Back to Journals » Cancer Management and Research » Volume 13
Analysis of the Spectrum and Characteristics of Pediatric Cancer Based on Hospital Information Systems in China
Authors Zhou H , Wu Z , Wang H, Yu W, Huang J, Zhou L, Yu D, Hou T, Lv Y, Chen C , Luo L, Shi J, Wang Z
Received 29 August 2020
Accepted for publication 5 January 2021
Published 11 February 2021 Volume 2021:13 Pages 1205—1214
DOI https://doi.org/10.2147/CMAR.S279427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Huining Zhou,1,* Zhengyi Wu,1,* Hui Wang,1 Wenya Yu,1 Jiaoling Huang,1 Liang Zhou,1 Dehua Yu,2 Tianchun Hou,1 Yipeng Lv,1 Chen Chen,3 Li Luo,4 Jianwei Shi,1,5 Zhaoxin Wang1,6
1School of Public Health, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China; 2Department of General Practice, Yangpu Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 3Shanghai Jing‘an District Jiangning Road Community Health Service Center, Shanghai, People’s Republic of China; 4School of Public Health, Fudan University, Shanghai, People’s Republic of China; 5Shanghai General Practice and Community Health Development Research Center, Shanghai, People’s Republic of China; 6General Practice Center, Nanhai Hospital, Southern Medical University, Foshan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianwei Shi; Zhaoxin Wang
School of Public Health, Shanghai Jiaotong University School of Medicine, 227 South Chongqing Road, Shanghai 200025, People’s Republic of China
Tel +86 188 1820 9852; +86 139 1853 7473
Email [email protected]; [email protected]
Purpose: The purpose of this study was to use the hospital information system to analyze the cancer profile and compare demographics, hospitalization, status of surgery and treatment cost of various cancer categories based on the electronic health record (EHR) of outpatient children with tumors in Shanghai, China.
Patients and Methods: Information was collected from 3834 inpatients aged 0– 18 who were diagnosed with malignant tumors in all 17 hospitals with pediatric wards in the Pudong New District of Shanghai from 2011 to 2016. All patients were classified according to the International Classification of Childhood Cancer-3 (ICCC-3). The chi-squared test was used to compare demographics, hospitalization information, status of surgery and treatment cost according to inpatients’ cancer category.
Results: In both the malignant non-solid tumor and solid tumor groups, males and those aged 0– 4 years were the dominant groups. Lymphocytic leukemia was the most common cancer in all inpatients (n=994, 25.93%), and the acute myeloid leukemia had the longest length of stay of inpatients (median=26.00 days). In both the non-solid and solid tumor groups, patients who received only one type of surgery had an advantage. The highest proportion of patients who had undergone surgery was found in non-Hodgkin lymphoma patients. In terms of total cost, surgical cost and medicine cost, the expenditure of central nervous system tumor patients was the highest. Astrocytoma had the highest total cost.
Conclusion: Leukemia is common in children with cancer in Pudong and should be given attention. Because the highest financial burden falls on patients with central nervous system tumors and acute myeloid leukemias, the government should take immediate and targeted measures for these cancers in particular.
Keywords: malignant tumors, disease burden, demographics, hospitalization information, epidemiology
Introduction
Advances in early detection, treatment, and supportive care have resulted in higher survival rates in increasing numbers of pediatric patients. According to the study by Johnston et al, a total of 360,114 cases of childhood cancer occurred globally in 2015.1–3 The standardized rate of childhood cancer in Europe and North America was approximately 178 cases/million and 218 cases/million in West and Central Africa.4 A study in Italy showed that, during 2003–2008, the cancer incidence rate for children aged 14 or younger was 164 cases per million, while the cancer incidence rate in children 15–19 years old was 269 cases per million.5 In the economically developed countries of Australia, Ireland, Switzerland, and the United States, the incidence of childhood cancer was 140–160 per million children.6–8 Despite differences in the incidence of childhood cancer worldwide, cancer remains the second leading cause of child mortality in developed countries.9
In addition to mortality issues, childhood cancer also comes with economic or financial costs and burden. Ghatak et al collected the expenditure details of 50 children with acute lymphoblastic leukemia and found that medical expenditure totaled $524 and non-medical expenditure was $207.10 A study in Mexico analyzed 449 admission cases of 101 children with acute lymphoblastic leukemia over 8 years and found that the hospitalization costs of children with acute lymphoblastic leukemia were cheaper in Mexico than those in high-income countries.11 Although some studies have examined hospitalization costs for various cancers, little is known regarding hospitalization costs for various cancer subcategories.
In China, from 2000 to 2010, the age-standardized morbidity rate of cancer for children aged 0 to 14 years was 87.1%, and the age-standardized mortality rate was 36.3%. The morbidity rate increased rapidly at a rate of 2.8% per year,12 which was much higher than that in the United States (0.6% per year) and Europe (1.1% per year).6,7,13 The 5-year total relative survival rate in China reached 71.9%, which was lower than that in developed countries (where it usually exceeds 80%).6,14,15 Liu et al analyzed the sex differences in the incidence of seven common tumors using data from the International Children’s Cancer Incidence Project (1990–2015). The results showed that there were more male patients with leukemia, lymphoma, central nervous system (CNS) tumors, neuroblastoma, retinoblastoma and liver tumors than female patients. However, among kidney tumor patients, the number of females was higher than that of males.16 In addition, most studies were performed without discussing data on hospitalizations for each type of childhood tumor. Therefore, little is known about the characteristics of cancer and financial costs for children in China.
In this study, we aimed to elucidate the demographic characteristics and disease burden in various categories of cancers using data from pediatric patients in Shanghai’s Pudong New District from 2011 to 2016. The 2019 Shanghai Statistical Yearbook showed that the characteristics of children in Pudong were similar to those of children in the whole of Shanghai, where children aged 0–14 years accounted for 12.07% of the total population, males accounted for 49.52%, and females accounted for 50.48%. In Pudong, children aged 0–14 years accounted for 13.46% of the total population, males accounted for 49.80%, and females accounted for 50.20%.17 The demographic indicators of Pudong are almost the same as those of Shanghai, so the children’s tumor data in Pudong reflect the situation in Shanghai to a certain extent. The findings of this study can provide a better understanding of the variations and characteristics in childhood cancer and offer insights for resource allocation and disease prevention and treatment for cancers with high disease burden.
Patients and Methods
Data Source
The electronic health record (EHR) is a secure and confidential health record for both outpatients and inpatients.18 In China, cancer treatment is strictly an inpatient procedure. Accordingly, in our study, we used the inpatients’ EHR data for analysis. Inpatient EHR data comprise two unified parts. The first part is the personal information of the patients; the patient or their family members provide information such as their sex, age, ID number, health insurance type and occupation. The second part includes the patient’s hospitalization information. This part is filled in by the patient’s attending physician and offers high reliability, including information such as diagnosis code (ICD-10), pathological diagnosis, cost and operation code.19 The retrospective data of this study came from the Health Information Center of Pudong New District, Shanghai. Before extraction, personal information was deleted and concealed through the Health Information center.
Study Participants
The retrospective data of 193,432 hospitalized children who were admitted to hospital between January 1, 2011 and December 31, 2016 were extracted from the EHR system in Pudong New District. The samples were chosen according to the following selection criteria: (1) the patient was hospitalized in a pediatric ward, (2) the ICD-10 code was C, (3) the patient was younger than 18 years old, (4) patient- and research-related information was available, and (5) the patients lived in Pudong. In our study, we analyzed each inpatient as the unit rather than each hospitalization. For patients who were hospitalized multiple times, their hospitalization data were integrated according to their ID information. A total of 3834 cases met the requirements.
Procedures
The patients included in this study were grouped according to the International Classification of Childhood Cancer-3 (ICCC-3). The ICCC facilitates the comparison of childhood cancer incidence rates.20 The first edition of the ICCC was based on the International Classification of Diseases, ICD-9, and the corresponding tumor-specific classification ICD-O-2,21 the ICCC was revised when the subsequent version of ICD and the corresponding ICD-O-3 were released.22 The revised ICCC-3 added definitions of 47 subgroups to the 12 main diagnostic groups. In this article, we examined 33 sub-categories; the remaining 14 sub-categories could not be analyzed because of a lack of patients. Furthermore, the groups were classified into a malignant non-solid tumor group and a solid tumor group for better comparison.
Measurements
Variables in this study included patient demographics. All patients were enrolled in Basic Medical Insurance for Urban Residents, and therefore the type of insurance was not analyzed as a variable. The patients were sub-grouped according to age (0–4, 5–9, 10–14 and 15–18 years old), according to the World Population Prospects document issued by the United Nations.23 The hospitalization information included length of stay (LOS) and number of the inpatient hospitalizations. Surgery status consisted of whether the patients underwent surgery during hospitalization (surgical status), type of surgery and the highest level of surgery. Type of surgery indicated the various kinds of surgeries during hospitalization, and the surgical level was based on the updated 2018 version of the surgical classification catalog issued by the Ministry of Health of China (2018 Ministry of Health Surgery Classification and Classification Catalog). In terms of cost, we measured the total cost, surgical cost and drug cost to reflect various disease burdens for each caner. Costs in the catalog are in US dollars (USD), so to facilitate readers’ understanding, for this study, costs are given in renminbi (RMB) at the current exchange rate of 1 RMB ≈ 0.1504 USD.
Statistical Analysis
All analyses were performed with SPSS software (version 21.0, Inc., Chicago, IL, USA). We conducted a descriptive statistical analysis of the patient demographics (e.g., sex, age), disease type, hospitalization information (LOS, number of hospitalizations), status of surgery, and cost (total cost, surgical cost, drug cost). The chi-squared test was used to compare the differences in demographics (sex, age), hospitalization information (LOS, number of hospitalizations), status of surgery, and cost by category of cancer according to the ICCC. We further compared the rankings of the 33 sub-categories of ICCC by characteristics.
Results
Demographics of the Inpatients
Table 1 lists the demographics of the patients with malignant non-solid tumors (leukemia) and malignant solid tumors in this study. In the two groups, the number of male children was higher (non-solid tumors: 63.22%, solid tumor: 61.51%). The proportion was highest in children aged 0–4 years in both groups (non-solid tumors: 50.93%, solid tumor: 59.66%). LOS in the non-solid tumor group (median=24.00 days) was higher than in the non-sold tumor group, and LOS in this group was highest in patients with acute myeloid leukemia (median=26.00 days). The proportion of patients who underwent surgical treatment of malignant was higher in the solid tumor group (59.52%) than in the non-solid tumor group (P<0.001). In the non-solid tumor group, level 3 surgery was the most commonly performed (n=197, 76.36%). However, level 4 surgery (n=577, 45.87%) was the most commonly performed in the malignant solid tumor group. The malignant solid tumor group had not only the highest total costs and drug costs but also the highest surgical costs compared with the non-solid tumor group (P<0.001).
 |
Table 1 Demographics of the Hospitalized Pediatric Malignant Solid Tumor Cases (n=3834) |
Distribution of the Classification of Cancers According to the ICCC
Figure 1 shows the specific distribution and rankings of all the tumors in this study. The top five malignant tumor groups were leukemia (43.69%), lymphoma (15.26%), soft tissue and other extraosseous sarcomas (12.36%), CNS tumors (7.33%) and kidney tumors (5.95%).
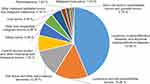 |
Figure 1 Specific distribution of the tumors in pediatric patients according to the ICCC. |
Comparison of Characteristics in Pediatric Cancer Patients According to Cancer Category
Table 2 shows the distribution of characteristics in pediatric cancer patients according to cancer type. In all tumor groups, the numbers of female patients with other malignant epithelial tumors and malignant melanoma, and retinoblastoma, were higher than those of male patients (P<0.05). With the lymphoma and reticuloendothelial tumors in the solid tumor group, patients in the 5–9-year-old age group were the largest, followed by the 0–4-year-old age group. In the non-solid tumor group, LOS was longest in the acute myeloid leukemia group (median=26 days). In the malignant solid tumor group, those with CNS tumors (median=17.00 days) had a longer LOS than other groups, and the shortest was 2.00 days (retinoblastoma). All patients in the retinoblastoma group underwent surgery. The CNS tumor group had the highest proportion of patients undergoing grade 4 surgery (79.92%). Regarding total cost, the acute myeloid leukemia group had the highest cost, which was slightly lower than that of CNS tumors in the solid tumor group by 44,731.37 (36,375.47–64,140.93) RMB. The total cost for patients with retinoblastoma was the lowest among all cancer types (median 6907.39 RMB). However, in the malignant tumor group, germ cell tumors, trophoblastic tumors, and gonadal tumors had the lowest surgical costs, with a median of 1370.00 RMB, which was higher than the subgroups in the leukemia group. The median cost of medicine in the acute myeloid leukemia group was 23,844.97 RMB (9467.02–3938.65 RMB), which was higher than that of the CNS tumor group in the malignant solid tumor group and more than that of other tumors; the lowest cost was for the retinoblastoma group at 797.98 (687.66–929.07) RMB.
 |
Table 2 Demographics of Pediatric Cancer Inpatients According to ICCC-3 |
Ranks of Various Characteristics in Pediatric Cancer Patients According to the ICCC-3
Figure 2 shows the ranking of the 39 sub-categories of cancer across various characteristics according to ICCC-3. Lymphoid leukemia was the most common cancer in all subgroups, followed by non-Hodgkin lymphomas. As for the proportion of male patients, myelodysplastic syndrome and other myeloproliferative diseases, other specified malignant bone tumors, and malignant melanomas, three groups were tied for first. In the 0–4-year-old group, the proportion of female patients was highest in adrenocortical carcinomas and thyroid carcinomas. Among the other specified malignant bone tumors, intracranial and intraspinal germ cell tumors most common. Hospitalizations were most frequent in patients with other unspecified malignant tumors. Surgical treatment was performed most frequently on patients with Non-Hodgkin lymphoma (not including Burkitt lymphoma). Patients with skin carcinomas most frequently received grade 1 surgical treatment. Patients with Hodgkin lymphoma most frequently received grade 2 surgical treatment. Total cost was highest for astrocytoma. In the CNS tumor category, ependymoma and choroid plexus tumor had the highest surgical costs.
 |
Figure 2 Ranking of the 39 cancer sub-categories by various characteristics. |
Discussion
In our research, we clarified several characteristics of children with malignant tumors in Shanghai’s Pudong New District. Leukemia was the most common tumor in this study. This result was consistent with those seen in other studies, which showed that leukemia accounts for 32% of childhood cancer in China, 36.0% of childhood cancer in Japan, and 29% of childhood cancer in France.24–26 Lymphoid leukemia was also the highest in the leukemia group and was the most common subgroup disease in childhood malignant tumors. This result was consistent with published research.27 Additionally, non-Hodgkin lymphoma was the second most common tumor in patients with malignant solid tumors, which is higher than in patients with Hodgkin’s disease. Consistent with our results, Ward et al reported that the prevalence of Hodgkin lymphoma was 4.514 and non-Hodgkin lymphoma was 6.442.15 In this study, the number of patients suffering from soft tissue and other extraosseous sarcomas was higher than that of the CNS tumors, which was different from that reported in Costa Rica.28 This interesting phenomenon might be due to the improvement of diagnostic level or changes in environmental factors.
The numbers of male patients with leukemia, lymphoma, CNS tumors, and liver tumors in this study were higher than those of female patients. This result is consistent with Liu’s analysis of the global sex differences in childhood tumors from 1990 to 2015.16 The predominance of males among children with cancer might be due to innate susceptibility, but as age increases, other factors might also play a role. For example, studies showed that in some sub-Saharan Africa regions, HIV infection affects the incidence of Burkitt’s lymphoma.29 Regarding the age of patients with malignant tumors, we found that in both the leukemia group and the malignant solid tumor group, patients aged 0–4 years old accounted for the largest proportion of patients with malignant tumors. Regarding all of the 11 types of malignant tumor, patients aged 15–18 years were the least common. In our study, the order of the top three tumors for each age group varied somewhat, but leukemia was most common in all age groups. Armstrong’s research results showed the same conclusion.30 Interestingly, in the malignant solid tumor group, the results showed that most patients with malignant bone tumors are under 10 years old, which is slightly lower than the findings of other studies.31,32 Additionally, another study analysis on the incidence and mortality of children’s tumors in the cancer registration area of Anhui Province in 2014 also showed that the incidence of bone tumors was the highest in the 5–10-year-old group, at 1.00/105.31,33
The most noteworthy finding in our study is that drug costs for children with cancer are high. In the subgroup analysis, the most expensive disease was acute myeloid leukemia, with a median drug cost as high as 23,844.97 RMB. For patients with acute myeloid leukemias, in keeping with the 10 disease diagnosis and treatment norms related to children’s blood diseases and malignant tumors issued by the National Health and Health Commission in China, doctors generally administered anthracyclines (eg, daunorubicin, dedaunomycin, mitoxantrone) combined with cytarabine for induction remission treatment.
In China, leukemia, lymphoma, CNS tumor, soft tissue sarcoma, kidney tumor, and reproductive system tumor are common tumors treated with chemotherapy. However, some drugs with better therapeutic effects, such as newly approved chemotherapeutics and targeted drugs, are included in the standard treatment guidelines in China and abroad. The more expensive treatment-essential drugs, such as pegaspase for the treatment of lymphoblastic lymphoma, are beneficial. Rituximab is used to treat high-risk mature B-cell lymphoma but is not covered by children’s cancer insurance. Therefore, to reduce drug costs for cancer patients, reasonable preventive drugs and imported drugs should be included in the medical insurance reimbursement drug catalog and the diagnosis and treatment catalog.
This study had several limitations. First, all of the samples were from Pudong, and thus the survey may not be representative of other areas, especially underdeveloped ones. The study should be extended to include a larger sample of pediatric institutions in more regions. Second, socioeconomic data such as household income cannot be obtained from EHR, and thus we cannot compare differences between socioeconomic groups in this study. Third, because there have been very few hospitalization cost investigations for childhood tumors in other regions of China, it is difficult to compare the current findings on hospitalization costs with those for other regions of China. Fourth, follow-up research needs to collect information on the classification of malignant tumors and the cost of radiotherapy from other data sources, as this would aid in further analysis of the disease burden of childhood tumors.
Conclusion
The study provides information on pediatric cancer patients from 17 hospitals in Pudong and shows the status and spectrum of childhood tumors to some extent. The results showed that the proportion of malignant tumor patients was highest in the 0–4-year-olds, boys, and patients with leukemia in Pudong New District of Shanghai, and the total hospitalization, and drug cost were the highest in acute myeloid leukemias and CNS tumors. Patients could benefit from increased government attention to the cost of treating patients with leukemia and CNS tumors.
Abbreviations
ICCC, International Childhood Cancer Classification; EHR, electronic health record; ICD, International Classification of Diseases; ICD-O, International Classification of Diseases for Oncology.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
We acquired written informed consent from the study participants. This study was approved by the Ethics Committees of Tongji University (ref: LL-2016-ZRKX-017). Participants’ personal information like ID and name was not available to the analysts in the research.
Acknowledgment
We sincerely acknowledge and appreciate the assistance of the Information Center of the Health and Family Planning Commission of the Pudong New District in Shanghai for their help in collecting the data. Huining Zhou and Zhengyi Wu are co-first authors of this paper.
Funding
Funding for this study was provided by the Natural Science Foundation of China (71603182 and 71774116), the Shanghai Pujiang Program (2019PJC072), the National Key R&D Program of China (2018YFC2000700 and 2018YFC1314700), the Shanghai Leading Talents (YDH-20170627), the Shanghai Health System Outstanding Talents Program (2018YQ52) and the Shanghai Public Health Outstanding Young Personnel Training Program (GWV-10.2-XD07).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Youlden DR, Baade PD, Green AC, Valery PC, Moore AS, Aitken JF. The incidence of childhood cancer in Australia, 1983–2015, and projections to 2035. Med J Aust. 2020;212(3):113–120. doi:10.5694/mja2.50456
2. Shabani M, Saeedi Moghaddam S, Ataeinia B, et al. Trends of national and subnational incidence of childhood cancer groups in Iran: 1990–2016. Front Oncol. 2020;9:1428. doi:10.3389/fonc.2019.01428
3. Jastaniah W, Essa MF, Ballourah W, et al. Incidence trends of childhood acute lymphoblastic leukemia in Saudi Arabia: increasing incidence or competing risks? Cancer Epidemiol. 2020;67:101764. doi:10.1016/j.canep.2020.101764
4. Johnston WT, Erdmann F, Newton R, Steliarova–Foucher E, Schüz J, Roman E. Childhood cancer: estimating regional and global incidence. Cancer Epidemiol. 2020;101662.
5. AIRTUM Working Group; CCM; AIEOP Working Group. Italian cancer figures, report 2012: cancer in children and adolescents. Epidemiol Prev. 2013;37:1–225.
6. Baade PD, Youlden DR, Valery PC, et al. Trends in incidence of childhood cancer in Australia, 1983–2006. Br J Cancer. 2010;102:620–626. doi:10.1038/sj.bjc.6605503
7. Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008;112:416–432. doi:10.1002/cncr.23169
8. Michel G, von der Weid NX, Zwahlen M, et al. Incidence of childhood cancer in Switzerland: the Swiss childhood cancer registry. Pediatr Blood Cancer. 2008;50(1):46–51. doi:10.1002/pbc.21129
9. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi:10.3322/caac.21149
10. Ghatak N, Trehan A, Bansal D. Financial burden of therapy in families with a child with acute lymphoblastic leukemia: report from north India. Support Care Cancer. 2016;24(1):103–108. doi:10.1007/s00520-015-2757-y
11. Jaime–Pérez JC, Fernández LT, Jiménez–Castillo RA, et al. Hospitalization rate and costs in acute lymphoblastic leukemia of childhood in a low–income group: financial impact in Northeast Mexico. Pediatr Blood Cancer. 2017;64(12):e26673. doi:10.1002/pbc.26673
12. Zheng R, Peng X, Zeng H, et al. Incidence, mortality and survival of childhood cancer in China during 2000–2010 period: a population–based study. Cancer Lett. 2015;363:176–180. doi:10.1016/j.canlet.2015.04.021
13. Mitra D, Shaw AK, Hutchings K. Trends in incidence of childhood cancer in Canada, 1992–2006. Chronic Dis Inj Can. 2012;32:131–139. doi:10.24095/hpcdp.32.3.03
14. Li J, Thompson TD, Miller JW, Pollack LA, Stewart SL. Cancer incidence among children and adolescents in the United States, 2001–2003. Pediatrics. 2008;121:1470–1477. doi:10.1542/peds.2007-2964
15. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi:10.3322/caac.21219
16. Liu Z, Yang Q, Cai N, Jin L, Zhang T, Chen X. Enigmatic Differences by Sex in Cancer Incidence: Evidence From Childhood Cancers. Am J Epidemiol. 2019;188(6):1130–1135. doi:10.1093/aje/kwz058
17. Shanghai Bureau of Statistics. 2019 Shanghai Statistical Yearbook. 2019.
18. Chen DD, Lian QG. Creation, update and application of the electronic health record in huajingzhen community health service center in Shanghai. Chin Gen Pract. 2010;13:2177–2178.
19. Steliarova–Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer. 2005;103(7):1457–1467. doi:10.1002/cncr.20910
20. Kramárová E, Stiller CA. The international classification of childhood cancer. Int J Cancer. 1996;68:759–765. doi:10.1002/(SICI)1097-0215(19961211)68:6<759::AID-IJC12>3.0.CO;2-W
21. Percy CL, Van H, Valerie Muir CS, World Health Organization. International classification of diseases for oncology. Pathology. 1990;23:782.
22. Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology. J Clin Pathol. 2000;30:782.
23. United Nations. World population prospects: the highlights. United Nations, department of economic and social affairs. Popul Div. 2019;1–46.
24. Ishihara H, Ohno Y, Fujii M, Hara J, Soda M. Epidemiological analysis of childhood cancer in Japan based on population–based cancer registries, 1993–2009. Jpn J Clin Oncol. 2017;47:660–663. doi:10.1093/jjco/hyx041
25. Lacour B, Clavel J. ILS concernent 1 enfant sur 440 avant l’âge de 15 ans Aspects épidémiologiques des cancers de I’enfant [Epidemiological aspects of childhood cancer]. Rev Prat. 2014;64:1264–1269.
26. Nakata K, Ito Y, Magadi W, et al. Childhood cancer incidence and survival in Japan and England: a population–based study (1993–2010). Cancer Sci. 2018;109:422–434. doi:10.1111/cas.13457
27. Steliarova–Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population–based registry study. Lancet Oncol. 2017;18:719–731. doi:10.1016/S1470-2045(17)30186-9
28. Erdmann F, Li T, Luta G, et al. Incidence of childhood cancer in Costa Rica, 2000–2014: an international perspective. Cancer Epidemiol. 2018;56:21–30. doi:10.1016/j.canep.2018.07.004
29. van den Bosch CA. Is endemic Burkitt’s lymphoma an alliance between three infections and a tumour promoter? Lancet Oncol. 2004;5:738–746. doi:10.1016/S1470-2045(04)01650-X
30. Armstrong GT. Long–term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14:298–303. doi:10.1016/j.ejpn.2009.12.006
31. Li L. Primary care and cancer. Family Med Community Health. 2017;5(2):101–102. doi:10.15212/FMCH.2017.0132
32. Jackson TM, Bittman M, Granowetter L. Pediatric malignant bone tumors: a review and update on current challenges, and emerging drug targets. Curr Probl Pediatr Adolesc Health Care. 2016;46(7):213–228. doi:10.1016/j.cppeds.2016.04.002
33. Dai D, Li W, Cha ZB, Chen YJ, Liu ZR. Analysis of childhood tumor incidence and death in 2014 in the tumor registration area of Anhui Province. China Child Health J. 2019;27(04):447–450.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
