Back to Journals » OncoTargets and Therapy » Volume 13
ANGPTL4 Promotes the Proliferation of Papillary Thyroid Cancer via AKT Pathway
Authors Yang L, Wang Y, Sun R, Zhang Y , Fu Y, Zheng Z, Ji Z, Zhao D
Received 7 November 2019
Accepted for publication 2 March 2020
Published 17 March 2020 Volume 2020:13 Pages 2299—2309
DOI https://doi.org/10.2147/OTT.S237751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Longyan Yang,1,* Yan Wang,1,* Rongxin Sun,1,* Yuanyuan Zhang,1 Ying Fu,1 Zhaohui Zheng,1 Zhili Ji,2 Dong Zhao1
1Beijing Key Laboratory of Diabetes Research and Care, Center for Endocrine Metabolism and Immune Diseases, Luhe Hospital Capital Medical University, Beijing 101149, People’s Republic of China; 2Department of General Surgery, Luhe Hospital Capital Medical University, Beijing 101149, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dong Zhao; Zhili Ji
Tel +86-10-69543901
Fax +86-10-69531069
Email [email protected]; [email protected]
Purpose: Although papillary thyroid carcinoma (PTC) is associated with a generally favorable prognosis, about 15% of patients present recurrence and distant metastasis in the next decade leading to death. Angiopoietin-like 4 (ANGPTL4) is secreted to circulation and belongs to the angiopoietin-like proteins. The expression of ANGPTL4 was increased in several solid tumor tissues compared to corresponding paracancerous tissues. ANGPTL4 was identified as pro-tumorigenic protein, including stimulating tumor cell growth, promoting tumor metastasis. However, the clinical significance and biological function of ANGPTL4 in PTC is still unclear. Hence, the purpose of this study was to evaluate the role of ANGPTL4 in PTC, investigating the possibility of whether ANGPTL4 could become a novel target for PTC therapy.
Methods: We investigated the expression level of ANGPTL4 and pAKT in PTC and paracancerous tissue by immunohistochemistry. We determined the effect of ANGPTL4 in PTC cell proliferation through cell counting kit-8 (CCK-8) and cell cycle by flow cytometry analysis. Furthermore, the correlation between ANGPTL4 expression levels and PTC cell proliferation from the TCGA data set was analyzed by GSEA. We explored the role of ANGPTL4 on the phosphorylation of AKT and proliferation in PTC cells via overexpression or knockdown assays and AKT inhibitor assay.
Results: In the present study, we found that ANGPTL4 was highly expressed in both protein and mRNA level in PTC compared with adjacent noncancerous tissues or benign nodule. ANGPTL4 expression increased according to thyroid tumor progression. ANGPTL4 level was positively correlated with the size of PTC. ANGPTL4 increased cell proliferation and decreased cell cycle arrest of PTC. Knockdown of ANGPTL4 inhibited the phosphorylation of AKT. ANGPTL4 regulated PTC cell proliferation through AKT signaling pathway.
Conclusion: Our findings suggested that ANGPTL4 was increased in PTC compared with adjacent noncancerous tissues, and ANGPTL4 increased cell proliferation and inhibited cell cycle arrest in PTC cells via promoting AKT phosphorylation. The study may provide fundamental information to suggest its suitability as a target for the treatment of PTC.
Keywords: papillary thyroid carcinoma, angiopoietin-like 4, AKT, proliferation
Introduction
Thyroid cancer develops from the tissues of the thyroid gland. It is the most common endocrine malignancy with 300,000 new cases per year worldwide, and 40,000 deaths per year approximately.1,2 Papillary thyroid cancer (PTC) is the most common type of thyroid cancer, comprising 80% of all cases.3 The incidence of PTC continues to rise worldwide.4 Currently, therapeutic options for thyroid cancer include surgery, thyroid hormone replacement and radioactive iodine ablation.5 Although PTC is associated with a generally favorable prognosis, about 15% of patients present recurrence and distant metastasis in the next decade leading to death.6,7 With the deepening of research for thyroid cancer, we recognize that the main cause of PTC is the dysregulation of many tumor-related genes.8 Therefore, the molecular mechanisms underlying the progression and metastasis of PTC still require intensive study.
The angiopoietin-like protein 4 (ANGPTL4) belongs to angiopoietin-like protein family. ANGPTL4 is secreted to circulation and regulates lipid metabolism via inhibiting lipoprotein (LPL). ANGPTL4 is produced by a variety of cells, including hepatocytes,9 adipocytes,10 skeletal muscle,11 macrophages,12 epithelial cells13 and so on. It is reported that ANGPTL4 promotes the migration and differentiation of endothelial cell, leading to the formation of new blood vessels.14,15 The expression of ANGPTL4 was increased in several solid tumors such as osteosarcoma,16 gastric cancer,17 renal cell carcinoma,18 non-small cell lung cancer,19 cervical cancer20 and so on compared with corresponding paracancerous tissues. ANGPTL4 was identified as pro-tumorigenic protein, including stimulating tumor cell growth and proliferation, promoting tumor metastasis.21 However, the clinical significance and biological function of ANGPTL4 in PTC are still unclear.
The mutations in the BRAF and RAS genes resulted in excessive activation of the mitogen-activated protein kinase (MAPK) pathway, which is an important oncogenic driver for PTC.22 Moreover, studies have revealed that phosphoinositide 3-kinase AKT (PI3K-AKT) pathway also drived carcinogenesis of PTC.23 Cell proliferation plays an important role in tumor progression and prognosis. Activation of PI3K-AKT signaling pathway results in high cell proliferation and vascularity.24 And abnormal activation of the PI3K-AKT pathway is crucial in initiation and progression of thyroid cancer.25 Therefore, in the present study, we investigated the different expression levels of ANGPTL4 in PTC, para-cancerous tissue and normal thyroid. We assessed the effect of ANGPTL4 on PTC cell proliferation and explored possible mechanism mediated by AKT pathway. This study will help to provide fundamental information as to its suitability as a target for the treatment of PTC.
Methods
Data Sets Collection and Thyroid Cancer Patient Samples
We obtained 32 pairs of papillary thyroid carcinoma and adjacent normal thyroid tissue from thyroidectomy conducted at the Luhe Hospital Capital Medical University between January 2016 and January 2017. All these confirmed thyroid cancer tissues and their adjacent normal thyroid tissues were embedded in paraffin wax. The clinicopathologic characteristics of patients with thyroid cancer are shown in Table 1. Ten fine needle aspiration thyroid samples (6 cases of benign thyroid node and 4 cases of malignant PTC) were collected for RNA-Seq and stored at −80°C. The clinical information is shown in Table 2. All patients in this study underwent histologic diagnoses. Tumors were staged based on the guidelines of the 7th edition of the American Joint Committee on Cancer Tumor-Node-Metastasis (AJCC-TNM) staging system. The clinical characteristics of patients were extracted from medical records. The research was approved by the Research Ethics Board of Luhe Hospital Capital Medical University and was carried out according to the World Medical Association Declaration of Helsinki. All patients included in the protocol signed a declaration of informed consent.
 |
Table 1 Clinicopathologic Characteristics of Patients with Thyroid Carcinoma |
 |
Table 2 Characteristics of Patients with Fine Needle Aspiration for Thyroid Nodule |
In addition, The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) dataset were used in this study. The mRNA expression data (RNA Seq v2) and clinical information for thyroid cancer patients in TCGA data set were downloaded from https://www.synapse.org and cBioPortal database (www.cbioportal.org), respectively. The dataset GSE3467 with information on mRNA expression form GEO (http://www.ncbi.nlm.nih.gov/geo/) was used to analyze ANGPTL4 expression level.
Cell Culture and Transfection
Cell was purchased from the National Infrastructure of Cell Line Resource (Beijing, China). TPC-1 and BCPAP thyroid cancer cells were cultured in RPMI 1640 medium (Gibico, USA), with 10% FBS (Gibico, USA) and 1% penicillin/streptomycin in a 37°C/5% CO2 incubator. Cell transfection was performed with Lipofectamine 2000 (Thermo Fisher–Invitrogen, USA) according to manufacturer’s instructions. Cells were transfected with ANGPTL4 small interfering RNA duplexes (Santa Cruz, USA) to decrease the level of ANGPTL4. Full-length cDNA encoding human ANGPTL4 were cloned into the vector GV366 plasmid (Shanghai Genechem Co., Ltd) with an HA-tag. Cells were transfected with HA-ANGPTL4 to increase the level of ANGPTL4. The cells were treated with 10% FBS at 37°C for indicated times. For stimulation of AKT signaling, cells were maintained in serum-free medium (Life Technologies, Inc., Carlsbad, CA, USA) for 24 hrs followed by stimulation with FBS at 37°C for 15 min.
Western Blot
Cells were harvested in sodium dodecyl sulfate (SDS) sample buffer. Equal amounts of proteins were separated by using 10% SDS-PAGE system, transferred onto the polyvinylidene fluoride membrane (Millipore, USA). The membranes were blocked with 5% non-fat dried milk. Then, the membrane was blotted with the primary antibodies: anti-ANGPTL4 antibody (1:1000, Santa Cruz, USA), the polyclonal rabbit anti-HA antibody (1:1000, MBL, Japan), Anti-AKT and anti-phospho-AKT (Ser473) (1:1000, Cell Signaling Technology, Danvers, MA, USA), anti-GAPDH antibody (1:3000, ZSGB-BIO, China), diluted with 1% non-fat dried milk in TBST. After washed with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:3000, ZSGB-BIO, China). The membranes were washed with TBST and scanned by ChemiDoc XRS+ chemiluminescence imaging system (Bio-Rad, USA). Each examination was tested in triplicate, and GAPDH was served as the internal reference.
Proliferation Assay
Cell Counting Kit-8 (CCK8) assay was performed to detect the cell proliferation rate. The cells were seeded at a density of 2000 cells/well into 96-well plates. Plates were then incubated for 0, 24, 48, 72, 96 and 120 hrs and viable cells were analyzed with Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) by using an Enspire microplate reader (Perkin Elmer, Waltham, MA, USA) at 450 nm.
Cell Cycle Analysis
Cells were fixed for overnight in ethanol at 4°C, and then incubated with propidium iodide for 10 min. Flow cytometry (BD Biosciences, San Jose, CA, USA) analysis was used to detect cell cycles. ModFit software was used to analyze the data.
Immunohistochemistry
Samples were subjected to standard deparaffinization, rehydration, and antigen retrieval for immunohistochemical staining. The slides were incubated with primary antibody against ANGPTL4 (LS-C331822, Life Span Bio Sciences, Inc). The staining score for primary tumor and adjacent normal tissue was recorded separately. The percentage of immunostaining and the staining intensity (0, negative; 1+, weak; 2+, moderate; and 3+, strong) were recorded. An H-score was calculated using the following formula: H-SCORE=∑ (PI×I) = (percentage of cells of weak intensity ×1)+(percentage of cells of moderate intensity ×2)+percentage of cells of strong intensity ×3). The maximum H-score would be 300, corresponding to 100% of cells with strong intensity.
Gene Set Enrichment Analysis
The ANGPTL4 expression levels of genes were analyzed using Gene Set Enrichment Analysis. The gene sets were obtained from Molecular Signatures Database from the Broad Institute (http://software.broadinstitute.org/gsea/msigdb). Tests were performed by using the default settings, and permutations number was set at 1000. False discovery rate <0.25 was considered statistically significant.
Cell Apoptosis, Wound-healing, Transwell, and Enzyme-Linked Immunosorbent Assays
The cell apoptosis, wound-healing, Transwell, and enzyme-linked immunosorbent assay methods are detailed in the Supplementary methods.
Statistical Analysis
Statistical analyses were performed using the SPSS 18.0 (SPSS Inc, Chicago, IL, USA). Results are described with mean±SD. The protein and mRNA expression levels from paired clinical samples were analyzed by two-tailed paired Student’s t-test. The mRNA levels of ANGPTL4 in stages in TCGA dataset were analyzed by one-way ANOVA. Cell proliferation curve was analyzed by repeated measurement ANOVA. Two-tailed unpaired Student’s t-test was used to determine the other results of statistical significance. Statistical significance was accepted for p< 0.05.
Results
ANGPTL4 Is Highly Expressed in Thyroid Cancer Compared with Adjacent Normal Thyroid Tissue
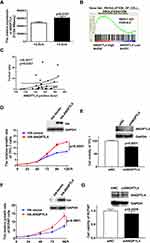
The expression of ANGPTL4 in human PTC tissues and adjacent noncancerous tissues was showed by immunohistochemistry (IHC) (Figure 1A). According to our IHC scoring system, ANGPTL4 IHC score for 32 cases of cancer was 156.9±3.184, and the score for 32 cases of adjacent normal thyroid tissue was 140.5±6.539. The data revealed that ANGPTL4 expression was upregulated in PTC tissues compared with adjacent noncancerous tissues (Figure 1B, p=0.0076). Next, we detected the mRNA level of ANGPTL4 for fine needle aspiration thyroid samples (6 cases of benign thyroid node and 4 cases of malignant PTC) by RNA-seq analysis. It was showed that ANGPTL4 mRNA was increased in maligant thyroid cancer comparing with benign thyroid tissue (Benign vs Maligant: 11.83±9.326 vs 1125±902.8, p=0.0143) (Figure 1C).
To further verify the findings, the mRNA level of ANGPTL4 in thyroid cancer and adjacent normal thyroid tissues were analyzed with GEO datasets and TCGA’s RNA-seq data of thyroid cancer patients. There were 9 paired thyroid cancer tissues and adjacent cancer tissues in GSE3467 datasets. Data from GSE3467 datasets showed that ANGPTL4 mRNA expression level was obviously upregulated in thyroid cancer tissues compared with adjacent non-tumor thyroid tissues (Figure 1D, p=0.0228). TCGA dataset contained 58 normal thyroid and 501 thyroid cancer. Analysis of ANGPTL4 mRNA level from TCGA dataset confirmed that ANGPTL4 was significantly higher in thyroid cancer tissues than normal thyroid tissues (Figure 1E, p<0.0001). Further analysis of ANGPTL4 mRNA levels in the paired thyroid cancer and adjacent normal thyroid tissues showed ANGPTL4 was obviously upregulated in thyroid cancer tissues compared with their paired adjacent non-tumor thyroid tissues (Figure 1F). ANGPTL4 is the protein secreted into circulation. Therefore, we analyzed the serum level of ANGPTL4 between thyroid nodule and thyroid cancer patients by ELISA, and found that there was no significant difference in serum level of ANGPTL4 between thyroid nodule and thyroid cancer patients (Supplemental Figure 1). It was suggested that ANGPTL4 in local thyroid tissue rather than in circulation played an important role in thyroid cancer.
ANGPTL4 Expression Increases According to Thyroid Tumor Progression
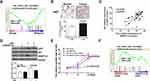
To explore the clinical relevance of ANGPTL4 in thyroid cancer development, the correlation between ANGPTL4 expression levels and tumor stages was evaluated. We analyzed the ANGPTL4 protein level, which was detected using H-score system for IHC in different stages of thyroid cancer patients. The patients of stage Ⅱ and Ⅲ were combined in one group due to the small number in each stage. The results showed that ANGPTL4 protein level was higher in the advanced stage of thyroid cancer than in stage Ⅰ (Figure 2A, p=0.0440). We further analyzed the level of ANGPTL4 mRNA in different stages of patients with thyroid cancer by using TCGA dataset. The results showed that along with the increase of thyroid tumor stage, ANGPTL4 mRNA expression level increased accordingly (Figure 2B, p=0.0283, one-way ANOVA and Tukey’s multiple comparison test). Moreover, ANGPTL4 mRNA from normal thyroid and different stages (I/II, III/IV) of thyroid cancer were divided into ANGPTL4 high/low expression groups based on the median values of ANGPTL4, respectively, and we found the ratio of ANGPTL4 high expression (white column) was increased according to the severity of thyroid cancer (Figure 2C). These data indicate that ANGPTL4 expression is associated with advanced tumor stage.
ANGPTL4 Increases Cell Proliferation of Thyroid Tumor
ANGPTL4 has been widely found to regulate proliferation of various cells. We found that ANGPTL4 was significantly increased in the group with the maximum diameter greater than 2.5 cm in TCGA dataset (Figure 3A, p=0.0181).To investigate the correlation between the levels of ANGPTL4 and proliferation signaling in thyroid cancer, data from TCGA dataset were further divided into high/low ANGPTL4 expression groups and then analyzed using gene set enrichment analysis (GSEA) method. The results showed that gene signatures of proliferation were enriched in patients with high level of ANGPTL4 (Figure 3B). Furthermore, we found that ANGPTL4 protein level measured with immunohistochemistry in 32 cases of thyroid cancer was positively correlated with the tumor size (Figure 3C, r=0.4017, p=0.0251). In order to confirm the role of ANGPTL4 in thyroid cancer cell proliferation, the TPC-1 thyroid cancer cells were transfected with ANGPTL4 expression constructs, and the overexpression or knock-down of ANGPTL4 was confirmed by Western blotting, separately (Figure 3D and E). Then, cell viability was measured, the ANGPTL4 overexpression cells were cultured in 96-well plates and stained with CCK8 at 24, 48, 72, 96 and 120 hrs. The results showed that the TPC-1 cell proliferation increased in ANGPTL4 over-expressed cells (Figure 3D, p<0.0001). By contrast, ANGPTL4 knock-down in TPC-1 cells led to decreased cell proliferation as judged by the CCK8 viability assays at 72 hrs (Figure 3E, p<0.0001).
To clarify the effect of promoting cell proliferation is not the cell line specific, another PTC cell line BCPAP was obtained. BCPAP cells were transfected with an expression construct carrying ANGPTL4 cDNA. After 48 hrs’ transfection, the level of ANGPT4 was confirmed by Western blotting (Figure 3F). ANGPTL4 over-expressed cells were cultured in 96-well plates and stained with CCK8 at 0, 24, 48, 72 and 96 hrs. We found that overexpression of ANGPT4 promoted the proliferation in BCPAP cell (Figure 3F, p<0.0001). By contrast, ANGPTL4 knock-down in BCPAP cells led to decreased cell viability as judged by the CCK8 assays at 72 h (Figure 3G, p=0.0229). The TPC-1 thyroid cancer cells were transfected with ANGPTL4 expression plasmid, and then the cell migration, cell invasion and cell apoptosis were investigated. The results showed overexpression of ANGPTL4 had no effect on thyroid cancer cell migration, invasion and cell apoptosis (Supplemental Figure 2A–C). These data confirmed that ANGPTL4 expression promoted thyroid cancer cell proliferation.
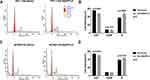
To explore the possible mechanism of ANGPTL4 promoting cell proliferation, we further investigated the effect of ANGPTL4 on cell cycle progression using flow cytometry. As shown in Figure 4A and B, after overexpression of ANGPTL4, the percentage of TPC-1 cells in G0/G1 phase significantly decreased from 57.08% to 55.01% (p=0.0003), whereas the distribution of cells in S phase increased from 37.00% to 46.81% (p=0.0087). To clarify the effect observed is not the cell line specific, we obtained another PTC cell line BCPAP. ANGPTL4 was overexpressed in BCPAP cells and we received a consistent result (Figure 4C, p=0.0193 and D, p=0.0193). These data demonstrated that overexpression of ANGPTL4 promoted cell cycle arrest at G1/S checkpoint and decreased cell cycle arrest at G0/1 checkpoint in thyroid cancer cells. Taken together, ANGPTL4 promoted cell growth and inhibited cell cycle arrest of thyroid cancer cells.
ANGPTL4 Promoted AKT Activation and AKT-Mediated Proliferation in Thyroid Cancer
The molecular mechanisms underlying ANGPTL4 promotion of thyroid cancer cell proliferation were studied. It is reported that MAPK and PI3K-AKT pathways drived carcinogenesis of PTC, and which could regulate cell proliferation. As we analyzed the activation of AKT signaling by GSEA, gene signatures of AKT signaling were enriched in malignant in comparison with benign (Figure 5A). Furthermore, the expression levels of pAKT in cancer tissue and adjacent tissue were measured by IHC, from which pAKT IHC score for cancer was 129.4±9.641, and the score for normal was 95.20±7.594 (Figure 5B, p=0.0001). And we found ANGPTL4 protein level was found significantly positively correlated with pAKT expression level (Figure 5C, r=0.5899, p<0.0001). We also measured the expression level of pERK1/2 in PTC tissues and adjacent tissues by IHC followed H-score analysis. But we found that ANGPTL4 protein level was not related with pERK1/2 expression level (Supplemental Figure 3). Next, we further investigated the effect of ANGPTL4 knockdown in the activation of AKT signaling in thyroid cancer cell. TPC-1 cells transfected with siNC/siANGPTL4 were treated with serum-free medium for 24 hrs followed by stimulation with FBS. The results showed that down-expression of ANGPTL4 led to inhibition of the expression of phosphorylated AKT (Figure 5D, p=0.0145). These results confirmed that ANGPTL4 promoted AKT activation in thyroid cancer.
In order to understand the role of ANGPTL4 in AKT-mediated proliferation, TPC-1 cells were transfected with HA-vector or HA-ANGPTL4, and then cultured for 1, 2 or 3 days for CCK8 proliferation assay. Consistent with the results in front, ANGPTL4 promoted the proliferation of thyroid cell (Figure 5E, p=0.0182). However, the proliferation rate of ANGPTL4 overexpressed cells in groups treated with AKT inhibitor was similar as compared with the TPC-1-HA-vector cells with AKT inhibitor, suggesting AKT inhibitors abolished the promotion effect of ANGPTL4 on cell proliferation (Figure 5E, p=0.3132). Next, we analyzed the correlation of AKT activation and ANGPTL4 expression in clinical samples in TCGA dataset by GSEA, and the result showed the gene signature of AKT activation was enriched in ANGPTL4 high-expression group (Figure 5F). These findings suggested that ANGPTL4 promoted AKT activation and AKT-mediated proliferation of thyroid cancer cell.
Discussion
Previous studies demonstrated that ANGPTL4 was associated with a variety types of cancers such as osteosarcoma,16 gastric cancer,17 renal cell carcinoma,18 non-small cell lung cancer,19 and cervical cancer,20 and played pivotal roles in tumor growth, proliferation and metastasis.21 ANGPTL4 was identified as a factor of lipid metabolism and angiogenesis which could be regulated by the nuclear hormone receptors PPARs, hypoxic conditions, fasting, and transforming growth factor β.26–29 More importantly, ANGPTL4 was suggested to be a novel diagnostic and prognostic biomarker for patients with renal cell carcinoma or cervical cancer.20,30 However, no reports have investigated the role of ANGPTL4 in PTC. Therefore, this study further explores the possibility of ANGPTL4 as a potential molecular target for thyroid cancer treatment.
In this study, we first evaluated the clinical significance of ANGPTL4 in PTC. We observed an obvious up-regulation of ANGPTL4 in 32 cases of human PTC tissues compared with adjacent noncancerous tissues by immunohistochemistry. Meanwhile, we collected 10 samples of thyroid cells for fine-needle aspiration cytology (4 patients of stage I PTC and 6 cases of benign thyroid tissues) and found that mRNA level of ANGPTL4 was increased in PTC. The mRNA level of ANGPTL4 was elevated in thyroid cancer compared with their adjacent tissues in TCGA’s RNA-seq data and GEO datasets of thyroid cancer patients. ANGPTL4 protein expression level as well as mRNA level increased accordingly with the increase of thyroid tumor stage. In addition, the gene signatures of proliferation were enriched in patients with high levels of ANGPTL4 using gene set enrichment analysis (GSEA) method. ANGPTL4 mRNA level was significantly increased in the group with the maximum diameter greater than 2.5 cm compared with the maximum diameter less than 2.5 cm in TCGA dataset. ANGPTL4 protein level was positively correlated with the tumor size in 32 cases of human PTC tissues. Furthermore, overexpression of ANGPTL4 promoted PTC cell proliferation, and knockdown of ANGPTL4 by siRNA inhibited PTC cell proliferation, and the mechanism of ANGPTL4 regulating PTC cell proliferation was ANGPTL4 inhibited cell cycle arrest of PTC cells. In addition, the gene signatures of AKT activated signaling were enriched in PTC in comparison with benign thyroid disease in 10 samples of thyroid cells for fine-needle aspiration cytology. ANGPTL4 protein level was significantly positively correlated with pAKT level in PTC tissue and adjacent tissue measured by IHC. Then, knockdown of ANGPTL4 by siRNA inhibited the phosphorylation of AKT in PTC cells. Moreover, overexpression or knockdown of ANGPTL4 increased or inhibited PTC cell proliferation. The gene signatures of PI3K-AKT signaling were enriched in patients with high levels of ANGPTL4 using gene set enrichment analysis (GSEA) method.
Our findings demonstrated that ANGPTL4 was increased in PTC compared with adjacent noncancerous tissues. The expression of ANGPTL4 was increased in several solid tumors compared with corresponding paracancerous tissues. Hypoxia is common in solid tumors. Moreover, studies have shown that hypoxia was an independent prognostic factor for malignant tumors.31 It was reported that hypoxia-inducible factor-1α (HIF-1α) gene expression was increased during hypoxic conditions in tumors, including PTC.32 Furthermore, ANGPTL4 is known to be a gene induced by hypoxia. Studies have shown that HIF-1α directly up-regulated the expression of ANGPTL4 in hepatocellular carcinoma cells.33 We analyzed the correlation between ANGPTL4 and HIF-1α in thyroid cancer. Consistent with previous results, we found that HIF-1α mRNA level was increased in thyroid cancer compared with normal in TCGA (Supplemental Figure 4A). Furthermore, the level of ANGPTL4 was positively correlated with the level of HIF1A in thyroid cancer in TCGA (Supplemental Figure 4B). We will study the regulation of HIF-1α on ANGPTL4 in PTC cells in the future.
According to previous reports, important prognostic factors in PTC included tumor size, depth of invasion, metastasis of lymph node, and distant metastasis.34 Among the factors, tumor size was very important, which was correlated with PTC cell proliferation. PI3K-AKT signaling is activated (AKT is phosphorylated) further which activates its downstream substrate cascade reaction, thus participating in glucose transport, cell survival, cell proliferation, glycolysis, protein synthesis, and anti-apoptotic process.35 It was reported that down-regulated ANGPTL4 significantly inhibited glucose uptake and AKT pathway, then inhibited proliferation in 3T3-L1 cells.36 Previous studies suggested that ANGPTL4 preserved vascular integrity via PI3 kinase/AKT signaling. This study demonstrated that ANGPTL4 promoted the phosphorylation of AKT, and then increased PTC cell proliferation.
Our previous study found that ANGPTL2, another member of angiopoietin-like protein family, promoted and enhanced proliferation, metastasis, and invasion of thyroid cancer cells and considered as a potential biomarker for diagnosis and prognosis of thyroid cancer patients. However, carcinogenesis is not a shared characteristic among angiopoietin-like protein family members. In our unpublished data, we found ANGPTL1 inhibited proliferation, migration and invasion of thyroid cancer cells, and ANGPTL5 and ANGPTL6 were unchanged between normal and thyroid cancer. Therefore, it is specific for ANGPTL4 in the role of promoting thyroid cancer cell proliferation.
However, there are some limitations in this study. First, the small sample size might decrease the statistical power of our results. In addition, we have investigated the expression and function of ANGPTL4 in PTC cells, but further investigation is needed to uncover the mechanism of the action of ANGPTL4 on PTC patients.
Conclusion
This study suggested that ANGPTL4 was increased in PTC compared with adjacent noncancerous tissues. ANGPTL4 level was positively correlated with the stage of PTC and PTC size. ANGPTL4 increased cell proliferation and inhibited cell cycle arrest in PTC cells via promoting AKT phosphorylation. The study will provide fundamental information as to its suitability as a target for the treatment of PTC.
Acknowledgments
This study was funded by Beijing Natural Science Foundation in China (Project Number: 7184222) and National Science Funding in China (Project Number: 81800768).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653. doi:10.1038/nrendo.2016.110
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi:10.3322/caac.v66.4
3. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi:10.1016/S0140-6736(16)30172-6
4. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. doi:10.1038/nrc3431
5. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
6. Grewal RK, Ho A, Schoder H. Novel approaches to thyroid cancer treatment and response assessment. Semin Nucl Med. 2016;46(2):109–118. doi:10.1053/j.semnuclmed.2015.10.010
7. Hadoux J, Pacini F, Tuttle RM, Schlumberger M. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol. 2016;4(1):64–71. doi:10.1016/S2213-8587(15)00337-X
8. Azar FK, Lee SL, Rosen JE. Medullary thyroid cancer: an update for surgeons. Am Surg. 2015;81(1):1–8.
9. Nakamoto M, Ishihara K, Watanabe T, et al. The glucocorticoid receptor regulates the ANGPTL4 gene in a CTCF-mediated chromatin context in human hepatic cells. PLoS One. 2017;12(1):e0169225. doi:10.1371/journal.pone.0169225
10. Gray NE, Lam LN, Yang K, et al. Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes. J Biol Chem. 2017;292(39):16135. doi:10.1074/jbc.A111.294124
11. Vienberg SG, Kleinridders A, Suzuki R, et al. Differential effects of angiopoietin-like 4 in brain and muscle on regulation of lipoprotein lipase activity. Mol Metab. 2015;4(2):144–150. doi:10.1016/j.molmet.2014.11.003
12. Liu N, Cui C, Sun Y, et al. Hydrogen peroxide promotes the expression of angiopoietin like 4 in RAW264.7 macrophages via MAPK pathways. Mol Med Rep. 2017;16(5):6128–6133. doi:10.3892/mmr.2017.7365
13. Meng Q, Qin Y, Deshpande M, et al. Hypoxia-inducible factor-dependent expression of angiopoietin-like 4 by conjunctival epithelial cells promotes the angiogenic phenotype of pterygia. Invest Ophthalmol Vis Sci. 2017;58(11):4514–4523. doi:10.1167/iovs.17-21974
14. Cazes A, Galaup A, Chomel C, et al. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res. 2006;99(11):1207–1215. doi:10.1161/01.RES.0000250758.63358.91
15. Chakraborty A, Kamermans A, van Het Hof B, et al. Angiopoietin like-4 as a novel vascular mediator in capillary cerebral amyloid angiopathy. Brain. 2018;141(12):3377–3388. doi:10.1093/brain/awy274
16. Zhang T, Kastrenopoulou A, Larrouture Q, et al. Angiopoietin-like 4 promotes osteosarcoma cell proliferation and migration and stimulates osteoclastogenesis. BMC Cancer. 2018;18(1):536. doi:10.1186/s12885-018-4468-5
17. Chen JW, Luo YJ, Yang ZF, et al. Knockdown of angiopoietin-like 4 inhibits the development of human gastric cancer. Oncol Rep. 2018;39(4):1739–1746. doi:10.3892/or.2018.6253
18. Le Jan S, Amy C, Cazes A, et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162(5):1521–1528. doi:10.1016/S0002-9440(10)64285-X
19. Zhu X, Guo X, Wu S, et al. ANGPTL4 correlates with NSCLC progression and regulates epithelial-mesenchymal transition via ERK pathway. Lung. 2016;194(4):637–646. doi:10.1007/s00408-016-9895-y
20. Nie D, Zheng Q, Liu L, et al. Up-regulated of angiopoietin-like protein 4 predicts poor prognosis in cervical cancer. J Cancer. 2019;10(8):1896–1901. doi:10.7150/jca.29916
21. Tan MJ, Teo Z, Sng MK, et al. Emerging roles of angiopoietin-like 4 in human cancer. Mol Cancer Res. 2012;10(6):677–688. doi:10.1158/1541-7786.MCR-11-0519
22. Crispo F, Notarangelo T, Pietrafesa M, et al. BRAF inhibitors in thyroid cancer: clinical impact, mechanisms of resistance and future perspectives. Cancers (Basel). 2019;11:9. doi:10.3390/cancers11091388
23. Nozhat Z, Hedayati M. PI3K/AKT pathway and its mediators in thyroid carcinomas. Mol Diagn Ther. 2016;20(1):13–26. doi:10.1007/s40291-015-0175-y
24. Alexandraki KI, Kaltsas G. Gastroenteropancreatic neuroendocrine tumors: new insights in the diagnosis and therapy. Endocrine. 2012;41(1):40–52. doi:10.1007/s12020-011-9562-2
25. Jiang Y, Hao S, Tian W, et al. PI3K inhibitors IC87114 inhibits the migration and invasion of thyroid cancer cell in vitro and in vivo. J Cell Biochem. 2018;119(5):4097–4102. doi:10.1002/jcb.v119.5
26. Cushing EM, Chi X, Sylvers KL, et al. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol Metab. 2017;6(8):809–818. doi:10.1016/j.molmet.2017.06.007
27. Gonzalez-Muniesa P, de Oliveira C, Pérez de Heredia F, Thompson MP, de Heredia FP, Thompson MP, Trayhurn P. Fatty acids and hypoxia stimulate the expression and secretion of the adipokine ANGPTL4 (angiopoietin-like protein 4/fasting-induced adipose factor) by human adipocytes. J Nutrigenet Nutrigenomics. 2011;4(3):146–153. doi:10.1159/000327774
28. Kaddatz K, Adhikary T, Finkernagel F, et al. Transcriptional profiling identifies functional interactions of TGF beta and PPAR beta/delta signaling: synergistic induction of ANGPTL4 transcription. J Biol Chem. 2010;285(38):29469–29479. doi:10.1074/jbc.M110.142018
29. Mandard S, Zandbergen F, Tan NS, et al. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279(33):34411–34420. doi:10.1074/jbc.M403058200
30. Dong D, Jia L, Zhou Y, et al. Serum level of ANGPTL4 as a potential biomarker in renal cell carcinoma. Urol Oncol. 2017;35(5):279–285. doi:10.1016/j.urolonc.2016.12.017
31. Baba K, Kitajima Y, Miyake S, et al. Hypoxia-induced ANGPTL4 sustains tumour growth and anoikis resistance through different mechanisms in scirrhous gastric cancer cell lines. Sci Rep. 2017;7(1):11127. doi:10.1038/s41598-017-11769-x
32. Li Y, Zhou X, Zhang Q, et al. Lipase member H is a downstream molecular target of hypoxia inducible factor-1alpha and promotes papillary thyroid carcinoma cell migration in BCPAP and KTC-1 cell lines. Cancer Manag Res. 2019;11:931–941. doi:10.2147/CMAR.S183355
33. Li H, Ge C, Zhao F, et al. Hypoxia-inducible factor 1 alpha-activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin beta1 signaling in human hepatocellular carcinoma. Hepatology. 2011;54(3):910–919. doi:10.1002/hep.24479
34. Gartland RM, Lubitz CC. Impact of extent of surgery on tumor recurrence and survival for papillary thyroid cancer patients. Ann Surg Oncol. 2018;25(9):2520–2525. doi:10.1245/s10434-018-6550-2
35. Tazzari PL, Cappellini A, Ricci F, et al. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21(3):427–438. doi:10.1038/sj.leu.2404523
36. Li M, Yang XJ, Zhang GY, et al. ANGPTL4 participates in gestational diabetes mellitus via regulating Akt pathway. Eur Rev Med Pharmacol Sci. 2018;22(16):5056–5062. doi:10.26355/eurrev_201808_15697
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.