB-negative bladder cancer. Anlotinib can increase the secretion of antitumor-related factors in GSDMB-positive bladder cancer such as TNF-a and CD107a. In addition, Anlotinib also induced an increase in GSDMB protein expression. Anlotinib treatment of GSDMB-positive bladder cancer decreased AKT and MEK protein expression, which were involved in Anlotinib signal transduction pathway.
Conclusion: Anlotinib has a strong antitumor effect on GSDMB-positive bladder tumors. This effect is mainly achieved by anlotinib stimulating the secretion of relevant antitumor factors by lymphocytes. The PI3K/AKT and MEK signal transduction pathways were inhibited by Anlotinib in bladder cancer expressing GSDMB protein.
Keywords: bladder cancer, Anlotinib, GSDMB, targeted therapy, pyroptosis
Introduction
Bladder cancer is the most common malignant disease of urogenital tumors in China, which seriously threatens human life and health. At present, the treatment of bladder cancer is mainly surgical treatment, supplemented by postoperative chemotherapy, immunotherapy or targeted therapy. Among them, targeted therapy has produced excellent benefits in the other two high-incidence tumors of the urogenital system (kidney cancer and prostate cancer) and has certain effectiveness in preventing postoperative recurrence, treating carcinoma in situ, preventing tumor progression, improving survival rate and prolonging survival time.
Tumor targeted therapy is the application of targeted drugs to specific gene mutations or biomarkers in patients, so as to achieve the purpose of inhibiting tumor growth or eliminating tumor cells. Fibroblast growth factor (FGF) can bind to fibroblast growth factor receptors 1–4 (FGFR1–4) tyrosine kinases, activating numerous intracellular processes and pathways.1 It has been proven that there are a large number of mutated Fibroblast growth factor receptor-3 (FGFR3) molecules in bladder cancer, which can be inhibited by mitogen-activated protein kinase (MAPK).2 Therefore, FGFRs can be novel actionable targets in treatment of bladder cancer. Recent studies on bladder cancer have shown that the mutation status of FGFR3 can be correlated with an immune-suppressing microenvironment and poor response to immunotherapy.3 However, there has not been much progress in targeted therapy for bladder cancer, and tyrosine kinase receptor inhibitors are a hot spot for targeted therapy drugs.4 Until 2019, the US Food and Drug Administration approved erdatinib, the first bladder cancer-targeting inhibitor, for the treatment of adult patients with locally advanced or metastatic bladder cancer with FGFR3 or FGFR2 gene changes and disease progression after platinum-based chemotherapy.5 Erdatinib is a once-daily oral pan-FGFR tyrosine kinase inhibitor and the first targeted therapy approved for metastatic bladder cancer.6 However, the drug requires testing whether patients have fusion mutations of FGFR2 and FGFR3, and its side effects are relatively large, which limits the application scope of this drug. Anlotinib, which is also a pan-target tyrosine kinase inhibitor, can cover more people with target mutations (including FGFR1-3 receptor mutations). Compared with erdatinib, its side effects are smaller, so it has a strong anti-tumor effect in various solid tumors.7 Anlotinib is a novel oral tyrosine kinase inhibitor that targets VEGFR1, VEGFR2/KDR, VEGFR3, C-Kit, PDGFR-α and fibroblast growth factor receptors (FGFR1, FGFR2 and FGFR3). The inhibition of the FGFR3 receptor is mainly through MAPK/ERK signaling pathway, which induces tumor cell death.
In addition to the common apoptosis and necrosis, there is also a pyroptosis mode of cancer cell death, which is mainly manifested by the cleavage of gasdermin protein (GSDM). Pyroptosis, also known as inflammatory cell necrosis, is a kind of programmed cell death, characterized by the expansion of cells until the cell membrane is broken, resulting in the release of cell content and activation of a strong inflammatory response. Pyroptosis is mainly mediated by the GSDM protein family. Some proteins of the cysteine aspartate protease family are activated by inflammasome, which causes GSDM protein cleavage and activation. The activated GSDM protein is translocated to the membrane, forming holes, cell swelling, cytoplasmic outflow, and finally leading to cell membrane rupture. Microscopically, pyroptosis cells show swelling with many vesicular projections. Pyroptosis is less swollen than necrotic cells. Under an electron microscope, it can be clearly seen that the pyroptotic cells form a large number of vesicles, namely pyroptosomes, before the rupture of the cytoplasmic membrane. Studies have shown that GSDMB protein can be activated by inflammatory factors such as Granzyme A (GZMA) and interferon-γ, thereby transforming normal cell apoptosis into pyroptosis.8
Materials and Methods
Searching for GSDMB Gene Related Information in TCGA Database
The distribution of GSDMB gene in tumor tissues and adjacent tissues was retrieved from TCGA bladder cancer database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga), and the relationship between GSDMB expression and tumor stage, overall survival rate, immunotherapy response, tumor recurrence and progression rate was analyzed.
Cell Transfection
The pLVX plasmid carrying GSDMB gene and lentivirus-packaged 293T cells were co-incubated, and the IMDM medium (12440053, Gibco) was changed 24 h after transfection with LIPO-2000 and incubated for another 24 h. Then the viral medium was co-cultured with MB49 bladder cancer cell line. After 72 h of puromycin screening, transfected positive cells were collected for Western-blot analysis to confirm the successful introduction of GSDMB gene into MB49 cell line.
Anlotinib Treatment
Anlotinib is obtained from Chia Tai Tianqing Company. C57BL/6 mice were divided into four groups (group 1: GSDMB-negative MB49 cells bearing + normal saline gavage; group 2: GSDMB-negative MB49 cells bearing + anlotinib gavage; group 3: GSDMB-positive MB49 cells bearing + normal saline gavage; Group 4: GSDMB-positive MB49 cells bearing + anlotinib gavage; bearing cell number was 5*10^4, dose of Anlotinib for using was 3 mg/kg). One week after the completion of tumor bearing, 2 weeks of constant gavage were performed, and the mice were sacrificed after 1 week of observation. Tumor volume was measured every 3 days (tumor volume formula V = π * width * width * length/6).
Flow Cytometry
To obtain single-cell suspensions from xenografted tumors, tumor tissues were dissociated with gentleMACS™ Dissociator (130-093-235, Miltenyi Biotec) and digested with tumor dissociation kit (Miltenyi Biotec, Germany) according to the manufacturer’s instruction. For stimulation, the cells were co-cultured with GolgiStop (1.0 μg/mL), phorbol myristate acetate (PMA) (1.0 μg/mL) and Ionomycin (10 μg/mL) (Sigma-Aldrich, Merck, Germany) for 4 h. For staining, anti-mouse CD3 (Catalogue 563123, BD Biosciences), anti-mouse CD4 (Catalogue 563331, BD Biosciences), anti-mouse CD8 (Catalogue 552877, BD Biosciences), anti-mouse GranzymeA (clone GzA-3G8.5, eBiosciences), anti-mouse GranzymeB (clone NGZB, Invitrogen), anti-mouse IL-2 (clone JES6-5H4, BD Biosciences), anti-mouse IL-10 (clone JES5-16E3, eBiosciences), anti-mouse TNF-a (clone MP6-XT22, BioLegend), anti-mouse CD107-a (clone 1D4B, BD Biosciences), anti-mouse PD-L1 (clone MIH5, BD Biosciences) and anti-mouse Ki67 (clone B56, BD Biosciences) were used. Samples were then detected by BD LSR Fortessa flow cytometer.
Western Blots Analysis
The protein samples were heated and inactivated, then separated by gel electrophoresis. After transmembrane, the membranes were blocked with 5% BSA for 1 h, followed by incubation with different primary antibodies at 4°C for 12 h. The primary antibodies used in the study included GSDMB (ab215729, Abcam), MEK (8727, Cell Signaling Technology), p-MEK (9154, Cell Signaling Technology), AKT (4691, Cell Signaling Technology), p-AKT (13038, Cell Signaling Technology). The membrane was further incubated with conjugated secondary antibodies and washed 6 times in TBST (5 min each time). Finally, the membrane was incubated with HRP-conjugated antibodies and detected by the ECL System (Millipore).
Immunochemical Staining
Paraffin-embedded tissue sections were used for immunochemical staining. Antibodies for immunochemical staining were as follows: FGFR1 (PA5-25979, Thermo Fisher Scientific), FGFR2 (PA1-24763, Thermo Fisher Scientific), FGFR3 (MA5-32620, Thermo Fisher Scientific), FGFR4 (PA5-119550, Thermo Fisher Scientific).
Statistics
All statistical analyses were performed by SPSS v.21 (IBM SPSS Software, USA). A one-way analysis of variance was performed for the comparison of tumor weight, cytokines between four groups and tumor stage. Survival analysis was performed for the Overall Survival. P < 0.05 was considered a significant statistical difference.
Results
The Overall Survival Rate of Bladder Cancer Patients with High GSDMB Expression is Better Than That of Patients with Low GSDMB Expression
By searching 412 cases of bladder cancer gene and clinical information in the TCGA database, it was found that the overall survival rate of bladder cancer patients was strongly positively correlated with the expression of GSDMB gene. The overall survival rate of bladder cancer patients with high GSDMB gene expression is better than that of patients with low GSDMB gene expression (P=0.00088), and the expression level in tumor tissue is much higher than that in normal tissue (P=0.007). In addition, GSDMB gene expression was closely correlated with age and tumor stage, with higher gene expression in patients younger than 60 years with early stage bladder cancer (Figure 1).
 |
Figure 1 Clinical manifestation of GSDMB gene. (A) Correlation between GSDMB gene expression and overall survival rate; (B) correlation of GSDMB gene expression with age and tumor stage. |
Anlotinib is More Effective in the Treatment of GSDMB-Positive Bladder Cancer Than GSDMB-Negative Bladder Cancer
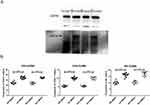
We have successfully imported the GSDMB gene into the GSDMB-negative MB49 bladder cancer cell line, transformed it into a GSDMB-positive bladder cancer cell line, and carried out WB verification. The results showed that the GSDMB gene could be expressed in the MB49 cell line after transfection (Figure S1; Detection of GSDMB protein before and after GSDMB gene transfection). Tumor-bearing mice were treated with saline or anlotinib. One week after the end of gavage, the mice were sacrificed, and the bladder cancer tissues were weighed for comparison. The experiment was repeated three times. The results showed that there was no significant difference in tumor volume and weight between group 1 and group 3, while there was a significant difference in tumor volume and weight between group 2 and group 4 (P<0.05). Anlotinib was more effective in the treatment of GSDMB-positive bladder cancer than GSDMB-negative bladder cancer. Treatment without Anlotinib did not induce tumor growth inhibition (Figure 2).
Anlotinib Can Induce the Secretion of Pyroptosis-Related Factors Which Up-Regulated the Expression of GSDMB Protein
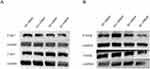
Western-blot detection of tumor cells in GSDMB-positive bladder cancer bearing group showed that the expression of GSDMB protein in group 4 (Anlotinib gavage group) was significantly higher than that in group 3 (normal saline gavage group). Anlotinib induced an increase in the expression of the pyroptosis protein GSDMB (Figure 3A). Flow cytometry results showed that inducing pyroptosis, GZMA secretion was increased in CD8+T cells and GZMB secretion was increased in CD8+T cells and NK cells (Figure 3B).
Anlotinib Can Increase the Secretion of Antitumor-Related Factors in GSDMB-Positive Bladder Cancer
After the treatment of GSDMB-positive bladder cancer with Anlotinib, the flow cytometry results showed that the secretion of IL-2 and IL-10 in CD4+T cells and CD8+T cells increased (Figure 4A). In addition, the secretion of TNF-α by CD4+T cells and CD8+T cells was increased, and the secretion of CD107a by CD8+T cells and NK cells was increased (Figure 4B). The results of flow cytometry suggest that the proportion of PD-L1 and Ki67 decreased, which was conducive to exert the anti-tumor effect of T cells, and indicated that the proliferation of tumor cells was inhibited (Figure 4C). Anlotinib can significantly increase the secretion of inflammatory factors and tumor-killing factors in GSDMB-positive bladder cancer and improve the anti-tumor effect. In addition, for tumor cells themselves, the application of Anlotinib in the expression of GSDMB protein significantly down-regulated the ratio of PD-L1 and Ki67, leading to a significant decline in the survival and proliferation of tumor cells.
Anlotinib Can Block PI3K/AKT and MEK/ERK Signaling Pathways in GSDMB-Positive Bladder Cancer
In GSDMB-positive bladder cancer treated with anlotinib, phosphorylated AKT and MEK protein levels were decreased. Relatively, there were no significant changes in total AKT and MEK protein levels (Figure 5).
Anlotinib Inhibits Angiogenesis in GSDMB-Positive Bladder Cancer
Immunohistochemical staining of FGFR1-4 in tumor tissue showed that the expression of FGFR2 and FGFR3 was down-regulated after Anlotinib treatment, and the expression of FGFR3 was down-regulated most significantly in GSDMB-positive bladder cancer treatment group, while the expression of FGFR1 and FGFR4 have no difference between four groups. Anlotinib treatment of GSDMB-positive bladder cancer inhibited tumor angiogenesis by down-regulating FGFR3 receptor expression (Figure S2; expression of FGFR1-4 receptors in bladder cancer was detected by IHC. (A) FGFR1; (B) FGFR2; (C) FGFR3; (D) FGFR4).
Discussion/Conclusion
Depending on the degree of tumor invasion, bladder cancer can be divided into muscle-invasive type and non-muscle-invasive type. Muscle-invasive type of bladder cancer is mainly treated with radical cystectomy, while the non-muscle-invasive type of bladder cancer can be treated with transurethral resection of bladder tumor and assisted with intravesical chemotherapy and immunotherapy. The advent of immunotherapy has significantly transformed the treatment of bladder cancer (bladder cancer is currently the most approved cancer for PD-1/PD-L1 immune checkpoint inhibitors, and pembrolizumab and opdivo have become the standard treatment for metastatic bladder cancer). For patients who have not received immunotherapy before, at present, only those with high PD-L1 expression are recommended to use immunotherapy drugs.9
As a result, there remains a significant proportion of patients who do not benefit from immunotherapy, opening the way for targeted therapy. Related studies have shown that the mutation rate of FGFR3 gene in bladder cancer is extremely high, and this receptor is an important target molecule for targeted therapy.2
Among the targeted therapy drugs, tyrosine kinase inhibitors (RTKs) have been playing an important role. Compared with other RTK inhibitors (including sorafenib, sunitinib, pazopanib, etc.), Anlotinib can inhibit more targets, and 2-week oral therapy is commonly used in clinical practice at present.10 In a Phase III clinical trial, anlotinib treatment improved progression-free survival and overall survival in patients with advanced non-small cell lung cancer compared with placebo treatment, and has been approved as a third-line drug for the treatment of advanced non-small cell lung cancer.11 In addition, a randomized phase IIB trial showed that Anlotinib significantly prolonged median progression-free survival in patients with advanced soft tissue sarcomas.12 Anlotinib has also shown marvelous efficacy in patients with advanced medullary thyroid carcinoma and metastatic renal cell carcinoma.13 Anlotinib is tolerably similar to other tyrosine kinase inhibitors; however, Anlotinib has a significantly lower incidence of grade III or higher side effects than sunitinib.14 In addition, studies have shown that Anlotinib can change the tumor immune microenvironment by down-regulating the expression of PD-L1 on vascular endothelial cells.15 In our study, we found that Anlotinib was very effective in inhibiting growth of bladder cancer. Furthermore, Anlotinib could enhance the function of immune cells in the tumor microenvironment by inducing secretion of antitumor-related factors.
Multiple signaling pathways mediated by Anlotinib (MEK/ERK, PI3K/AKT and RAF/MRK signaling pathways) can exert angiogenesis and suppress cell proliferation, thereby inducing tumor cell death.16 However, recent studies have shown that the death mode of tumor cells is not only necrosis and apoptosis but also pyroptosis.17 The effect of PD-1/PD-L1 inhibitor in colon cancer mouse model with GSDMB expression was better than that in mice without GSDMB expression. The results showed that degranzyme from cytotoxic lymphocytes lysed and activated GSDMB to induce pyroptosis of target cells. This pyroptosis effect promoted cytotoxic lymphocyte-mediated anti-tumor effect, and the high expression of GSDMB in the digestive system suggested the importance of GSDMB-mediated pyroptosis in intestinal cells and will guide the treatment of related cancers.8 Studies have shown that during the development of colon cancer, the expression of GSDME protein in colon cancer cells is decreased compared with adjacent normal cells, which promotes the proliferation of cancer cells. As an important biomarker in the process of pyroptosis, GSDME protein can be cleaved by inflammatory cysteine aspartate protease when colon cancer cells are exposed to external stimuli, resulting in pyroptosis.18 In the study of bladder cancer-related pyroptosis mechanism, upregulated GSDMB combined with ubiquitin-specific peptidase-24 (USP24) can enhance the growth and invasion ability of bladder cancer cells. Studies have found that GSDMB can bind to signal transducer and activator of transcription-3 (STAT3) and activate this signaling pathway. USP24/GSDMB/STAT3 signaling axis may be a new targeted signaling pathway for bladder cancer treatment.19
In conclusion, anlotinib could exert a strong inhibitory effect on GSDMB-positive bladder tumors according to our cancer cell line research. Anlotinib can inhibit the growth of GSDMB-positive bladder tumors by stimulating the secretion of inflammatory factors and regulating the tumor microenvironment. The inhibitory effect of tumor growth is mainly achieved by blocking the MEK/ERK and PI3K/AKT signaling pathways. The research results can not only provide new ideas for the comprehensive treatment of bladder cancer but also provide a theoretical basis for the application research of pyroptosis protein in other tumors besides transformation prospects.
Cell Lines
The 293T cell was purchased from National Collection of Authenticated Cell Cultures (Cat No. SCSP-502). The MB49 cell line was donated by Professor Lee Longcheng’s laboratory at University of California, San Francisco.20 The use of the MB49 cell was approved by the Ethics Committee of Xinhua Hospital.
Statement of Ethics
All the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The animal study protocol was approved by the Ethics Committee of Xinhua Hospital. Animal experiments were performed in accordance with the Health Guidelines of the National Institutes for the Care and Use of Laboratory Animals. The human data from TCGA (publicly available database) has been approved by the Ethics Committee of Xinhua Hospital Affiliate to Shanghai Jiaotong University School of Medicine.
Acknowledgments
We appreciate Chia Tai Tianqing Company for providing Anlotinib.
Funding
This research was funded by The People’s Hospital of SND, grant number SGY2021B04.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Szklener K, Chmiel P, Michalski A, Mańdziuk S. New directions and challenges in targeted therapies of advanced bladder cancer: the role of FGFR inhibitors. Cancers. 2022;14(6):1416.
2. van Rhijn BWG, Mertens LS, Mayr R, et al. FGFR3 mutation status and FGFR3 expression in a large bladder cancer cohort treated by radical cystectomy: implications for anti-FGFR3 treatment?(†). Eur Urol. 2020;78(5):682–687. doi:10.1016/j.eururo.2020.07.002
3. Wang L, Gong Y, Saci A, et al. Fibroblast growth factor receptor 3 alterations and response to PD-1/PD-L1 blockade in patients with metastatic urothelial cancer. Eur Urol. 2019;76(5):599–603. doi:10.1016/j.eururo.2019.06.025
4. Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70(5):404–423. doi:10.3322/caac.21631
5. Siefker-Radtke AO, Necchi A, Park SH, et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a Phase 2 study. Lancet Oncol. 2022;23(2):248–258. doi:10.1016/S1470-2045(21)00660-4
6. Xiao JF, Caliri AW, Duex JE, Theodorescu D. Targetable pathways in advanced bladder cancer: FGFR signaling. Cancers. 2021;13:19. doi:10.3390/cancers13194891
7. Chu T, Zhong R, Zhong H, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16(4):643–652. doi:10.1016/j.jtho.2020.11.026
8. Zhou Z, He H, Wang K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:6494. doi:10.1126/science.aaz7548
9. Jing W, Wang G, Cui Z, et al. FGFR3 destabilizes PD-L1 via NEDD4 to control T-cell-mediated bladder cancer immune surveillance. Cancer Res. 2022;82(1):114–129. doi:10.1158/0008-5472.CAN-21-2362
10. Su Y, Luo B, Lu Y, et al. Anlotinib induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint blockade in neuroblastoma. Clin Cancer Res. 2022;28(4):793–809. doi:10.1158/1078-0432.CCR-21-2241
11. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 Phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
12. Chi Y, Fang Z, Hong X, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018;24(21):5233–5238. doi:10.1158/1078-0432.CCR-17-3766
13. Sun Y, Du F, Gao M, et al. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid. 2018;28(11):1455–1461. doi:10.1089/thy.2018.0022
14. Pal SK, Tangen C, Thompson IM, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021;397(10275):695–703. doi:10.1016/S0140-6736(21)00152-5
15. Liu S, Qin T, Liu Z, et al. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 2020;11(5):309. doi:10.1038/s41419-020-2511-3
16. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi:10.1186/s13045-018-0664-7
17. Fang Y, Tian S, Pan Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. doi:10.1016/j.biopha.2019.109595
18. Tan G, Lin C, Huang C, et al. Radiosensitivity of colorectal cancer and radiation-induced gut damages are regulated by gasdermin E. Cancer Lett. 2022;529:1–10. doi:10.1016/j.canlet.2021.12.034
19. He H, Yi L, Zhang B, et al. USP24-GSDMB complex promotes bladder cancer proliferation via activation of the STAT3 pathway. Int J Biol Sci. 2021;17(10):2417–2429. doi:10.7150/ijbs.54442
20. Jin X, Ma J, Liang X, et al. Pre-instillation of tumor microparticles enhances intravesical chemotherapy of nonmuscle-invasive bladder cancer through a lysosomal pathway. Biomaterials. 2017;113:93–104. doi:10.1016/j.biomaterials.2016.10.036
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.