Back to Journals » International Medical Case Reports Journal » Volume 10
Anisohypermetropia as a sign of unilateral glaucoma in the pediatric population
Authors Tan DKL, Teh GH, Ho CL, Quah BL
Received 15 February 2017
Accepted for publication 22 April 2017
Published 15 June 2017 Volume 2017:10 Pages 203—207
DOI https://doi.org/10.2147/IMCRJ.S134809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Deborah KL Tan,1,2 Gillian H Teh,2,3 Ching Lin Ho,2,4 Boon Long Quah1,2
1Department of Paediatric Ophthalmology and Adult Strabismus, Singapore National Eye Centre, 2Singapore Eye Research Institute, 3Department of General Cataract and Comprehensive Ophthalmology, 4Department of Glaucoma, Singapore National Eye Centre, Singapore
Abstract: Childhood glaucoma poses a diagnostic and therapeutic challenge to ophthalmologists. Difficulty in examination and limitations on ability to perform structural and functional testing of optic nerve make diagnosis and verification of glaucoma control difficult in children. It is well known that an excessive loss of hyperopia is a useful sign in alerting the examining ophthalmologist to the possible diagnosis of glaucoma. We present an interesting case of juvenile onset glaucoma presenting with anisohypermetropic amblyopia in one eye and normal vision in the fellow eye that has glaucoma. It is an unusual case as the left eye with abnormal vision from hypermetropic amblyopia, though by itself requiring treatment, was a red herring for a potentially blinding condition in the fellow eye with normal vision and lower and less amblyogenic hyperopia on examination. We believe that glaucomatous enlargement of the right eye resulted in significant loss of hyperopia in that eye and in turn contributed to anisohypermetropic amblyopia in the left eye. To the best of our knowledge, this is the first reported case of juvenile onset glaucoma presenting with anisohypermetropic amblyopia in one eye and normal vision in the fellow eye that has glaucoma.
Keywords: childhood glaucoma, anisometropia, anisohypermetropia, amblyopia, myopic shift
Introduction
Childhood glaucoma poses a diagnostic and therapeutic challenge to ophthalmologists. Difficulty in examination and limitations on ability to perform structural and functional testing of optic nerve make diagnosis and verification of glaucoma control difficult in children. We have found an excessive loss of hyperopia to be a useful sign in alerting the examining ophthalmologist to the diagnosis of juvenile open-angle glaucoma (JOAG).
Case
A 6-year-old Japanese boy with an unremarkable birth and medical history was referred to our clinic for decreased vision in the left eye that was picked up during routine health checkup in school. Otherwise, he did not complain of any blurred vision. His parents noticed he adopted a left face turn, when watching television, of ~1 year duration. There was no significant family history of ocular disease, and his parents were emmetropic.
On examination, visual acuity was 6/6 in the right eye and 6/45 in the left eye. There was no relative afferent pupillary defect, and eyes were orthotropic with full extraocular motility. Anterior segment was unremarkable with clear corneas and deep anterior chamber. Cyclorefraction was performed revealing hypermetropic anisometropia of +1.5 D sphere in the right eye and +5.5 D sphere in the left eye. Initial impression was left amblyopia secondary to high hyperopia; however, careful dilated fundal examination also revealed optic disc asymmetry of cup–disc ratio 0.6 in the right eye and 0.4 in the left eye (Figure 1). Intraocular pressures (IOPs) were then measured by iCare tonometer and found to be averaging 35 mmHg and 15 mmHg in the right and left eye, respectively, with repeated examinations demonstrating similar abnormally high readings in the right eye. Horizontal corneal diameters were ~11.0–11.5 mm in the right eye and 11.0 mm in the left eye. Axial length was 22.65 mm in the right eye and 21.14 mm in the left eye, and central corneal thickness was averaging 0.594 mm in the right eye and 0.578 mm in the left eye.
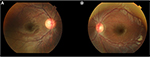  | Figure 1 Optic disc asymmetry of cup–disc ratio 0.6 in the right eye (A) and 0.4 in the left eye (B). |
A diagnosis of right JOAG and left anisometropic amblyopia was made. He was started on Gutt Cosopt bd in the right eye, and also prescribed glasses, with advice for patching of the right eye 6 hours a day. On review about a week later, his IOP was normal at 9 mmHg in the right eye and 11 mmHg in the left eye. He was reviewed regularly with IOP maintained in the low teens while on Gutt Cosopt bd. Optic nerve head topography on Heidelberg retinal tomograph (HRT) and optical coherence tomography of the optic nerve head and peripapillary retinal nerve fiber layer were normal except for rim area asymmetry noted on HRT (Figure 2A and B). Humphrey visual field was attempted but unreliably performed (Figure 3A and 3B). Patching of the left eye was eventually discontinued as amblyopia resolved with visual acuity improving to and maintaining at 6/6. The patient’s parent has given written informed consent to have the case details and accompanying images published.
  | Figure 3 Humphrey visual field of patient’s left (A) and right (B) eyes. |
Discussion
Childhood glaucoma is an uncommon pediatric condition often associated with significant visual impairment.1,2 According to the latest consensus by the World Glaucoma Association, childhood glaucoma is classified as primary or secondary. Primary congenital glaucoma and JOAG constitute the primary childhood glaucoma.3 Primary congenital glaucoma is caused by isolated angle anomalies and consists of 3 subcategories categorized by age of onset – neonatal (0–1 month), infantile (>1–24 months) and late onset (>2 years).3 JOAG presents anywhere from childhood to early adulthood and presents much as adult primary open-angle glaucoma.3 There is no consensus on the age limits for diagnosing JOAG, and the difference between adult and juvenile forms of open-angle glaucoma based on age is regarded as arbitrary.4 Secondary childhood glaucomas are associated with non-acquired ocular or systemic anomalies.
JOAG is an uncommon subset of pediatric glaucoma and is usually transmitted in an autosomal dominant fashion, most commonly involving the myocilin protein.5,6 Myocillin protein is found in trabecular meshwork cells, trabecular beams and juxtacanalicular connective tissues, mutations of which lead to accumulation of misfolded proteins and endoplasmic reticulum stress that compromise the trabecular meshwork cells that regulate IOP.7 Two types of juvenile glaucoma have been described; one is not associated with any gonioscopic abnormalities and the other is associated with iridocorneal angle abnormalities and is termed “goniodysgenesis”.8–11 A recent population-based study reported the incidence of JOAG to be 0.38 per 100,000 residents between 4 and 20 years of age, and an epidemiological study from Dallas Glaucoma Registry reported that JOAG comprised ~4% of all childhood glaucomas.12,13 There have been reports of a male preponderance, association with myopia and severe elevation of IOP with large diurnal fluctuations.8,14–21 Early identification and treatment of glaucoma in children are vitally important as these patients have longer life expectancy than the typical glaucoma patients.
However, early diagnosis may be difficult for many reasons. Careful examination of the optic nerve head, measurements of IOPs and visual field assessment are often challenging in these young patients. Furthermore, these patients with juvenile glaucoma are often without symptoms despite the increased IOPs. Signs and symptoms of congenital glaucoma such as epiphora, photophobia, blepharospasm, Haab’s stria, corneal clouding and increasing corneal diameter are often not seen with juvenile glaucoma. Hence, in a child whose sclera is still vulnerable to the effects of elevated IOP, proxies of persistent elevated IOP such as enlarging corneal diameter, increasing axial length and progressive myopia also need to be taken into consideration and assessed regularly. Therefore, a marked change or significant inter-eye difference in refractive error may be an indicator of juvenile glaucoma, which should prompt us to perform meticulous examination of the optic nerve head and IOPs. With the advent of iCare rebound tonometry and its greater tolerability in children, there is now increased success of obtaining an IOP measurement in the pediatric population.
Anisometropia is not uncommonly seen in the pediatric population and not necessarily attributable to glaucoma.22 However, it is noteworthy that 31% of a sample of patients with primary congenital glaucoma showed at least 2.0 D of anisometropia and that 100% of patients have at least this amount of anisometropia if unilateral primary congenital glaucoma is diagnosed.1,23 In our presented case, the patient was hyperopic with 4.0 D difference between each eye; although the anisometropia may have been contributed in part by the left eye not emmetropizing as normally expected in our patient’s age group, we believe that glaucomatous enlargement of the right eye resulted in significant loss of hyperopia in that eye and in turn contributed to anisohypermetropic amblyopia in the left eye. The diagnostic limitation of our case was that gonioscopy was not performed and diagnosis of JOAG was based on the preponderance of evidence. The level of IOP with possible optic nerve head changes suggestive of glaucoma indicated immediate treatment, and the IOP fell within normal limits once glaucoma-lowering drops were started. Subsequent optic nerve heading imagings were normal.
There are little data available on the therapeutic options of JOAG, possibly due to the rarity of the condition. Some studies suggested that juvenile glaucoma may need primary surgical treatment.24,25 However, since JOAG has also been postulated to be a subset of adult POAG with an earlier age of onset, it may be possible to give these patients a trial of medical therapy.26 Gupta et al20 found in their cohort of high-pressure JOAG patients that medical therapy alone could control IOP and prevent glaucomatous progression in 52% of their patients over a 5-year follow-up. Success of medical therapy could be related to the severity of angle dysgenesis. Those presenting at younger age may have greater trabeculodysgenesis with more severe disease that requires early surgery compared to those who present later.9 Filtering surgery in the juvenile age group are known to have lower success rates than among adults.27 Trabeculectomy without mitomycin in the juvenile age group has been reported to have a success rate of 68% over a 3-year follow-up.28 Trabeculectomy with mitomycin improves surgical success among JOAG but has higher risk of postoperative hypotonic maculopathy and long-term bleb-related infections.28,29 Post-trabeculetomy cataract formation in juvenile glaucoma is also known to occur with the same frequency as in adult glaucomas.30 Given the longer life expectancy of these patients, decisions for undertaking surgery in these young patients will have to be taken with caution.
This is an unusual case as the eye with abnormal vision from amblyopia, though by itself requiring treatment, was a red herring for a potentially blinding condition in the fellow eye with normal vision on examination. All patients must be thoroughly examined when asymmetry is detected between each eye instead of assuming the common diagnosis of anisohypermetropic amblyopia. The importance of detailed fundal examination even in the presence of good central vision cannot be further emphasized. To the best of our knowledge, this is the first reported case of juvenile onset glaucoma presenting with anisohypermetropic amblyopia in one eye and normal vision in the fellow eye that has glaucoma.
Conclusion
IOP measurement and optic disc appearance are fundamental features of the examination of a child with glaucoma. However, rapid changes in refractive status and axial length are helpful in both diagnosing and monitoring of childhood glaucoma, while sclera remains vulnerable to the effects of elevated IOP. Development of an excessive loss of hyperopia in our pediatric patients is a useful sign for identifying glaucoma suspects. Measures to ensure prompt and adequate evaluation are important to confirm the diagnosis of glaucoma and for early treatment to minimize the degree of visual impairment.
Disclosure
The authors report no conflicts of interest in this work.
References
Broughton WL, Parks MM. An analysis of treatment of congenital glaucoma by goniotomy. Am J Ophthalmol. 1981;91(5):566–572. | ||
Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–393. | ||
Weinreb RG, Papadopoulos M, Grigg J. Childhood Glaucoma. World Glaucoma Association Consensus Series 9. Amsterdam, the Netherlands: Kugler Publications; 2013. | ||
Wiggs JL, Damji KF, Haines JL, Pericak-Vance MA, Allingham RR. The distinction between juvenile and adult-onset primary open-angle glaucoma. Am J Hum Genet. 1996;58:243–244. | ||
Johnson AT, Drack AV, Kwitek AE, et al. Clinical features and linkage analysis of a family with autosomal dominant juvenile glaucoma. Ophthalmology. 1993;100:524–529. | ||
Wiggs JL, Haines JL, Paglinauan C, Fine A, Sporn C, Lou D. Genetic linkage of autosomal dominant juvenile glaucoma to 1q21-q31 in three affected pedigrees. Genomics. 1994;21(2):299–303. | ||
Anholt RR, Carbone MA. A molecular mechanism for glaucoma: endoplasmic reticulum stress and the unfolded protein response. Trends Mol Med. 2013;19(10):586–593. | ||
Goldwyn R, Waltman SR, Becker B. Primary open-angle glaucoma in adolescents and young adults. Arch Ophthalmol. 1970;84:579–582. | ||
Jerndal T. Congenital glaucoma due to dominant goniodysgenesis: a new concept of the heredity of glaucoma. Am J Hum Genet. 1983;35(4):645–651. | ||
Barkan O. Pathogenesis of congenital glaucoma. Gonioscopic and anatomic observation of the angle of the anterior chamber in the normal eye and in congenital glaucoma. Am J Ophthalmol. 1955;40:1–11. | ||
Worst JGF. The Pathogenesis of Congenital Glaucoma. Assen: Van Gorcum; 1966. | ||
Aponte EP, Diehl N, Mohney BG. Incidence and clinical characteristics of childhood glaucoma: a population-based study. Arch Ophthalmol. 2010;128(4):478–482. | ||
Fung DS, Roensch MA, Kooner KS, Cavanagh HD, Whitson JT. Epidemiology and characteristics of childhood glaucoma; results from the Dallas Glaucoma registry. Clin Ophthalmol. 2013;7:1739–1746. | ||
Pressman SH, Crouch ER. Pediatric aphakic glaucoma. Ann Ophthalmol. 1983;15(6):568–573. | ||
Perkins ES. Glaucoma in the younger age groups. Arch Ophthlmol. 1960;64:882–891. | ||
Merritt JC, Reid LA, Smith R, Harris DF. Diurnal intraocular pressure in juvenile open-angle glaucoma. Ann Ophthalmol. 1979;11:253–260. | ||
Lotufo D, Ritch R, Szmud L, Burris JE. Juvenile glaucoma, race, and refraction. JAMA. 1989;261(2):249–252. | ||
Patel GR, Cooper SN. The relation of myopia with congenital glaucoma. All India Ophthalmol Soc. 1969;17:208–215. | ||
Park SC, Kee C. Large diurnal variation of intraocular pressure despite maximal medical treatment in juvenile open angle glaucoma. J Glaucoma. 2007;16(1):164–168. | ||
Gupta V, Ov M, Rao A, Sharma A, Sihota R. Long-term structural and functional outcomes of therapy in juvenile-onset primary open-angle glaucoma: a five-year follow-up. Ophthalmologica. 2012;228(1):19–25. | ||
Kwun Y, Lee EJ, Han JC, Kee C. Clinical characteristics of juvenile-onset open angle glaucoma. Korean J Ophthalmol. 2016;30(2):127–133. | ||
Tanlamai T, Goss DA. Prevalence of monocular amblyopia among anisometropic amblyopes. Am J Optom Physiol Opt. 1979;56:704–715. | ||
Robin AL, Quigley HA, Pollack IP, Maumenee AE, Maumenee IH. An analysis of visual acuity, visual fields, and disk cupping in childhood glaucoma. Am J Ophthalmol. 1979;88(5):847–858. | ||
Jerndal T. Goniodysgenesis and hereditary juvenile glaucoma: a clinical study of a Swedish pedigree. Acta Ophthalmol Suppl. 1970;107:3–10. | ||
Wiggs JL, Del Bono EA, Schuman JS, Hutchinson BT, Watson DS. Clinical features of five pedigrees genetically linked to the juvenile glaucoma locus on chromosome 1q21-q31. Ophthalmology. 1995;102(12):1782–1789. | ||
Chandler PA, Grant WM. Juvenile Open Angle Glaucoma. Philadelphia, PA: Lea & Febiger; 1965. | ||
The Advanced Glaucoma Intervention Study (AGIS). Risk factors for failure of trabeculectomy and argon laser trabeculoplasty. Am J Ophthalmol. 2002;134:481–498. | ||
Tsai JC, Chang HW, Kao CN, Lai IC, Teng MC. Trabeculectomy with mitomycin versus trabeculectomy along for juvenile primary open angle glaucoma. Ophthalmologica. 2003;217:24–30. | ||
Poulsen EJ, Allingham RR. Characteristics and risk factors of infections after glaucoma filtering surgery. J Glaucoma. 2000;9(6):438–443. | ||
Adelman RA, Brauner SC, Afshari NA, Grosskreutz CL. Cataract formation after initial trabeculectomy in young patients. Ophthalmology. 2003;110(3):625–629. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.