Back to Journals » Journal of Pain Research » Volume 12
Anesthetic management in untreated Bland-White-Garland syndrome: a case report and literature review
Authors Guo Q , Chen YJ, Huang H
Received 5 January 2019
Accepted for publication 27 June 2019
Published 16 July 2019 Volume 2019:12 Pages 2167—2176
DOI https://doi.org/10.2147/JPR.S200534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Qiao Guo, Yuan-Jing Chen, He Huang
Department of Anesthesiology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, People’s Republic of China
Abstract: Bland-White-Garland syndrome (BWGS) is a rare congenital coronary artery malformation. In adult patients with BWGS, left coronary artery is supplied by collateral vessels from dilated right coronary artery. When high-pressure coronary flow drains into the low-pressure pulmonary artery with little ventricle perfusion, it causes a “coronary steal”. In this study, a 53-year-old man with untreated BWGS receiving choledochotomy under general anesthesia was presented. The patient suffered from chronic biliary calculi, atrial fibrillation, complete left bundle branch block, and chronic heart failure. The anesthetic management for choledochotomy in this patient presented a special challenge. Moreover, relevant literature search was performed for all the case reports of BWGS published in PubMed and MEDLINE from 1990 to 2018. In addition, a summary of underlying pathophysiology and anesthetic implications of patients with BWGS was provided.
Keywords: anesthesia, Bland-White-Garland syndrome, left main coronary artery, right coronary artery, pulmonary artery, coronary steal
Background
Bland-White-Garland syndrome (BWGS), also known as anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), is a rare congenital malformation affecting 1 in every 300,000 live births and represents 0.24–0.46% of all congenital cardiac diseases. It is characterized by high mortality due to chronic myocardial ischemia, heart failure, ventricular arrhythmias, and sudden death. If the adequate collateral circulation between right and left coronary artery exists, only about 10–15% of the patients survive to adulthood.1,2 However, in 80–90% of the BWGS cases, sudden cardiac death occurs within the first year of life. The survival depends greatly on an adequate collateral circulation between the left and right coronary arteries. Moreover, identification of dilated collaterals is often the first indication of pathology. Survival to adulthood is unusual, and patients over the age of 50 are rare.
Here, a rare case of a patient with BWGS will be presented, who also suffered from chronic biliary calculi. Although his cardiac function was in poor status and the risk of anesthesia was extremely high, choledocholithiasis was the only chance for him to survive. This case is rare because it describes the anesthetic management of a patient with BWGS, and as far as the author knows there are very few studies that focus on the anesthetic management of patients with BWGS, especially ones undergoing noncardiac surgery.
Case presentation
A 53-year-old man, diagnosed with choledocholithiasis, was scheduled for a choledochotomy under general anesthesia in our hospital. Written informed consent has been obtained by the patient and case details and accompanying images are allowed to be published by the patient and our hospital.
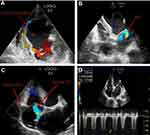
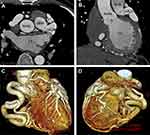
The patient presented with a 10-month history of whole body yellowing and severe abdominal pain and survived a cardiac arrest 1 year ago. His medication included furosemide, spironolactone, and perindopril. The blood pressure was 110/65 mmHg, heart rate 72 beats/min, oxygen saturation 99%. Blood tests showed hemoglobin: 99 g/L, albumin: 35.7 g/L, APTT: 45.9 s, TT: 14.9 s, total bilirubin: 55.3 umol/L, gamma-glutamyltransferase: 554 u/L, PRO-BNP: 3262 pg/mL. The electrocardiogram showed fibrillation with a ventricular rate of 96 beats per minute (Figure 1), complete left bundle branch block, occasional premature ventricular beats. Transthoracic echocardiography (TTE) showed left main coronary artery (LMCA) originating from the pulmonary artery (PA), systolic and diastolic blood flow signals at the origin of LMCA, slightly decreased left ventricular function (ejection fraction 52%), enlargement of the ventricle and atrium, moderate pulmonary hypertension with pulmonary artery pressure of 56 mmHg, expansion of the right coronary artery (RCA), and severe mitral and tricuspid regurgitation (Figure 2). Then, PA computed tomography angiography (CTA) and double source CT coronary angiography revealed a congenital anomalous LMCA originating from the PA with no identified connection to the aortic root and a severely dilated RCA originating from the normal sinus of Valsalva along with abundant intercoronary collaterals circulation (Figure 3). The diagnosis of BWGS was confirmed. Due to the poor hepatic function, he could not wait for cardiac surgery. Furthermore, he refused to undergo cardiac surgery because of its high risk.
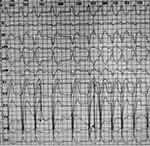 |
Figure 1 Electrocardiogram showed fibrillation, complete left bundle branch block, and occasional premature ventricular beats. |
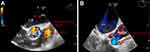
A temporary pacemaker was implanted prior to the induction of anesthesia. The arterial blood gas analysis was normal (PH=7.39, PaCO2=35 mmHg, PaO2=88 mmHg, HCO3=22.4 mmol/L, HCT=33%, K=3.4 mmol/L). The patient was put under general endotracheal anesthesia with steady intravenous induction. Fentanyl 8 µg/kg, atracurium 0.2 mg/kg, propofol 1.5 mg/kg, and midazolam 0.05 mg/kg were administered with syringe pumps separately, and the duration of intravenous administration was more than 10 mins. The infusion rate was slightly adjusted according to the BIS value, so that the bispectral index (BIS) value was slowly descended from 100 to 60, and then the patient was intubated. Ultrasound‑guided transversus abdominis plane block and rectus sheath block were performed to reduce the pain stress response with 0.5% ropivacaine (0.5 mL/kg). Intraoperative transesophageal echocardiagraphy (TEE) showed severely dilated RCA and the LMCA originating from PA, 16 mm distal to the pulmonic valve, with the retrograde flow draining from dilated LMCA into the PA, causing coronary “steal phenomenon” (Figure 4).
The anesthesia was maintained by sevoflurane supplied at 1 minimum alveolar concentration, and continuous infusion of 1% propofol with infusion rate around 15–20 mL/hr. Anesthetic depth was accurately monitored by BIS (50–60). Oxygen was given by pressure-controlled mechanical ventilation (airway pressure 13 cm H2O, oxygen/air ratio 1:1, end-tidal CO2 35–38 mmHg). The mean arterial pressure was maintained above 80 mmHg and the heart rate between 70 and 90/min, with cardiac function monitored by TEE. Norepinephrine 0.03–0.08 µg/kg/min and dopamine 3–6 µg/kg/min were required during the surgery.
The laparotomy lasted 2 hrs. The patient was extubated shortly after surgery and was transported to intensive care unit. His cardiac function remained stable and he was transferred to the general ward on postoperative day 1. He was subsequently discharged 7 days after surgery. A 6-month follow up showed the patient was convalescencing smoothly.
Literature review
Recent literature on anesthetic management of patients with BWGS were reviewed by conducting a thorough search on PubMed and MEDLINE of all the case reports of Bland-White-Garland syndrome published from 1950 to 2018. The keywords used included “Bland-white-Garland Syndrome”, “ALCAPA”, “coronary anomaly”, “anaesthesia”, “anomalous origin of the left coronary artery from the pulmonary artery”. The references of the selected literature have also been included. In addition, cases without definitive diagnosis of BWGS, reports only about surgical procedures, ultrasound or radiologic diagnosis were excluded.
Our literature search showed a total of 6 cases related to anesthetic management between 1996 and 2008. The characteristics of the reported cases of BWGS patients undergoing general anesthesia are listed in Table 1, and the clinical course of anesthetic management in Table 2. These patients were between the ages of 7 weeks and 68 years. Cardiovascular surgery with CPB took place in 5 cases (5/6), and noncardiac surgery took place only in 1 case (1/6). Suspicion and diagnostic modalities of BWGS were completed before surgery (5/6), during or after the surgery (1/6). Five cases had symptoms of myocardial ischemia (5/6).
 |
Table 1 Characteristic of reported cases of BWGS undergoing general anesthesia |
 |
Table 2 Clinic course of anesthetic management |
Discussion
The hemodynamic changes of BWGS are based on the pressure imbalance. Although the abnormal connection between coronary artery and PA exists during the fetal period, since there is a pressure balance between pulmonary and systemic circulation, the direction of blood flow in the left coronary artery (LCA) is still anterograde. After birth, the pressure of pulmonary circulation decreases rapidly. This “pressure imbalance” between the systemic and pulmonary circulation leads to a significant decrease of the antegrade blood flow and oxygen concentration in the LCA, leading to myocardial ischemia. Infant-type BWGS causes death of infants. If the lateral branches between the left and right coronary arteries can form rapidly, the blood of the RCA enters the LCA through the collateral branches, and the perfusion of the left ventricle can be improved. The mechanism of the high-pressure coronary flow draining into the low-pressure PA with little ventricle perfusion is called the “coronary steal”.7 Moreover, the perfusion of the left ventricle caused by this retrograde blood flow is still inadequate. Therefore, patients are at high risk of myocardial infarction, left ventricular dysfunction, mitral regurgitation, recurring ventricular arrhythmia, and even sudden death. Our patient had no obvious physiological abnormalities after birth and did not present with clinical symptoms throughout his childhood. He has a right dominant coronary artery, which is an important factor for the survival of BWGS patients into adulthood, since this may be related to the increased blood oxygen supply from the right dominant coronary artery through the collateral vessels to the left coronary artery.
Coronary steal can be diagnosed by clinical symptoms, ECG, cardiac ultrasound, and angiography.8,9 Coronary steal causes complications as the result of decreasing myocardial perfusion, especially when there is an increased myocardial oxygen demand combined with reduced coronary perfusion, as well as a sudden drop in the pulmonary vascular resistance. This is why careful management is required during mechanical ventilation to prevent a sudden drop in pulmonary vascular resistance, which can be done by maintaining a reasonable arterial saturation without causing hyperoxia or hypocapnia.5,6 Subsequently, unintentional excessive ventilation should be avoided during mechanical ventilation to prevent the coronary steal. Diastolic blood pressure and diastolic duration are the two most important factors to determine the coronary blood flow. It is important to increase diastolic pressure and decrease heart rate in order to maintain coronary perfusion. A sharp increase of systemic vascular resistance should be prevented, as it would influence the forward output of the left ventricle, increasing mitral regurgitation.
This is a rare case about description of the anesthetic management of a patient with BWGS, especially ones undergoing noncardiac surgery. The difference or originality of this case compared to other case reports regarding anesthetic management for patients with BWGS syndrome is the concern about myocardial blood supply and coronary steal phenomenon throughout the whole anesthetic process. Anesthetic management was done to ensure adequate coronary perfusion and prevent coronary steal.
We searched both successful and unsuccessful cases that were relevant and organized our understanding of the underlying pathophysiologies and anesthetic implications with BWGS (summarized in Table 3). BWGS can be diagnosed by cardiac ultrasound, CTA, left heart catheterization, cardiac magnetic resonance imaging, and angiography, which were significant for anomalous LMCA originating from the PA and an enlarged right coronary artery10–13 Genomics correlates of this rare disease are particularly missing, four mutations in three candidate genes potentially related to the ALCAPA phenotype, reporting the existence of mutations in CFTR, MEN1, and PKP2.14 The anesthetic technique influences myocardial ischemia with BWGS. Fentanyl, has vagotonic effects that sometimes result in severe bradycardia, combined with propofol the result is particularly hypotensive.15 Bolus injection for anesthesia induction was not adopted, because blood concentration would rise sharply, and then the side effects of all the drugs are superimposed, leading to unwanted tachycardia and hypotension.16–19 Volatile agent for maintaining anesthesia is believed to have pleiotropic effects on protecting against myocardial ischemia, as well as maybe reduce the incidence of intraoperative awareness.6,20,21 The possible problems about the co-administration of inhalation and intravenous anesthetics for this patient are the decreases in systemic vascular resistance, which would decrease diastolic blood pressure and drop in left coronary artery blood flow from intercoronary collaterals.5,22 Less inhalation sevoflurane might have led to greater hemodynamic stability, so only 1% sevoflurane was used in this patient. Inotropes such as dopamine, dobutamine, and epinephrine should be used with extreme caution as they might result in tachycardia.21 Adequate dose of noradrenaline can increase coronary perfusion pressure by stimulation of alpha-adrenoreceptors.
 |
Table 3 Anesthetic consideration and management of patients with BWGS |
In addition to routine monitoring, other equipment needs should be considered. Anudeep Jafra6 suggested the routine use of TTE in managing perioperative care when the underlying cardiac disease is rare, but the evidence base is insufficient. Anesthesiologists with a cardiac and echocardiography background can successfully perform TTE or TEE, which provides valuable new diagnostic information guiding changes in perioperative management.23 In our case, echocardiography was performed to assess the preload and ventricular function.
For surgical procedures that may promote bradycardia, and particularly in patients with pre-existing severe cardiovascular disease (ie, NYHA C and D), cardiac pacing is indicated.24 Since sudden death may occur in adult type BWGS, especially in patients with preexisting bundle branch block, the usage of temporary transvenous or transcutaneous pacing as a routine prophylactic measure is suggested, though it is an invasive procedure. In our patient, bradycardia did occur during the laparotomy, which may have been the result of anesthetics’ vagal reflex, and when the heartbeat became slower than 70 per/min, the pacemaker was turned on.
Conclusion
Chronic ischemia in adult type BWGS leads to impaired myocardial function, which has potential for devastating outcomes. A successful management of anesthesia in an adult with diagnosed, but untreated BWGS was described in this study. Anesthetic management was done to ensure adequate coronary perfusion, use a vasopressor, and prevent coronary steal. Comprehensive monitoring is suggested in perioperative management, as well as the routine use of pacemaker during surgery in managing this type of patients to prevent bradycardia and sudden cardiac arrest.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Minkovich LL, Brister SJ, Slinger PD. Transesophageal echocardiography in adult-type Bland-White-Garland syndrome. Anesth Analg. 2007;104(6):1348–1349. doi:10.1213/01.ane.0000261257.66969.28
2. Maddali MM, Al-delamie TY, Al-Maskari SN, Venkataraman M, Jasim DI. Unusual cause of chest pain at an unusual age. J Cardiothorac Vasc Anesth. 2011;25(3):501–504. doi:10.1053/j.jvca.2010.03.021
3. Kleinschmidt SGV, Molter G. The Bland-White-Garland syndrome. Clinical picture and anaesthesiological management. Paediatr Anaesth. 1996;6:65–68.
4. Roberts SM, Banbury T, Mehta A. A rare case of anomalous left coronary artery from the pulmonary artery (Bland-White-Garland Syndrome) in a 68-year-old woman. Semin Cardiothorac Vasc Anesth. 2017;21(2):186–190. doi:10.1177/1089253216659146
5. Fahy CJ, Ing RJ, Kern FH, O’Hare B, Redmond JM, Jaggers J. The anesthetic management and physiologic implications in infants with anomalous left coronary artery arising from the pulmonary artery. J Cardiothorac Vasc Anesth. 2012;26(2):286–290. doi:10.1053/j.jvca.2011.01.017
6. Jafra A, Arora S, Jayant A. The utility of targeted perioperative transthoracic echocardiography in managing an adult patient with anomalous origin of the left coronary artery-pulmonary artery for noncardiac surgery. Ann Card Anaesth. 2017;20(3):372–375. doi:10.4103/0971-9784.210402
7. Dimitrakakis G, Von Oppell U, Luckraz H, Groves P. Surgical repair of triple coronary-pulmonary artery fistulae with associated atrial septal defect and aortic valve regurgitation. Interact Cardiovasc Thorac Surg. 2008;7(5):933–934. doi:10.1510/icvts.2008.181388
8. Ahn S, Han A, Kim SY, et al. The incidence and risk factors of coronary steal after ipsilateral AVF in patients with a coronary artery bypass graft. J Vasc Access. 2017;18(4):290–294. doi:10.5301/jva.5000690
9. Kinoshita T, Asai T, Ishigaki T. Steal from skeletonized internal thoracic artery graft during hemodialysis after coronary artery bypass grafting. Heart Surg Forum. 2010;13(4):E254–E256. doi:10.1532/HSF98.20091191
10. Yau JM, Singh MR
11. Rodriguez-Gonzalez M, Tirado AM, Hosseinpour R, de Soto JS. Anomalous origin of the left coronary artery from the pulmonary artery: diagnoses and surgical results in 12 pediatric patients. Tex Heart Inst J. 2015;42(4):350–356. doi:10.14503/THIJ-13-3849
12. Bhalgat P, Naik A, Salvi P, et al. Cardiac magnetic resonance imaging, myocardial scar and coronary flow pattern in anomalous origin of left coronary artery from the pulmonary artery. Indian Heart J. 2018;70(2):303–307. doi:10.1016/j.ihj.2017.08.004
13. Takemoto K, Hirata K, Tanimoto T, et al. Combined non-invasive doppler echocardiography and coronary computed tomography lead to diagnosis of Anomalous Left Coronary Artery From the Pulmonary Artery (ALCAPA) Syndrome. Circ J. 2015;79(5):1136–1138. doi:10.1253/circj.CJ-14-1374
14. Hekim N, Batyraliev T, Trujillano D, et al. Whole exome sequencing in a rare disease: a patient with anomalous left coronary artery from the pulmonary artery (Bland-White-Garland Syndrome). Omics. 2016;20(5):325–327. doi:10.1089/omi.2016.0046
15. Daher M, Zanatta AR, Henz BD, Silva M, Santos S, Leite LR. Parada cardíaca súbita em anestesia geral como a primeira manifestação da origem anômala de artéria coronária esquerda. Rev Bras Anestesiol. 2012;62(6):881–884.
16. Liu MQ, Li FX, Han YK, et al. Administration of fentanyl via a slow intravenous fluid line compared with rapid bolus alleviates fentanyl-induced cough during general anesthesia induction. J Zhejiang Univ Sci B. 2017;18(11):955–962. doi:10.1631/jzus.B1600442
17. Bang JY, Kim S, Choi BM, Kim TY. Pharmacodynamic analysis of the influence of propofol on left ventricular long-axis systolic performance in cardiac surgical patients. J Korean Med Sci. 2019;34(16):e132. doi:10.3346/jkms.2019.34.e132
18. Sudfeld S, Brechnitz S, Wagner JY, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57–64. doi:10.1093/bja/aex127
19. Poterman M, Scheeren TWL, van der Velde MI, et al. Prophylactic atropine administration attenuates the negative haemodynamic effects of induction of anaesthesia with propofol and high-dose remifentanil: a randomised controlled trial. Eur J Anaesthesiol. 2017;34(10):695–701. doi:10.1097/EJA.0000000000000639
20. Fleisher LA, Andrew KEF, Auerbach D, Barnason SA, Joshua A. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;130(24):2215–45. doi:10.1161/CIR.0000000000000105
21. Kloesel B, Richtsfeld M, Konia M, Bass JL. Management and anesthetic considerations for patients with anomalous aortic origin of a coronary artery. Semin Cardiothorac Vasc Anesth. 2018. 1089253218793888. doi:10.1177/1089253218793888
22. Cimen NK, Kosem B, Cimen T, et al. Effects of remifentanil, nitroglycerin, and sevoflurane on the corrected QT and Tp-e intervals during controlled hypotensive anesthesia. J Clin Anesth. 2016;33:365–372. doi:10.1016/j.jclinane.2016.04.048
23. Cowie B. Focused cardiovascular ultrasound performed by anesthesiologists in the perioperative period: feasible and alters patient management. J Cardiothorac Vasc Anesth. 2009;23(4):450–456. doi:10.1053/j.jvca.2009.01.018
24. Marrocco-Trischitta MM, Mazzone P, Vitale R, Regazzoli D, Laricchia A, Chiesa R. Temporary transvenous pacemaker implantation during carotid endarterectomy in patients with trifascicular block. Ann Vasc Surg. 2016;34:206–211. doi:10.1016/j.avsg.2015.12.025
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.