Back to Journals » Journal of Hepatocellular Carcinoma » Volume 7
Angiogenesis in Hepatocellular Carcinoma; Pathophysiology, Targeted Therapy, and Role of Imaging
Authors Moawad AW , Szklaruk J , Lall C, Blair KJ, Kaseb AO, Kamath A, Rohren SA, Elsayes KM
Received 24 July 2019
Accepted for publication 24 December 2019
Published 23 April 2020 Volume 2020:7 Pages 77—89
DOI https://doi.org/10.2147/JHC.S224471
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Srikanta Dash
Ahmed W Moawad,1 Janio Szklaruk,1 Chandana Lall,2 Katherine J Blair,1 Ahmed O Kaseb,3 Amita Kamath,4 Scott A Rohren,5 Khaled M Elsayes1
1Department of Diagnostic Radiology, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA; 2Department of Radiology, University of Florida College of Medicine, Jacksonville, FL, USA; 3Department of Gastrointestinal Oncology, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA; 4Department of Radiology, Icahn School of Medicine at Mount Sinai West, New York, NY, USA; 5School of Medicine, Baylor College of Medicine, Houston, TX, USA
Correspondence: Khaled M Elsayes
Department of Radiology, The University of Texas MD Anderson Cancer Center, 1400 Pressler Street, Houston, TX 77030, USA
Tel +1 713-745-3025
Fax +1 713-794-4379
Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide, usually occurring on a background of liver cirrhosis. HCC is a highly vascular tumor in which angiogenesis plays a major role in tumor growth and spread. Tumor-induced angiogenesis is usually related to a complex interplay between multiple factors and pathways, with vascular endothelial growth factor being a major player in angiogenesis. In the past decade, understanding of tumor-induced angiogenesis has led to the emergence of novel anti-angiogenic therapies, which act by reducing neo-angiogenesis, and improving patient survival. Currently, Sorafenib and Lenvatinib are being used as the first-line treatment for advanced unresectable HCC. However, a disadvantage of these agents is the presence of numerous side effects. A major challenge in the management of HCC patients being treated with anti-angiogenic therapy is effective monitoring of treatment response, which decides whether to continue treatment or to seek second-line treatment. Several criteria can be used to assess response to treatment, such as quantitative perfusion on cross-sectional imaging and novel/emerging MRI techniques, including a host of known and emerging biomarkers and radiogenomics. This review addresses the pathophysiology of angiogenesis in HCC, accurate imaging assessment of angiogenesis, monitoring effects of anti-angiogenic therapy to guide future treatment and assessing prognosis.
Keywords: angiogenesis, hepatocellular carcinoma, anti-angiogenic therapy, Sorafenib
Introduction
Primary liver tumors are the 6th most commonly diagnosed cancers and the second most common cause of cancer deaths around the world.1 Hepatocellular carcinoma (HCC) accounts for about 90% of primary liver tumors with the highest disease burden in sub-Saharan Africa and Asia where Hepatitis B virus (HBV) infection is endemic.2 Most of these cases, especially in Asia and Africa, present at an advanced stage, typically beyond the capability of curative treatment. Greater than 70% of HCC is diagnosed at later stages, typically when unresectable. The main treatment options for unresectable HCC include loco-regional therapy with trans-arterial chemoembolization (TACE) or systemic therapy with agents such as Sorafenib.
Anti-angiogenic therapy is currently the recommended therapy for advanced stage disease given the highly vascular nature of HCC.3 We will focus our discussion on pathophysiological concepts of angiogenesis in HCC, and the role of imaging in the assessment of angiogenesis and monitoring the response to treatment of patients with HCC treated with anti-angiogenic therapy.
Epidemiology of HCC
Generally, HCC results from a series of liver insults either acute or sub-acute which progress slowly into fibrosis and subsequent cirrhosis. Less commonly, HCC develops without previous liver cirrhosis. In the cirrhotic liver, HCC is the end result of a progressive hepatic carcinogenesis sequence, beginning with regenerative nodules, dysplastic nodules and final evolution into HCC.4 Most of the patients with HCC have an associated history of viral hepatitis infection. HBV and Hepatitis C virus (HCV) account for 50% and 25% of all cases of HCC, respectively.5 Obesity and diabetes mellitus have also been associated with an increased risk of HCC due to the development of non-alcoholic steatohepatitis (NASH).6 Chronic alcohol consumption can be synergistic with hepatitis in the development of HCC.7 Chronic aflatoxin exposure is another risk factor for HCC but this is less common in the USA, mostly occurring in Asia and Africa.8
Pathophysiology of Angiogenesis in HCC
Angiogenesis refers to the expansion and remodeling of the primary embryonic vascular network. This occurs physiologically in adults during the menstrual cycle and during processes such as wound healing. Understanding the pathophysiology of angiogenesis is critical in the management of patients with HCC. Under non-pathological conditions, angiogenesis is a highly ordered and tightly regulated process with complex but balanced interactions between pro-angiogenic and anti-angiogenic factors. These factors can be divided into secreted factors and membrane bound factors.9 Secreted factors include vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), angiopoietins (Ang), and platelet-derived growth factors (PDGF). Membrane bound factors include neuropilin receptors (NRP). Uncontrolled angiogenesis can be seen in the setting of solid tumors and diabetic retinopathy.10
Tumor-induced angiogenesis is mediated by two essential factors, over-expression of angiogenic factors, as well as inhibition of anti-angiogenic factors, resulting in increased tumor vascular burden with abnormal blood vessels which lack normal vascular structure with deficient pericytes, smooth muscle cells, and intact basement membrane.11
Knowledge of the particular effect of angiogenic factors has been important in the development of the pharmaceutical/therapeutic options in managing uncontrolled angiogenesis such as that found in tumors. These factors may be used either via stimulation or inhibition of angiogenesis to have a direct effect on cancer treatment.12
Vascular Endothelial Growth Factor (VEGF)
VEGF, the most well-known angiogenic factor, is a secreted factor and a key regulator.13 VEGF is normally expressed in the human body at low levels but has high expression in tumors. It was found that about 91% of advanced HCCs show elevated VEGF expression, relative to normal conditions.14,15 VEGF-A is the isoform responsible for angiogenesis and vascular remodeling, it binds to tyrosine kinase-related receptors (VEGFR).16 There are at least 3 known members of these receptors: VEGFR-1, VEGFR-2, and VEGFR-3. VEFGR 1 and 2 are considered the most important receptors in angiogenesis. The levels of VEGFR-2 have been correlated with a worse outcome in HCC.17,18 Binding of VEGF with its receptor stimulates a transduction signal that leads to proliferation and migration of endothelial cells (EC), as well as induction of angiogenesis.19,20 Bevacizumab is an example of a VEGF-A antibody which has been widely used in brain and colorectal cancers, among others.21
Fibroblast Growth Factor (FGF)
FGFs are a family of growth factors containing several members which interact with tyrosine kinase receptors (FGF receptors (FGFR) 1 through 4).22 FGFs and its receptors have numerous functions including differentiation as well as maintenance of neovascularization initiated by VEGF.23 This is mediated by the enzyme Heparanase which induces angiogenesis, new vessel formation and stimulation of endothelial cell invasion to induce distant metastasis.23,24
FGFRs are normally expressed in adult cells with FGFRs 3 and 4 being the most commonly expressed by normal hepatocytes.25 Clinical studies demonstrate the importance of FGF subtype-19 (FGF19) in tumor-induced angiogenesis, where it was shown that FGF19/FGFR4 complex was overexpressed in adult HCC.26 In addition, administration of FGF19 neutralizing antibodies prevents HCC development in mice.27
A recent pre-clinical study showed that the FGF signaling pathway maintains survival of murine HCC after angiogenesis inhibition, confirming the integral role of FGR/FGFR in HCC induced angiogenesis. Furthermore, it suggested the need of dual inhibition of VEGF and FGF axes to enhance cancer cell death.28
Angiopoietins and Tie Receptors
Angiopoietins (Ang) are secreted proteins that play an important role in HCC. These proteins interact with Tie receptors, which are membrane bound tyrosine kinase receptors. The Ang/Tie complex enhances vascular stability and induces apoptosis and cellular matrix destabilization in the absence of VEGF.29,30 Over-expression of Ang-2, relative to Ang-1, is found to be correlated with HCC. It was hypothesized that the role of Ang/Tie complex in the development of HCC is much more important than the VEGF system, which makes HCC a suitable neoplasm for anti-angiogenic therapy.31
Platelet-Derived Growth Factor (PDGF)
PDGFs are secreted growth factors closely related to VEGF32 and are important in HCC-induced angiogenesis. PDGF binds to tyrosine kinase receptors; PDGF receptors (PDGFR) α and β.33 Binding of the growth factors with their corresponding receptors leads to activation of signaling cascade which leads to upregulation of VEGF and recruitment of perivascular cells.34 The role of the PDGF/PDGFR complex in angiogenesis was found to contribute to tumor development, along with its autocrine role in the stimulation of cancer cells.35 Overexpression of PDGF-C and B subtypes was found to correlate with liver fibrosis and progression of dysplastic nodules to HCC in mice models.36,37
Neuropilin Receptors (NRP)
The two homologs of NRP family (namely NRP-1 and NRP-2) each has a different action and role.38 In the liver, NRP-1 receptors are expressed in veins and capillaries and bind with VEGF to act as co-receptors for VEGF.39–41 Hepatic NRP-1 was found to bind hepatocytes growth factor (HGF) which has potent angiogenic activity for hepatocytes.42 NRP-1 co-localizes with PDGF receptor-β, resulting in tumoral spread.43
Anti-Angiogenic Therapy of HCC
Treatment options for advanced HCC are limited, usually improving overall survival rather than curing the disease. These options include chemotherapy, radio-embolization, immune check-point therapy, and anti-angiogenic therapy.44
Anti-angiogenic therapy was developed with the rationale that these drugs lead to destruction of the abnormally structured blood vessels resulting in tumor hypoxia and shrinkage.45 However, Jain et al introduced the vascular normalization hypothesis that anti-angiogenic therapy restores the integrity and structure of the tumor-induced blood vessels without destroying these vessels.46
Anti-angiogenic induced reduction in tumoral vascular burden induces hypoxic tumoral microenvironment. This hypoxia leads to accumulation of certain chemokines including programmed death receptor ligand 1 (PD-L1) which suppress immune cells and induces resistance to anti-angiogenic therapy, recent advances include the combination of immune checkpoint therapy with anti-angiogenic therapy to treat advanced HCC.47,48
To date, the US Food and Drug Administration (FDA) has approved few anti-angiogenic drugs for HCC. Table 1 summarizes these drugs and their indications.49–58
 |
Table 1 List of Anti-Angiogenic Drugs That are FDA Approved for Treatment of HCC |
Sorafenib is an oral multi-kinase inhibitor that targets the activity of VEGF-2, PDGFR-β, and other signaling cascades, and has been the only approved systemic therapy for HCC for over a decade. Sorafenib upregulates P53 and decreases expression of matrix metalloprotease-2 (MMP-2) which suppresses cellular proliferation and invasion.59,60 It is the only anti-angiogenic drug incorporated into the American Association for the Study of Liver Diseases (AASLD) guidelines for treatment advanced HCC (unresectable HCC).44,61,62 (Figure 1). Although its indication of use is still vague, it is the first line of treatment in advanced HCC (with macro-vascular invasion or metastatic disease).44
 |
Figure 1 Indication of HCC resection as stated by ASSLD guidelines for treatment HCC 2018. Data from Poon et al.62 |
Lenvatinib is a more potent anti-angiogenic drug than Sorafenib, which acts through multi-kinase inhibition of VEGFR (more potent than Sorafenib), FGF receptors and PDGFR. Dual suppression of VEGF and FGF pathways results in concomitant suppression of both neo-angiogenesis and tumor growth. Lenvatinib is used in unresectable HCC with comparable results in overall survival between both Lenvatinib and Sorafenib.63 Lenvatinib provides an advantage over Sorafenib with a longer time to progression as well as progression-free survival in patients enrolled in the trial.64
Regorafenib and Cabozantinib are tyrosine kinase inhibitors which are the only anti-angiogenic drugs that were seen to be advantageous as a second-line therapy in patients who progressed on Sorafenib. Regorafenib and Cabozantinib show statistically significant improvement in overall survival and progression-free survival over the use of placebo in these patients.54,55,65
Ramucirumab is a monoclonal antibody that inhibits the VEGF axis through blocking the activation of VEGFR-2. The FDA recently approved the use of Ramucirumab based on Phase 3 trial (REACH-2) that demonstrated effectiveness of this drug in patients who progressed on Sorafenib with AFP ≥ 400 ng/mL. It showed an increase in overall survival and progression-free survival with comparable results as Cabozantinib and Regorafenib.57,58
Imaging of HCC for Evaluation of Angiogenesis
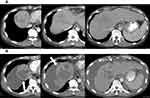
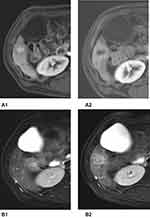
Monitoring anti-angiogenic therapy for HCC is crucial in management and improves the effectiveness and efficiency of patient care.66 The primary response of anti-angiogenic therapy depends predominantly on intra-tumoral vascularity changes (Figure 2). This response is unlike cytotoxic drugs which result in the killing of tumor cells directly, leading to tumor necrosis and hemorrhage with monitoring of post-treatment effect depending solely on size and volume (as in RECIST 1.1 and WHO classification). Using this same method to monitor response may provide an erroneous treatment response during anti-angiogenic treatment67 (Figure 3).
Routine Imaging Studies for Angiogenesis Evaluation
Magnetic resonance imaging (MRI) is rapidly proving to be a superior modality in oncologic imaging with technologic improvements in acquisition and image refinement. Several studies have demonstrated that MRI has better sensitivity and specificity for both detection and characterization of HCC compared to computed tomography (CT) and ultrasound imaging.68–70
Imaging of angiogenesis is best performed using dynamic contrast-enhanced multi-phasic CT and MRI. The enhancement pattern of HCC depends on changes that occur due to angiogenesis during the process of carcinogenesis. Normally, the main blood supply of the liver is from the portal vein with little contribution from the hepatic artery. In HCC, especially in advanced cases, vascular supply is typically only from the hepatic artery with no contribution from the portal system (Figure 4).71 Detection of tumoral vascularity depends on intravenous injection of contrast media that causes differential enhancement between the tumor and the normal liver parenchyma.
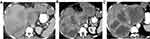
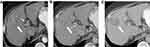
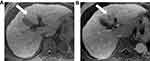
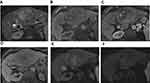
The classic enhancement pattern according to Liver Imaging Reporting and Data System (LI-RADS®) v2018 and AASLD is tumoral hyper-enhancement in the arterial phase since normal hepatic parenchyma is supplied mainly by the portal vein while HCC depends primarily on hepatic arterial supply. The classic pattern of HCC is washout out in the portal/delayed phase which contrasts with the background parenchymal enhancement in these phases relative to the HCC, which becomes hypo-attenuated relative to normal liver (Figure 5).61,72,73 This classic enhancement pattern is present in only 28% of HCC measuring 1–2 cm. At this size, HCCs even may be hypo-vascular and appear hypo-attenuated in cross-sectional imaging, which is a diagnostic dilemma (Figure 6). These findings may be due to the fact that these lesions contain few unpaired arteries.74,75 The degree of tumoral enhancement can be monitored using treatment with Sorafenib, due to its antagonistic effect on blood vessels. MRI has an advantage over CT in monitoring treatment response since enhancement effects on MRI are more robust than on CT imaging. Tumoral necrosis appears as non-enhancing areas with increased necrosis-to-viable tumor ratio. Dynamic contrast-enhanced MRI is more sensitive than CT in diagnosis of HCC as MRI offers various ancillary sequences sensitive for detection and characterization, including diffusion weighted imaging (DWI) (in addition to the signal changes on T1 and T2 weighted images).76 T1 and T2 weighted images (WI) can show signal changes following therapy as early as 2–4 weeks. Tumoral response usually appears as focal/diffuse increased signal on both T1 and T2 weighted sequences due to necrosis, hemorrhage or both (Figure 7). These changes in signal must be interpreted with caution as Sorafenib usually induces intra-tumoral hemorrhage which affects the signal of T1WI and T2WI according to the phase of hemorrhage (early, subacute or late phases).77,78 DWI is an important imaging biomarker to monitor Sorafenib therapy. HCC usually shows diffusion restriction with high signal on DWI. Cellular changes following Sorafenib therapy lead to decreased signal on DWI and increased ADC values i.e. decreased diffusion restriction79 (Figure 8).
Specific Imaging Studies for Angiogenesis
The need for specific tools to assess anti-angiogenic response emerges from the lack of quantitative studies to track early changes in angiogenesis.
Perfusion Cross-Sectional Imaging
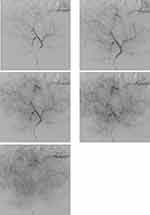
Hepatic perfusion imaging can be used for monitoring the response of anti-angiogenic therapy and depends predominantly on the vascular burden of the tumor. Perfusion imaging is obtained using contrast-enhanced studies with quantification of the blood flow at the capillary level per unit time, depending on the hemodynamic circulation of the contrast agent. Quantitative studies are shown to be more sensitive in detecting response to anti-angiogenic therapy in the form of vascular burden changes, compared to RECIST and also detects changes much earlier than mRECIST.66,80
Hepatic Perfusion CT scan (pCT) includes a pre-contrast series followed by sequential scanning of the tumor in two phases: an early phase within 40–60 seconds from the time of contrast administration (30–60 mL of iodine-based contrast agent must be injected rate ≥5 mL/sec followed by 50 mL saline flush) and a delayed phase within 2–10 mins. Perfusion CT requires a multi-detector scanner (≥16 detector configuration) with high temporal resolution (one image per second) to ensure proper extraction of the perfusion parameters.81,82
Perfusion MRI (pMRI) of the liver is a dynamic T1W imaging technique that can provide quantitative data of tumor microvasculature, detecting vasculature changes in the tumor, prior to and after therapy. Pre-contrast T1 mapping is usually performed in the oblique axis (to include all vessels) followed by gadolinium-based extracellular contrast injection. Contrast enhancement is evaluated on the late arterial, portal venous and delayed dynamic phases. 3D gradient echo techniques such as fast spoiled gradient echo, fast low angle shot (FLASH) with variable flip angles with parallel imaging are recommended for image acquisition due to a higher signal-to-noise ratio and less scan time.83,84 In either modality, the scan should include the main portal vein, aorta and the region of interest (HCC under therapy in our case).
Quantification of the perfusion parameters can be obtained by either model-free approach or model-based approach; model-free approach depends on capturing tissue enhancement rate in relation to contrast passage through the tissues.
Perfusion parameters are extracted from the maximum slope of time-to-intensity curve of hepatic artery and portal vein to derive only hepatic perfusion index (HPI). Model-based approach built according to tracer kinetic physiological models. The most commonly used models in liver perfusion images are dual compartmental model (deconvolution analysis) and distributed parameter model (for further details about these kinetic models, see reference85). The difference between both models depends on the contrast concentration gradient between the intravascular and interstitial space.
The “Dual Compartment Model” assumed an equal distribution of the contrast between both spaces while the “Distributed Parameter Model” takes into consideration the tracer concentration gradient between both spaces, especially in cases of HCC due to the presence of immature tumoral vessels. Being computationally simpler, the Dual Compartment Model is more commonly used with the main drawbacks being the underestimation of the permeability surface area product and inability to calculate the mean transit time (MTT), which is an important parameter for monitoring anti-angiogenic therapy.85–87
Perfusion studies have recently been shown to be valid for assessment of response to anti-angiogenic drugs using the parametric perfusion maps and extracting parameters from the kinetic model (Table 2). These values reflect the flow of contrast between the extracellular and intracellular compartments, thus indicating tumoral blood flow and vascular permeability.88–91
 |
Table 2 Perfusion Parameter Obtained in the Previous Studies for Monitoring Treatment with Sorafenib |
Generally in both pCT and pMRI, there is a trend for a significant decrease in the levels of blood flow (BF), BV, HPI and ALP with significant increase in MTT.80,81
K-trans is the most important parameter extracted from pMRI, which is independently affected by blood flow, vessel surface area, and vessel permeability. In pre-clinical & clinical trials, K-trans is associated with higher microvascular density and its level is correlated with the aggressive nature of the liver nodules being highest in HCC compared with regenerative nodules.92–96
Research in perfusion imaging is ongoing in preclinical and clinical trials and is a promising radiological marker for monitoring response to anti-angiogenic therapy.
Use of Contrast Enhanced US (CEUS) in Diagnosis of HCC
CEUS is a potential third modality besides MRI and CT to diagnose HCC, but it has not been approved in the USA since cholangiocarcinoma may show the same vascular enhancement pattern as HCC.97 CEUS, however, has its uses in certain circumstances. According to LI-RADS, CEUS can be used for (a) further evaluation of focal lesions ≥10 mm detected on unenhanced US, (b) further assessment of probably HCC on CT/MRI (LI-RADS 3/4) and (c) detection of arterial hyper-enhancement when there is a mismatch on a prior CT or MRI. Microbubble injection during US is safe which may be an advantage over CT and MR where contrast agents can have potential risks in some patients.98
Conclusion
Recent imaging advancements are now being used routinely in monitoring angiogenesis which is an essential and integral part of treatment in advanced HCC. Ongoing clinical trials and novel treatments are promising, many using a combination of immune therapy, loco-regional therapy and anti-angiogenic therapy for advanced HCC management. Recent advances in MR techniques and other radiological biomarkers are currently being used to monitor changes in angiogenesis in advanced HCC.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mohammadian M, Mahdavifar N, Mohammadian-hafshejani A. Liver cancer in the world: epidemiology, incidence, mortality and risk factors. WCRJ. 2018;5(2):e1082.
2. Mittal S, El-serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl(0)):S2–S6. doi:10.1097/MCG.0b013e3182872f29
3. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917. doi:10.1016/S0140-6736(03)14964-1
4. Seeff LB. Introduction: the burden of hepatocellular carcinoma. Gastroenterology. 2004;127(5,Supplement 1):S1–S4. doi:10.1053/j.gastro.2004.09.010
5. Janevska D, Chaloska-ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732–736. doi:10.3889/oamjms.2015.111
6. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA-R, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978. doi:10.1002/hep.23527
7. Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155(4):323–331. doi:10.1093/aje/155.4.323
8. Waly Raphael S, Yangde Z, Yuxiang C. Hepatocellular carcinoma: focus on different aspects of management. ISRN Oncol. 2012;2012:421673. doi:10.5402/2012/421673
9. Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282(5):C947–C970. doi:10.1152/ajpcell.00389.2001
10. Klagsbrun M, D’Amore PA. Regulators of angiogenesis. Annu Rev Physiol. 1991;53:217–239. doi:10.1146/annurev.ph.53.030191.001245
11. Gupta MK, Qin R-Y. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9(6):1144. doi:10.3748/wjg.v9.i6.1144
12. Nagy J, Chang S, Dvorak A, Dvorak H. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100(6):865–869. doi:10.1038/sj.bjc.6604929
13. Karamysheva AF. Mechanisms of angiogenesis. Biochem Biokhimiia. 2008;73(7):751–762. doi:10.1134/S0006297908070031
14. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi:10.1056/NEJM198612253152606
15. Kaseb AO, Hanbali A, Cotant M, Hassan MM, Wollner I, Philip PA. Vascular endothelial growth factor in the management of hepatocellular carcinoma. Cancer. 2009;115(21):4895–4906. doi:10.1002/cncr.v115:21
16. Shibuya M. Vascular Endothelial Growth Factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–1105. doi:10.1177/1947601911423031
17. Maharaj AS, Saint-geniez M, Maldonado AE, D’amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168(2):639–648. doi:10.2353/ajpath.2006.050834
18. Kim RD, Lazaryan A, Aucejo F, et al. Vascular endothelial growth factor receptor 2 (VEGFr2) expression and recurrence of hepatocellular carcinoma following liver transplantation: the cleveland clinic experience. J Clin Oncol. 2008;26(15_suppl):4594. doi:10.1200/jco.2008.26.15_suppl.4594
19. Carmeliet P, De Smet F, Loges S, Mazzone M. Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol. 2009;6(6):315–326. doi:10.1038/nrclinonc.2009.64
20. Suchting S, Freitas C, le Noble F, et al. The notch ligand delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104(9):3225–3230. doi:10.1073/pnas.0611177104
21. Zhu AX, Holalkere NS, Muzikansky A, Horgan K, Sahani DV. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13(2):120–125. doi:10.1634/theoncologist.2007-0174
22. Chae YK, Ranganath K, Hammerman PS, et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2017;8(9):16052. doi:10.18632/oncotarget.v8i9
23. Compagni A, Wilgenbus P, Impagnatiello MA, Cotten M, Christofori G. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res. 2000;60(24):7163–7169.
24. Vlodavsky I, Elkin M, Pappo O, et al. Mammalian heparanase as mediator of tumor metastasis and angiogenesis. Isr Med Assoc J. 2000;2(Suppl):37–45.
25. Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45(7):1005–1019. doi:10.1177/002215549704500710
26. Cheng A-L, Shen Y-C, Zhu AX. Targeting fibroblast growth factor receptor signaling in hepatocellular carcinoma. Oncology. 2011;81(5–6):372–380. doi:10.1159/000335472
27. Sawey ET, Chanrion M, Cai C, et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by oncogenomic screening. Cancer Cell. 2011;19(3):347–358. doi:10.1016/j.ccr.2011.01.040
28. Hoshi T, Watanabe Miyano S, Watanabe H, et al. Lenvatinib induces death of human hepatocellular carcinoma cells harboring an activated FGF signaling pathway through inhibition of FGFR–MAPK cascades. Biochem Biophys Res Commun. 2019;513(1):1–7. doi:10.1016/j.bbrc.2019.02.015
29. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. doi:10.1038/nrm2639
30. Le CT, Laidlaw G, Morehouse CA, et al. Synergistic actions of blocking angiopoietin-2 and tumor necrosis factor-alpha in suppressing remodeling of blood vessels and lymphatics in airway inflammation. Am J Pathol. 2015;185(11):2949–2968. doi:10.1016/j.ajpath.2015.07.010
31. Mitsuhashi N, Shimizu H, Ohtsuka M, et al. Angiopoietins and Tie‐2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology. 2003;37(5):1105–1113. doi:10.1053/jhep.2003.50204
32. Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398(2):284–290. doi:10.1006/abbi.2001.2707
33. Magnusson PU, Looman C, Ahgren A, Wu Y, Claesson-welsh L, Heuchel RL. Platelet-derived growth factor receptor-beta constitutive activity promotes angiogenesis in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2007;27(10):2142–2149. doi:10.1161/01.ATV.0000282198.60701.94
34. Laschke MW, Elitzsch A, Vollmar B, Vajkoczy P, Menger MD. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006;21(1):262–268. doi:10.1093/humrep/dei308
35. Raica M, Cimpean AM. Platelet-Derived Growth Factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals (Basel). 2010;3(3):572–599. doi:10.3390/ph3030572
36. Campbell JS, Johnson MM, Bauer RL, et al. Targeting stromal cells for the treatment of platelet-derived growth factor C-induced hepatocellular carcinogenesis. Differentiation. 2007;75(9):843–852. doi:10.1111/j.1432-0436.2007.00235.x
37. Maass T, Thieringer FR, Mann A, et al. Liver specific overexpression of platelet-derived growth factor-B accelerates liver cancer development in chemically induced liver carcinogenesis. Int J Cancer. 2011;128(6):1259–1268. doi:10.1002/ijc.v128.6
38. Nakamura F, Goshima Y. Structural and functional relation of neuropilins. Adv Exp Med Biol. 2002;515:55–69.
39. Berge M, Allanic D, Bonnin P, et al. Neuropilin-1 is upregulated in hepatocellular carcinoma and contributes to tumour growth and vascular remodelling. J Hepatol. 2011;55(4):866–875. doi:10.1016/j.jhep.2011.01.033
40. Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001;109(1):115–119. doi:10.1016/S0925-4773(01)00518-4
41. Pellet-many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411(2):211–226. doi:10.1042/BJ20071639
42. Panigrahy D, Adini I, Mamluk R, et al. Regulation of soluble neuropilin 1, an endogenous angiogenesis inhibitor, in liver development and regeneration. Pathology. 2014;46(5):416–423. doi:10.1097/PAT.0000000000000121
43. Xu ZC, Shen HX, Chen C, et al. Neuropilin-1 promotes primary liver cancer progression by potentiating the activity of hepatic stellate cells. Oncol Lett. 2018;15(2):2245–2251. doi:10.3892/ol.2017.7541
44. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
45. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi:10.1056/NEJM197111182852108
46. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi:10.1126/science.1104819
47. Liu G-M, Li X-G, Zhang Y-M. Prognostic role of PD-L1 for HCC patients after potentially curative resection: a meta-analysis. Cancer Cell Int. 2019;19(1):22. doi:10.1186/s12935-019-0738-9
48. Shun L, Liu X, Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019;24(Suppl 1):S3–S10. doi:10.1634/theoncologist.2019-IO-S1-s01
49. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390.
50. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
51. FDA Approves Lenvatinib for Unresectable Hepatocellular Carcinoma. U.S. Food & Drug administration (FDA); 2018.
52. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
53. FDA Expands Approved Use of Stivarga to Treat Liver Cancer. U.S. Food & Drug administration (FDA); 2017.
54. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
55. Abou-alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
56. FDA Approves Cabozantinib for Hepatocellular Carcinoma. U.S. Food & Drug administration (FDA); January 2019.
57. FDA Approves Ramucirumab for Hepatocellular Carcinoma. U.S. Food & Drug administration (FDA); May 2019.
58. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
59. Cervello M, Bachvarov D, Lampiasi N, et al. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle. 2012;11(15):2843–2855. doi:10.4161/cc.21193
60. Wei JC, Meng FD, Qu K, et al. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol Sin. 2015;36(2):241–251. doi:10.1038/aps.2014.122
61. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
62. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–382. doi:10.1097/00000658-200203000-00009
63. Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018;7(1):1–19. doi:10.1159/000487148
64. Cheng A-L, Finn RS, Qin S, et al. Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients (pts) with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2017;35(15_suppl):4001. doi:10.1200/JCO.2017.35.15_suppl.4001
65. Personeni N, Pressiani T, Santoro A, Rimassa L. Regorafenib in hepatocellular carcinoma: latest evidence and clinical implications. Drugs Context. 2018;7:212533. doi:10.7573/17404398
66. Kim KW, Lee JM, Choi BI. Assessment of the treatment response of HCC. Abdom Imaging. 2011;36(3):300–314. doi:10.1007/s00261-011-9683-3
67. Jiang T, Kambadakone A, Kulkarni NM, Zhu AX, Sahani DV. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST). Invest Radiol. 2012;47(1):11–17. doi:10.1097/RLI.0b013e3182199bb5
68. Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2014;10(3):153–161.
69. European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi:10.1016/j.jhep.2011.12.001
70. Elsayes KM, Hooker JC, Agrons MM, et al. 2017 Version of LI-RADS for CT and MR imaging: an update. RadioGraphics. 2017;37(7):1994–2017. doi:10.1148/rg.2017170098
71. Asayama Y, Yoshimitsu K, Nishihara Y, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. Am J Roentgenol. 2008;190(1):W28–W34. doi:10.2214/AJR.07.2117
72. Ludwig DR, Fraum TJ, Cannella R, et al. Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of liver imaging reporting and data system v2018. Abdom Radiol (NY). 2019. doi:10.1007/s00261-019-01948-x
73. American College of Radiology. CT/MRI LI-RADS® v2018. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018.
74. The International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49(2):658–664. doi:10.1002/hep.22709
75. Yoon SH, Lee JM, So YH, et al. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009;193(6):W482–W489. doi:10.2214/AJR.08.1818
76. Kim MJ, Choi JI, Lee JS, Park JW. Computed tomography findings of sorafenib-treated hepatic tumors in patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(7):1201–1206. doi:10.1111/j.1440-1746.2011.06709.x
77. Horger M, Lauer UM, Schraml C, et al. Early MRI response monitoring of patients with advanced hepatocellular carcinoma under treatment with the multikinase inhibitor sorafenib. BMC Cancer. 2009;9:208. doi:10.1186/1471-2407-9-208
78. Choi J-I, Imagawa DK, Bhosale P, et al. Magnetic resonance imaging following treatment of advanced hepatocellular carcinoma with sorafenib. Clin Mol Hepatol. 2014;20(2):218–222. doi:10.3350/cmh.2014.20.2.218
79. Schraml C, Schwenzer NF, Martirosian P, et al. Diffusion-weighted MRI of advanced hepatocellular carcinoma during sorafenib treatment: initial results. AJR Am J Roentgenol. 2009;193(4):W301–W307. doi:10.2214/AJR.08.2289
80. Kaufmann S, Thaiss WM, Schulze M, et al. Prognostic value of perfusion CT in hepatocellular carcinoma treatment with sorafenib: comparison with mRECIST in longitudinal follow-up. Acta radiologica. 2018;59(7):765–772. doi:10.1177/0284185117732805
81. Kim SH, Kamaya A, Willmann JK. CT perfusion of the liver: principles and applications in oncology. Radiology. 2014;272(2):322–344. doi:10.1148/radiol.14130091
82. Hayano K, Lee SH, Sahani DV. Imaging for assessment of treatment response in hepatocellular carcinoma: current update. Indian J Radiol Imaging. 2015;25(2):121–128. doi:10.4103/0971-3026.155835
83. Thng CH, Koh TS, Collins DJ, Koh DM. Perfusion magnetic resonance imaging of the liver. World J Gastroenterol. 2010;16(13):1598–1609. doi:10.3748/wjg.v16.i13.1598
84. Abdullah SS, Pialat JB, Wiart M, et al. Characterization of hepatocellular carcinoma and colorectal liver metastasis by means of perfusion MRI. J Magn Reson Imaging. 2008;28(2):390–395. doi:10.1002/jmri.v28:2
85. Lee T-Y. Functional CT: physiological models. Trends Biotechnol. 2002;20(8):S3–S10. doi:10.1016/S0167-7799(02)02035-8
86. Cheong LHD, Lim CCT, Koh TS. Dynamic contrast-enhanced CT of intracranial meningioma: comparison of distributed and compartmental tracer kinetic models—initial results. Radiology. 2004;232(3):921–930. doi:10.1148/radiol.2323031198
87. Cuenod CA, Balvay D. Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging. 2013;94(12):1187–1204. doi:10.1016/j.diii.2013.10.010
88. Chen BB, Shih TT. DCE-MRI in hepatocellular carcinoma-clinical and therapeutic image biomarker. World J Gastroenterol. 2014;20(12):3125–3134. doi:10.3748/wjg.v20.i12.3125
89. Zech CJ, Reiser MF, Herrmann KA. Imaging of hepatocellular carcinoma by computed tomography and magnetic resonance imaging: state of the art. Dig Dis. 2009;27(2):114–124. doi:10.1159/000218343
90. Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast‐enhanced t1‐weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–232. doi:10.1002/(ISSN)1522-2586
91. Jahng G-H, Li K-L, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol. 2014;15(5):554–577. doi:10.3348/kjr.2014.15.5.554
92. O’connor JPB, Jackson A, Parker GJM, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9:167. doi:10.1038/nrclinonc.2012.2
93. Zhang W, Chen HJ, Wang ZJ, Huang W, Zhang LJ. Dynamic contrast enhanced MR imaging for evaluation of angiogenesis of hepatocellular nodules in liver cirrhosis in N-nitrosodiethylamine induced rat model. Eur Radiol. 2017;27(5):2086–2094. doi:10.1007/s00330-016-4505-1
94. Li L, Wang K, Sun X, et al. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit. 2015;21:376–382. doi:10.12659/MSM.892534
95. Sahani DV, Jiang T, Hayano K, et al. Magnetic resonance imaging biomarkers in hepatocellular carcinoma: association with response and circulating biomarkers after sunitinib therapy. J Hematol Oncol. 2013;6:51. doi:10.1186/1756-8722-6-51
96. Campos M, Candelária I, Papanikolaou N, et al. Perfusion magnetic resonance as a biomarker for sorafenib-treated advanced hepatocellular carcinoma: a pilot study. GE Port J Gastroenterol. 2019. doi:10.1159/000493351
97. Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51(6):2020–2029. doi:10.1002/hep.23600
98. American college of Radiogoy. CEUS LI-RADS® v2017. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CEUS-LI-RADS-v2017.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.