Back to Journals » Drug Design, Development and Therapy » Volume 13
Andrographolide attenuates bupivacaine-induced cytotoxicity in SH-SY5Y cells through preserving Akt/mTOR activity
Received 10 January 2019
Accepted for publication 3 April 2019
Published 16 May 2019 Volume 2019:13 Pages 1659—1666
DOI https://doi.org/10.2147/DDDT.S201122
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Huiyuan Zhang,1 Weiwei Wang,1 Qian Du2
1Department of Neurology, Liaocheng People’s Hospital, Liaocheng, Shandong, 252000, People’s Republic of China; 2EEG Room, Liaocheng People’s Hospital, Liaocheng, Shandong 252000, People’s Republic of China
Background: Bupivacaine (Bup) is the most commonly used local anesthetic. However, Bup induces cytotoxicity, especially in older patients. Recent reports have indicated that andrographolide (Andro) exhibits protective effects on human neurons. Nevertheless, whether Andro can inhibit Bup-induced cytotoxicity remains unclear. As such, we investigated the effect of Andro on Bup-induced cytotoxicity of SH-SY5Y cells in the present study.
Methods: Western blotting was used to examine expression of Bax, Bcl2, active caspase 3, p-Akt, and p-mTOR in SH-SY5Y cells. In addition, ELISA was used to detect levels of total glutathione and reactive oxygen species in cells.
Results: We found that Andro attenuated Bup-induced cytotoxicity of SH-SY5Y cells. In addition, Andro inhibited Bup-induced apoptosis via downregulating the expression of Bax and active caspase 3 and upregulating the proteins Bcl2, p-Akt, and p-mTOR in SH-SY5Y cells. Moreover, Andro alleviated Bup-induced oxidative damage in SH-SY5Y cells via downregulating the level of reactive oxygen species and upregulating of the level of total glutathione. More significantly, inhibition of Akt abolished the protective effect of Andro in Bup-treated SH-SY5Y cells.
Conclusion: Our findings indicated that Andro played a neuroprotective role via preserving Akt/mTOR activity and increasing antioxidative status in Bup-treated SH-SY5Y cells. Therefore, Andro may be a potential agent for the treatment of human cytotoxicity induced by Bup.
Keywords: andrographolide, bupivacaine, apoptosis, Akt, cytotoxicity
Introduction
Local anesthetics have been found to induce brain neural injury in human patients.1 Local anesthetics can induce permanent injury in young patients, and even affect neurobehavioral outcomes.2 Bupivacaine (Bup) is a common local anestheticused for postoperative pain relief.3 A recent study indicated that 5% Bup can induce histopathological abnormalities in a rat model.4 Meanwhile, injection of Bup can also lead to serious sciatic nerve damage in rats.5 Even with a normal or lower dose, Bup can induce neurotoxicity in cells.6 As such, it is urgent that we develop novel effective methods for the treatment of local anesthetic neurotoxicity. .
Andrographolide (Andro) is a natural diterpenoid extracted from the tradition Chinese herbal medicine Andrographis paniculate.7 Andro has been revealed to exhibit a variety of biological activities, including antitumor, anti-inflammatory, antivirus, and antioxidation actions.8–12 A previous study indicated that Andro can stimulate neurogenesis in the adult hippocampus.13 Liang et al found that Andro may exhibit neuroprotective effects in nervous system diseases.14 Meanwhile, a recent study showed that Andro exerted strong neuroprotective effects in a mouse model of Parkinson’s disease.15 However, it remains unclear whether Andro provides neuroprotection against Bup. In addition, the Akt-signaling pathway participates in regulating cell growth, survival, and death.16 Studies have indicated Bup-induced apoptosis in neural injury via inactivation of the Akt pathway.17,18
Therefore, our main purpose was to investigate the effect of Andro on Bup-induced neurotoxicity in SH-SY5Y cells. In this study, an in vitro model of Bup-induced cytotoxicity was first established. Then, mechanisms by which Andro regulates Bup-induced injury in SH-SY5Y cells were evaluated.
Methods
Cell cultures
The human neuroblastoma cell line SH-SY5Y was purchased from the American Type Culture Collection (Rockville, MD, USA). SH-SY5Y cells were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS(Thermo Fisher Scientific) and 100 U/mL penicillin–streptomycin at 37°C in a humidified 5% CO2 incubator. Cell-culture medium was changed daily. Andro (365645) and Bup (B5274) were obtained from Sigma-Aldrich (St Louis, MO, USA). AZD5363 was purchased from MedChem Express (Monmouth Junction, NJ, USA).
CCK8 assay
A CCK8 assay kit (Beyotime, Haimen, China) was used to determine cell viability. SH-SY5Y cells were seeded into a 96-well plate at a density of 5×103 cells/well overnight. When cell confluence had reached about 80%, SH-SY5Y cells were incubated with Bup, Andro, or AZD5363. After that, 10 μL CCK8 solution was added to each well and cultured at 37°C for another 3 hours. OD values were measured using a microplate reader (Thermo Fisher Scientific) at 450 nm.
Immunofluorescence assay
SH-SY5Y cells were seeded into 24-well plates at a density of 4×105 cells/well overnight. For treatment 1 (Figure 2A), SH-SY5Y cells were incubated with Andro (0 or 200 μM) for 12 hours. Then, the culture medium was changed and cells incubated with Bup (0 or 400 μM) for another 48 hours at 37°C. After that, cells were prefixed in 4% paraformaldehyde at room temperature for 20 minutes and fixed in cold methanol for 10 minutes at −20°C. Later, cells were incubated with primary antibodies for anti-Ki67 (1:1,000; Abcam, Cambridge, UK) () and DAPI (1:1,000; Abcam) at 4°C overnight.
For treatment 2 (Figure 5A), SH-SY5Y cells were incubated with Andro (0 or 200 μM) for 12 hours. Then, the culture medium was changed and cells incubated with 10 nM AZD5363 for 1 hour. Later, the culture medium was changed and cells incubated with Bup (0 or 400 μM) for another 48 hours. Next, cells were incubated with primary antibodies for anti-p-Akt (1:1,000; Abcam) and DAPI (1:1,000) at 4°C overnight. After that, cells were incubated with goat antirabbit IgG secondary antibodies (1:5,000; Abcam) at 37°C for 1 hour. Cells were visualized with fluorescence microscopy (Olympus, Tokyo, Japan).
Flow-cytometry analysis of cell apoptosis
SH-SY5Y cells were seeded on six-well plates at a density of 5×105 cells/well overnight. SH-SY5Y cells were incubated with Bup, Andro, or AZD5363. After that, collected cells were washed with cold PBS and resuspended in 500 μL binding buffer at 4°C. Staining with annexin V–FITC–PI (Thermo Fisher Scientific) was used to detect cell apoptosis according to the manufacturer’s specifications. Results were measured with flow cytometry (BD Bioscience, San Jose, CA, USA).
Western blot analysis
Total cellular protein was quantified with a BCA protein assay kit (Beyotime). An equal amount of protein samples (40 μg/lane) was separated by polyacrylamide-gel electrophoresis. Then, proteins were transferred to polyvinylidene fluoride membranes (Thermo Fisher Scientific). After blocking with 5% nonfat milk for 50 minutes, membranes were incubated with primary antibodies overnight at 4°C. After that, membranes were incubated with secondary antibody for 1 hour at room temperature. Primary antibodies used were anti-Bax (1:1,000), anti-Bcl2 (1:1,000), anti–active caspase 3 (1:1,000), anti-p-Akt (1:1,000), anti-p-mTOR (1:1,000), and anti-β-actin (1:1000). The secondary antibody was goat antirabbit IgG (1:5,000). All these antibodies were provided by Abcam. Finally, images protein bands were captured with an electrochemiluminescence reagent (Santa Cruz Biotechnology). The density of blots for targets was normalized to β-actin.
Glutathione assay
ELISA kits (Beyotime) were used to detect levels of intracellular reduced glutathione (GSH) and oxidized GSH (GSSG). Absorbance was read at 450 nm by a microplate reader (Thermo Fisher Scientific). The level of total GSH was the sum of GSH and GSSG.
Intracellular ROS assay
A reactive oxygen species (ROS) assay kit (Beyotime) was used to detect the level of intracellular ROS. Intracellular ROS level was alsodetected with a microplate reader (Thermo Fisher Scientific).
Statistical analysis
Each group was subjected to least three independent experiments, and all data are expressed as means ± SD. Student’s t-test was used to analyze comparison betweens two groups. Comparisons among multiple groups were made with one-way ANOVA followed by Dunnett’s test. P<0.05 was considered statistically significant.
Results
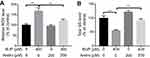
Effects of Bup or andro on growth of SH-SY5Y cells
The chemical structure of Andro is indicated in Figure 1A. CCK8 assays were used to investigate the effects of Bup or Andro on the growth of SH-SY5Y cells. As shown in Figure 1B, Bup significantly inhibited the viability of SH-SY5Y cells in a dose-dependent manner. Since 400 μM Bup induced about 50% growth inhibition, 400 μM Bup was utilized in the following experiments. Meanwhile, Andro had a very limited effect on cell proliferation when the concentration reached 200 μM (Figure 1C). However, 400 μM Andro had significant cytotoxicity. Therefore, 200 μM Andro was utilized in the following experiments.
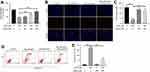
Andro alleviated Bup-induced cytotoxicity in SH-SY5Y cells via inhibition of apoptosis
As indicated in Figure 2A, the cytotoxicity of 400 μM Bup on SH-SY5Y cells was significantly alleviated by Andro (100 or 200 μM) treatment. Next, a Ki67 immunofluorescence assay was used to determine cell viability as well. The results showed Bup markedly inhibited cell proliferation, which was notably reversed by 200 μM Andro (Figure 2, B and C). In addition, flow cytometry was applied to explore further the apoptosis in SH-SY5Y cells. The rate of early and late apoptotic stage in Bup-treated SH-SY5Y cells was about 10% and 20.00%, respectively. However, the rate of early- and late-apoptosis stages in Bup-treated SH-SY5Y cells was markedly decreased to about 4% and 10% in the presence of Andro (Figure 2, D and E), respectively. These results indicated that Andro significantly decreased Bup-induced apoptosis in SH-SY5Y cells. As such, Andro inhibited Bup-induced cytotoxicity via inhibition of apoptosis in SH-SY5Y cells.
Andro attenuated Bup-induced cytotoxicity in SH-SY5Y cells via increasing antioxidative status
Oxidative status is an important cell characteristic included in the response to cellular environment transformation. Generation of ROS and GSH mediates apoptosis in neuroblastoma SH-SY5Y cells.19,20 As shown in Figure 3, A and B, Bup significantly increased the level of ROS and decreased the level of total GSH in SH-SY5Y cells compared with the control group. However, levels of ROS and GSH in SH-SY5Y cells recovered to normal status in the presence of Andro compared with the Bup-treated group (Figure 3, A and B). These data suggested that Andro attenuated Bup-induced cytotoxicity via increasing antioxidative status in SH-SY5Y cells.
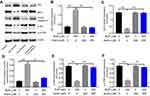
Andro inhibited Bup-induced cytotoxicity in SH-SY5Y cells via activating p-Akt and p-mTOR
To explore further the effects of Andro on Bup-induced apoptosis in SH-SY5Y cells, Western blotting was performed. It has been reported that Bcl2 and caspase 3 are proapoptotic proteins, while Bax is an antiapoptotic protein.21 In addition, the Akt–mTOR pathway plays an important role in cell survival and death.22 As indicated in Figure 4, Bup significantly increased Bax and active caspase 3 expression and decreased levels of Bcl2, p-Akt, and p-mTOR in cells compared with the control group. However, these effects were markedly reversed in the presence of Andro (Figure 4). Therefore, these data suggested that Andro attenuated Bup-induced cytotoxicity in SH-SY5Y cells via activating the Akt–mTOR pathway.
Akt inhibitor abrogated the protective effect of Andro in Bup-treated SH-SY5Y cells
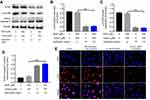
To investigate further whether Andro attenuated Bup-induced cytotoxicity via the Akt pathway, the Akt-selective inhibitor AZD5363 was used. As indicated in CCK8 assays, the protective effect of Andro in SH-SY5Y cells against Bup was abrogated by AZD5363 treatment; however, AZD5363 notably increased Bup-induced cytotoxicity (Figure 5A). Meanwhile, the antiapoptotic effect of Andro in Bup-treated SH-SY5Y cells was also reversed by AZD5363 treatment (Figure 5, B and C). These data suggested that the Akt inhibitor abrogated the protective effect of Andro in Bup-treated SH-SY5Y cells.
In addition, AZD5363 abolished the upregulation of p-Akt and p-mTOR in cells, even in the presence of Andro (Figure 6, A–C). Meanwhile, the expression of active caspase 3 in cells was significantly increased with treatment by AZD5363. Similarly, immunofluorescence assays indicated the expression of p-Akt in SH-SY5Y cells was significantly inhibited by AZD5363, even in the presence of Andro (Figure 6, D and E). All these data confirmed that Andro inhibited Bup-induced cytotoxicity of SH-SY5Y via preserving the Akt–mTOR pathway, which was abrogated by AZD5363.
Discussion
In the present study, we found that Andro protected SH-SY5Y cells from Bup-induced cytotoxicity. Pretreatment with 200 μM Andro significantly suppressed Bup-induced cytotoxicity, apoptosis, and ROS accumulation. A study has shown that Bup induced neurotoxicity in neuroblastoma Neuro2a cells via activating apoptosis.23 Wen et al showed that Bup induced neural injury in SH-SY5Y cells,24 consistent with the results of this study. In addition, we found that 200 μM Andro had no cytotoxicity on SH-SY5Y cell proliferation. Consistently with previous research, ginkgolide B had no effect on cell proliferation, while pretreatment with ginkgolide B protected SH-SY5Y cells from Bup-induced neurotoxicity.25 Furthermore, Chen et al indicated that paeoniflorin attenuated Bup-induced neurotoxicity via downregulation of Bax and caspase 3 levels n SH-SY5Y cells.21 Harato et al also showed that Bup induced apoptosis in mouse neuro2a cells via increasing the expression of caspase 3 and inducing ROS generation.26 In this study, Andro reversed Bup-induced cytotoxicity in SH-SY5Y cells via downregulation of the level of proapoptotic Bax and caspase 3 proteins and upregulation of the level of the antiapoptotic Bcl2 protein. Our results are consistent with previous reports that Andro can reverse Bup-caused neurotoxicity via inhibiting apoptosis in SH-SY5Y cells.
An important element of cellular survival is oxidoreductive homeostasis. In neuronal cells, the course of cell apoptosis is associated with ROS and GSH generation.27,28 Park et al found that Bup-induced apoptosis was associated with the production of ROS.29 Lu et al showed Bup-induced apoptosis in SH-SY5Y cells via induction of ROS production and activation of the AMPK pathway.30 However, inhibition of GSH can induce apoptosis. Our findings are consistent with previous reports that Bup increased ROS generation and decreased total GSH level in SH-SY5Y cells. Moreover, Andro is considered an antioxidant,31 and we demonstrated the effects of Andro on ROS and total GSH content in Bup-treated SH-SY5Y cells. We found that ROS content was reduced and the level of total GSH increased with Andro treatment. Supporting our finding, Andro-attenuated hypoxia-induces the production of ROS.32 As such, Andro recover ROS and GSH to normal levels in Bup-treated SH-5Y5Y cells and played an important role in maintaining oxidoreductive homeostasis.
Akt, a serine/threonine-specific protein kinase, can modulate cell death and survival in multiple cellular processes.33 The primary direct downstream effector protein of Akt is mTOR.33 mTOR is a main nodal point of regulation for ribosome biogenesis and protein translation.33 The Akt-signaling pathway has been found to be involved in Bup-induced neural injury.34 In this study, we observed that Bup markedly decreased the expression of p-Akt and p-mTOR. This is consistent with Zhao et al’s report that Bup induced neurotoxicity in SH-SY5Y cells via inhibiting the PI3K–Akt pathway.34 Importantly, we found that Andro attenuated Bup-induced cytotoxicity via preserving Akt/mTOR activity in SH-SY5Y cells. Chen et al also found that Andro inhibited endothelial cell apoptosis through activation of the PI3K–Akt signaling pathway.35 Therefore, it is presumed that Andro rescued Bup-induced cytotoxicity via preserving Akt/mTOR activity in SH-SY5Y cells. To verify these data, the Akt-selective inhibitor AZD5363 was used in this study. AZD5363 abrogated the protective effect of Andro in Bup-treated SH-SY5Y cells. These results suggested that Andro exerted its neuroprotective effects through preserving the Akt–mTOR signaling pathway. However, a limitation of the current study was the lack of animal experiments, which hindered the clinical use of Andro.
Conclusion
In summary, this study indicated that Andro alleviated Bup-induced cytotoxicity in SH-SY5Y cells via inhibition of apoptosis and ROS generation and activation of the Akt–mTOR signaling pathway. Therefore, Andro may be a potential agent for the treatment of human cytotoxicity induced by Bup.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang K, Yang S, Luo C. TNF-alpha and TNF-R1 regulate bupivacaine-induced apoptosis in spinal cord dorsal root ganglion neuron. Eur J Pharmacol. 2018;833:63–68. doi:10.1016/j.ejphar.2018.05.034
2. Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatr Anaesth. 2011;21(7):716–721. doi:10.1111/j.1460-9592.2010.03506.x
3. Li YJ, Zhao W, Yu XJ, et al. Activation of p47phox as a mechanism of bupivacaine-induced burst production of reactive oxygen species and neural toxicity. Oxid Med Cell Longev. 2017;2017:8539026. doi:10.1155/2017/8539026
4. Takenami T, Wang G, Nara Y, et al. Intrathecally administered ropivacaine is less neurotoxic than procaine, bupivacaine, and levobupivacaine in a rat spinal model. Can J Anaesth. 2012;59(5):456–465. doi:10.1007/s12630-012-9685-9
5. Sen O, Sayilgan NC, Tutuncu AC, et al. Evaluation of sciatic nerve damage following intraneural injection of bupivacaine, levobupivacaine and lidocaine in rats. Braz J Anesthesiol. 2016;66(3):272–275. doi:10.1016/j.bjane.2014.09.012
6. Lu J, Xu SY, Zhang QG, Xu R, Lei HY. Bupivacaine induces apoptosis via mitochondria and p38 MAPK dependent pathways. Eur J Pharmacol. 2011;657(1–3):51–58. doi:10.1016/j.ejphar.2011.01.055
7. Tao L, Zhang L, Gao R, et al. Andrographolide alleviates acute brain injury in a rat model of traumatic brain injury: possible involvement of inflammatory signaling. Front Neurosci. 2018;12:657. doi:10.3389/fnins.2018.00044
8. Peng Y, Wang Y, Tang N, et al. Andrographolide inhibits breast cancer through suppressing COX-2 expression and angiogenesis via inactivation of p300 signaling and VEGF pathway. J Exp Clin Cancer Res. 2018;37(1):248. doi:10.1186/s13046-018-0926-9
9. Shen YC, Chen CF, Chiou WF. Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect. Br J Pharmacol. 2002;135(2):399–406. doi:10.1038/sj.bjp.0704493
10. Xia YF, Ye BQ, Li YD, et al. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J Immunol. 2004;173(6):4207–4217.
11. Wang D, Guo H, Chang J, et al. Andrographolide prevents EV-D68 replication by inhibiting the acidification of virus-containing endocytic vesicles. Front Microbiol. 2018;9:2407. doi:10.3389/fmicb.2018.02407
12. Li B, Jiang T, Liu H, et al. Andrographolide protects chondrocytes from oxidative stress injury by activation of the Keap1-Nrf2-Are signaling pathway. J Cell Physiol. 2018;234(1):561–571. doi:10.1002/jcp.26769
13. Varela-Nallar L, Arredondo SB, Tapia-Rojas C, Hancke J, Inestrosa NC. Andrographolide stimulates neurogenesis in the adult hippocampus. Neural Plast. 2015;2015:935403. doi:10.1155/2015/935403
14. Liang Y, Li M, Lu T, Peng W, Wu JH. Andrographolide promotes neural differentiation of rat adipose tissue-derived stromal cells through Wnt/beta-catenin signaling pathway. Biomed Res Int. 2017;2017:4210867. doi:10.1155/2017/4210867
15. Zhang Z, Lai D, Wang L, et al. Neuroprotective effects of the andrographolide analogue AL-1 in the MPP(+)/MPTP-induced Parkinson‘s disease model in vitro and in mice. Pharmacol Biochem Behav. 2014;122:191–202. doi:10.1016/j.pbb.2014.03.028
16. Wang X, Zhang X, Cheng Y, et al. Alpha-lipoic acid prevents bupivacaine-induced neuron injury in vitro through a PI3K/Akt-dependent mechanism. Neurotoxicology. 2010;31(1):101–112. doi:10.1016/j.neuro.2009.10.010
17. Fan YL, Li HC, Zhao W, et al. Curcumin attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells via activation of the Akt signaling pathway. Neurochem Res. 2016;41(9):2425–2432. doi:10.1007/s11064-016-1955-4
18. Wang LY, Li X, Han YZ. Neuroprotection by epigallo catechin gallate against bupivacaine anesthesia induced toxicity involves modulation of PI3/Akt/PTEN signalling in N2a and SH-SY5Y cells. Int J Clin Exp Med. 2015;8(9):15065–15075.
19. Bastola T, An RB, Kim YC, Kim J, Seo J. Cearoin induces autophagy, ERK activation and apoptosis via ROS generation in SH-SY5Y neuroblastoma cells. Molecules. 2017;22(2). doi:10.3390/molecules22020242
20. Waly M, Power-Charnitsky VA, Hodgson N, et al. Alternatively spliced methionine synthase in SH-SY5Y neuroblastoma cells: cobalamin and GSH dependence and inhibitory effects of neurotoxic metals and thimerosal. Oxid Med Cell Longev. 2016;2016:6143753. doi:10.1155/2016/6143753
21. Chen L, Li Q, Wang H, et al. Paeoniflorin attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells via suppression of the p38 MAPK pathway. J Cell Biochem. 2018;120:7015–7023.
22. Cao P, Liu B, Du F, et al. Scutellarin suppresses proliferation and promotes apoptosis in A549 lung adenocarcinoma cells via AKT/mTOR/4EBP1 and STAT3 pathways. Thorac Cancer. 2019;10(3):492–500. doi:10.1111/1759-7714.12962
23. Liang Y, Ji J, Lin Y, He Y, Liu J. The ganglioside GM-1 inhibits bupivacaine-induced neurotoxicity in mouse neuroblastoma Neuro2a cells. Cell Biochem Funct. 2016;34(6):455–462. doi:10.1002/cbf.3208
24. Wen X, Zhong J, Zhang T, et al. Role of calmodulin-dependent protein kinase II in bupivacaine hydrochloride-induced injury of SH-SY5Y cells. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35(8):1133–1136.
25. Li L, Zhang QG, Lai LY, et al. Neuroprotective effect of ginkgolide B on bupivacaine-induced apoptosis in SH-SY5Y cells. Oxid Med Cell Longev. 2013;2013:159864. doi:10.1155/2013/159864
26. Harato M, Huang L, Kondo F, et al. Bupivacaine-induced apoptosis independently of WDR35 expression in mouse neuroblastoma Neuro2a cells. BMC Neurosci. 2012;13:149. doi:10.1186/1471-2202-13-149
27. Fiskum G. Mechanisms of neuronal death and neuroprotection. J Neurosurg Anesthesiol. 2004;16(1):108–110.
28. Park J, Lee J, Yeom Z, Heo D, Lim YH. Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci Rep. 2017;7(1):14520. doi:10.1038/s41598-017-15163-5
29. Park CJ, Park SA, Yoon TG, et al. Bupivacaine induces apoptosis via ROS in the Schwann cell line. J Dent Res. 2005;84(9):852–857. doi:10.1177/154405910508400914
30. Lu J, Xu SY, Zhang QG, Lei HY. Bupivacaine induces reactive oxygen species production via activation of the AMP-activated protein kinase-dependent pathway. Pharmacology. 2011;87(3–4):121–129. doi:10.1159/000323402
31. Wu T, Peng Y, Yan S, et al. Andrographolide ameliorates atherosclerosis by suppressing pro-inflammation and ROS generation-mediated foam cell formation. Inflammation. 2018;41(5):1681–1689. doi:10.1007/s10753-018-0812-9
32. Lin HC, Su SL, Lu CY, et al. Andrographolide inhibits hypoxia-induced HIF-1alpha-driven endothelin 1 secretion by activating Nrf2/HO-1 and promoting the expression of prolyl hydroxylases 2/3 in human endothelial cells. Environ Toxicol. 2017;32(3):918–930. doi:10.1002/tox.22293
33. Sun L, Dong Y, Zhao J, Yin Y, Zheng Y. The CLC-2 chloride channel modulates ECM synthesis, differentiation, and migration of human conjunctival fibroblasts via the PI3K/Akt signaling pathway. Int J Mol Sci. 2016;17:6. doi:10.3390/ijms17060910
34. Zhao W, Liu Z, Yu X, et al. iTRAQ proteomics analysis reveals that PI3K is highly associated with bupivacaine-induced neurotoxicity pathways. Proteomics. 2016;16(4):564–575. doi:10.1002/pmic.201500202
35. Chen JH, Hsiao G, Lee AR, Wu CC, Yen MH. Andrographolide suppresses endothelial cell apoptosis via activation of phosphatidyl inositol-3-kinase/Akt pathway. Biochem Pharmacol. 2004;67(7):1337–1345. doi:10.1016/j.bcp.2003.12.015
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.