Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Analysis of the Peri-Operative Cost of Non-Traumatic Major Lower Extremity Amputation in Jordan
Authors Aljarrah Q , Bakkar S , Aleshawi A, Al-Gharaibeh O, Al-Jarrah M , Ebwayne R , Allouh M , Abou-Foul AK
Received 28 September 2019
Accepted for publication 18 December 2019
Published 9 January 2020 Volume 2020:12 Pages 13—21
DOI https://doi.org/10.2147/CEOR.S232779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Qusai Aljarrah,1 Sohail Bakkar,2 Abdelwahab Aleshawi,3 Omar Al-Gharaibeh,3 Mooath Al-Jarrah,3 Radi Ebwayne,1 Mohammed Allouh,4 Ahmad K Abou-Foul5
1Department of Surgery, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan; 2Department of Surgery, Faculty of Medicine, The Hashemite University, Zarqa 13133, Jordan; 3School of Medicine, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan; 4Department of Anatomy, College of Medicine & Health Sciences, United Arab Emirates University, Al Ain 17666, United Arab Emirates; 5Department of Otolaryngology, Head and Neck Surgery, Imperial College Healthcare NHS Trust, St Mary’s Hospital, London W2 1NY, UK
Correspondence: Qusai Aljarrah
Department of Surgery, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan
Tel +962775593131
Fax +96227200627
Email [email protected]
Purpose: Non-traumatic major lower extremity amputation (NMLEA) is a commonly performed procedure that presents a substantial cost burden. Patients who undergo NMLEA are usually considered as a high-risk group with significant comorbidities, which translates into a protracted peri-operative course and increased health-care costs. The primary aim of this study was therefore to perform a contemporary peri-operative cost analysis of NMLEA performed in our center. We are a major tertiary referral hospital that provides vascular surgery services to the entire northern counties in Jordan. We also aimed to assess the various factors that influence the cost of NMLEA in less economically developed countries.
Methods: Records of all patients who underwent NMLEA at King Abdullah University Hospital between January 2012 and December 2017 were retrieved. Total inpatient cost was calculated and analyzed against different patients’ variables.
Results: A total of 140 patients underwent NMLEA between 2012 and 2017 in our facility. Below-knee amputations accounted for 110 cases, while above-knee amputations included 30 patients. Approximately two-thirds of the cases (61.4%) were males, with average age of the patients being approximately 62.9 years. The commonest comorbidities were diabetes mellitus and hypertension, which were recorded in 89.3% and 80.3% of the patients, respectively. The average operative time was 133.0 ± 10.8 mins, and the average length of stay (LOS) was 6.7±0.4 days. The mean cost for amputations was 4904.7± 429.3 United States dollars. Multiple linear regression analysis demonstrated that LOS and admission-to-operation time were the independent predictors of cost.
Conclusion: Delayed amputations and prolonged LOS remain the most important determinants for the peri-operative cost of NMLEA. When amputation is deemed inevitable, an expedited multidisciplinary approach may possibly reduce undue delays and result in cost-effective delivery of this age-old remedy.
Keywords: health care cost, amputation, ischemia, diabetic foot syndrome, length of stay
Introduction
Non-traumatic major lower extremity amputation (NMLEA) represents a substantial economic burden to any healthcare system.1–9 The annual cost of lower extremity amputation in the USA is estimated at $4.3 billion, accounting for 75% of the toll of diabetic foot syndrome (DFS).8,10 Corresponding data from the National Health Service (NHS) in England estimates expenditure of £972 million to £1.13 billion on health-care costs related to foot ulceration and amputation in diabetics in 2014‒2015; this is equivalent to 0.72‒0.83% of the entire NHS budget11 and accounted for higher expenditures than the combined annual cost of the three most common cancers in the UK.12 In the Middle East and North Africa (MENA) region, approximately 18,345 NMLEAs are annually performed.13 It is also projected that the prevalence of NMLEA will rise significantly, especially with the global pandemics of diabetes mellitus (DM) and metabolic syndrome, and the ageing populations worldwide.2,7,8,14,15
In Jordan, like in other less-developed countries, expanding health-care demands are undoubtedly challenged by scarce resources, and this is exacerbated by the worsening geo-politico-economic crises in the neighboring Syria and Iraq. This has led to a local paradigm shift to cost containment strategies while ensuring quality assurance at point of delivery; the aim is “intensive but not expensive care”.
There is paucity of research regarding the predictors of total inpatient cost following NMLEA particularly from MENA region. While the length of stay (LOS) has been consistently linked to increased amputation cost, other potential variables are difficult to associate due to various potential confounding factors. That is especially true when comparing different health-care systems due to variations in clinical practice, reimbursement systems, attitudes to inpatient stay, costs, and prices.1,2,4,9,16–19
The aim of this current study was to retrospectively assess peri-operative cost of NMLEA during inpatient surgical stay in a major university hospital that serves the north of Jordan and refugees from neighboring countries. We also hope to improve our understanding of various factors that influence the peri-operative financial cost of NMLEA. This will be of help to health-care providers who aim to establish a cost-effective amputation service in other developing populations.
Materials and Methods
Medical records of all patients treated in our center, King Abdullah University Hospital (KAUH), with major lower extremity amputation between January 2012 and December 2017 were retrospectively reviewed. KAUH is a major tertiary referral center that provides vascular surgery services to the entire northern region of Jordan, including refugee camps from neighboring Syria and Iraq. We included only adult patients who underwent amputations for acute or critical lower-limb ischemia (ALI or CLI), and patients with infected septic DFS. Patients who had amputations for other indications (trauma or tumors) were excluded from this study. This study was approved by our local institutional review board.
We used the definitions of CLI and ALI as described in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).20 ALI was defined by the presence of sensorimotor deficit and absent Doppler signals at ankle level (ICD-9-CM code 459.9). CLI was defined as the presence of rest pain or tissue loss (ICD-9-CM code 440.22–24). Major lower extremity amputation was defined as any amputation above the ankle joint (ICD9-CM: codes 84.13–19) and included below-knee amputation (BKA) and above-knee amputation (AKA). The definition of DFS used by the World Health Organization was utilized in our study: “ulceration of the foot (distally from the ankle and including the ankle) associated with neuropathy and different grades of ischemia and infection”,21 and included ICD-9-CM codes 250.80, 250.82 with 785.4, 681.11, 681.10, 681.7, 681.9, 682.6, 682.7, 730.0, and 730.1
Information collected included demographic details, comorbidities, operative data, and LOS. The last documented laboratory results before the amputation were also used for the analysis. Serum albumin and glycated hemoglobin (HbA1c) levels were evaluated as numerical variables, with normal ranges of 3.5‒5.5 g/dL and 4‒5.6%, respectively.
The demographic data collected include sex and age. The comorbidities considered comprise hypertension, DM, cerebrovascular disease, ischemic heart disease, chronic kidney disease, and Buerger’s disease. The operative parameters include type of amputation (AKA or BKA), site of amputation (right or left limb), indication for amputation (CLI, ALI, or infected DFS), type of anesthesia utilized (spinal anesthesia (SA) or general anesthesia (GA)), the surgeons’ specialty (vascular or non-vascular surgeon), the operative time (from induction of anesthesia until the end of amputation), and the time from admission to operation. LOS was measured from admission to discharge. We only analyzed cumulative costs of NMLEA that resulted from direct care of the patients, which were incurred from time of admission until discharge from the hospital. These costs included theatre costs, expenses of in-house services, ward costs, cost of laboratory investigations, and cost of medications. Indirect costs that resulted from lost work hours, residual disability, prosthesis, and rehabilitation costs were excluded from our analysis.
The costs were adjusted for inflation to the base year of the analysis (2017), using the methods described by Turner et al.22 Local currency inflation rates were used and then exchanged to United States dollars (USD).
Statistical Analysis
Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS) software, version 21.0 (IBM, Armonk, New York, USA), with statistical significance set at P < 0.05. Data was presented as frequency distributions for categorical variables and mean ± standard error of the mean (SE) for continuous variables. Data was tested at 5% level of significance. Pearson χ2 test was used to investigate the significance of association between categorical variables, while Student’s t-test and one-way analysis of variance (ANOVA) were applied to examine the significance level for continuous normally distributed variables. If a significant relationship was found, then a posthoc residual analysis for categorical variables and a Fisher’s least significant difference test for continuous variables were applied to determine the exact significance between groups for each variable.
Results
Patients’ Characteristics
The study included 140 patients who satisfied the inclusion and exclusion criteria in the 6-year period of the study. Approximately two-thirds of the patients (61.4%) were males, with a male: female ratio of 1.6:1 (Table 1). The average age of the amputee patients was 62.9 ± 1.1 years. There was a high prevalence of comorbid chronic medical conditions. The most common comorbidity was diabetes mellitus (89.3%), followed by hypertension (80.3%). As expected for amputee patients who had vasculopathy, ischemic heart disease and cerebrovascular disease were noted in 46.4% and 27.7%, respectively.
 |
Table 1 Patients’ Demographics and Characteristics |
The most common indication for amputation was DFS with 72.9% of cases, followed by ALI and CLI, which accounted for 12.9% and 11.7% of amputations, respectively (Table 2). Below-knee amputations accounted for 110 cases, while above-knee amputations were done in 30 patients. The majority of amputations were performed by vascular surgeons, while surgeons from other specialties like general or orthopedic surgery performed around 41% of the cases. The average time from admission to operation was 8.3 days, with only 30.6% of the amputations performed within 48 hrs of admission. Figure 1 outlines the various causes of delayed amputation in our institution. The mean operative time was 133.0 ± 10.8 mins, and the average LOS was 6.7±0.4 days. The mean cost for amputations was 4904.7± 429.3 USD.
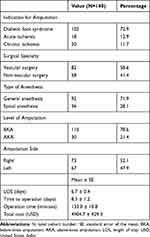 |
Table 2 Patients’ Variables, Including Indication for Amputation, Timing, Intra-Operative Data, and Inpatient Stay |
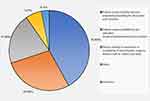 |
Figure 1 A pie chart outlining the reasons for delays in performing amputation in our institution (N=93). |
Factors Affecting Cost
The cost of NMLEA was significantly associated with the indication for amputation in Table 3, length of hospitalization Table 4, time from admission to operation Table 4, and type of anesthesia Table 3. Patients who underwent amputation for ALI had a significantly higher cost than those who had DFS or chronic ischemia (P=0.039). Patients who had their operation under GA incurred higher cost compared to those who had it under SA (P=0.019). Moreover, a significantly (P<0.001) positive correlation was found between time from admission to operation and cost (cost increased by 323.6 USD for each additional day of admission). LOS also correlated significantly (P<0.05) with cost (cost increased by 179.1 USD for each day). After controlling for all factors in the model, multiple linear regression analyses demonstrated that LOS and length from admission to operation were the most important cost influencers for NMLEA. In this model, one standard deviation (SD) increase in duration from admission to operation was associated with 0.85 increase in the SD of cost (for everyday delay in performing the amputation, the cost increased by 362.9 USD; P<0.05). Similarly, increase of one SD in LOS was associated with 0.53 increase in the SD of the cost (for everyday increase in the total LOS, the cost increased by 434.28 USD; P<0.05). Type of anesthesia was the only significant factor associated with both higher total inpatient costs (P =0.019) and longer LOS (P=0.021) Table 5.
 |
Table 3 Factors Influencing the Total Cost of Amputation |
 |
Table 4 Univariate Linear Analysis for the Continuous Factors That Influence the Total Cost of Amputation |
 |
Table 5 Factors Influencing Length of In-Patient Stay |
Discussion
This study aimed to perform a contemporary peri-operative cost analysis of NMLEA in our center, and to assess factors that affect cost of NMLEA. In a less economically developed country, such as Jordan, the financial burden of NMLEA can be substantial due to limited health-care resources. As demonstrated in our results (Figure 1), other challenging factors may result in delayed treatment, and hence increased costs. These factors include patients’ preconceptions and attitudes towards amputation, which may render the consenting process challenging and subsequently lengthen time to operation. Secondly, lack of amputation support services can force the amputees to become self-dependent, especially with the presence of negative impact from the society that stigmatizes amputation. Thirdly, the impact of poorly controlled DM can lead to limb loss at younger age, compared to what occurs in more developed nations. Younger amputees will subsequently rely on the health-care services for many more years to maintain active and productive lives.2,14,23–25
With all these challenges facing the healthcare system, designing and implementing cost-effective services is paramount.1,2,14 In Jordan, the incidence of DM is rising at an alarming rate, with diabetic lower extremity complications accounting for a substantial amount of healthcare expenditure.26,27 This picture is not very different in more developed countries, like the US where NMLEA is ranked as the sixth most expensive surgery performed.5,9,17 Moreover, the cost of lower extremity amputation in diabetics has been identified as the most expensive complication of DM, compared to cardiac-, cerebrovascular-, and renal-related hospitalizations.5,9,17,18,28,29 Therefore, identifying factors that are linked with increased total inpatient cost among our cohort of amputees may lead to quality improvement programs and the development of intensive but inexpensive amputation services in other developing nations.
In the current study, we identified that delaying NMLEA is the most important variable that affects total inpatient cost. Only one-third of our patients had their operation within 48 hrs of admission. The Vascular Society of Great Britain and Ireland guidelines on major amputation surgery recommend that operative management should be executed in most patients within 48 hrs of the decision to operate.30 The United Kingdom’s (UK) National Diabetes Footcare Audit (NDFA) also clearly recommends that all patients with DFS should be referred promptly for early specialist assessment in accordance with National Institute for Health and Care Excellence (NICE) guideline.31,32 Our analysis showed that for every additional inpatient day, the cost of NMLEA increased by 4.8 fold (323.6 USD/day).
An important factor for delayed operations in our center is patients’ attitudes towards amputation. Many patients with clearly indicated amputation are reluctant to accept this undesirable outcome, and this usually leads to long operative delays. This may lead to disease progression and increased morbidity, which can affect LOS and increase the total cost of surgery. In a more developed healthcare system, early input from vascular clinical nurse specialists, who are trained to provide the necessary peri-operative counselling and psychological support, can prove invaluable and may shorten the time to operation and the total LOS.33 Moreover, delays associated with pre-operative optimization were also linked to delayed conduct of NMLEA.33 Consequently, devoting additional attention to outpatient medical optimization and adequate patient counselling, and establishing a coordinated amputation support team can lead to a quick and cost-effective amputation service.
Similar studies on the financial benefits of timely amputation are largely lacking in the literature. Driver et al9 conducted a thorough cost analysis of limb salvage pathways in diabetic patients and concluded that a guideline-based care for DFS is cost-effective when compared to usual care. The same authors also recommended a multidisciplinary approach when managing such patients, which results in shorter LOS and reduced cost.9
In 2014, a report by the UK’s National Confidential Enquiry into Patients’ Outcome and Death (NCEPOD) provided a great insight into the issues surrounding patient consent and operative management of lower limb amputation. The report acknowledged patients’ refusal to consent and delayed pre-operative optimization as the commonest reasons for delay in NMLEA.33
Our study demonstrated that acute limb ischemia was associated with increased cost. It is universally accepted that charges of limb salvage attempts are usually offset by rapid recovery compared to a more protracted course that an amputee requires.1,6 Interestingly, published literature identified dysvascular limb as a predictor of increased cost without differentiating between acute and chronic limb ischemia as separate clinical entities.8,13,14,16,29 Our results contradict the findings published by Singh et al34 who concluded that ALI is less expensive than CLI. In their analysis, ALI was associated with shorter LOS, and required less rehabilitation compared to CLI. Moreover, they identified unsuccessful limb salvage procedures as predictors of increased cost following NMLEA. We believe that rigorous attempts to salvage limbs with ALI before embarking on NMLEA, and usually the presence of poorly controlled pre-admission chronic conditions may legitimately incur extra cost when compared to other indications such as CLI or DFS.19 Moreover, less developed rehabilitation services in our region may explain the contradicting results to studies done in developed nations.34,35 In our region, patients with ALI who need amputation generally need longer time to accept the procedure, and that usually results in longer consenting process and delayed surgery.
Our findings also show that the type of anesthesia was a predictor that correlated with both increased LOS and total inpatient cost. NMLEAs performed under GA were 33% more expensive than those performed with SA. SA usually offers improved postoperative analgesia, and reduced nausea and vomiting, which may result in enhanced recovery and shorter LOS. Moreover, patients who require amputation under GA will generally need meticulous medical optimization and more preoperative investigations, which may delay the operation.36 It is important to note that the type of anesthesia does not make a significant difference to the post-operative clinical outcomes following NMLEA, and that choice should follow an informed joint decision with individualized consideration.37
Interestingly, NMLEA in elderly patients with associated comorbidities such as DM, cerebrovascular disease, and chronic kidney disease was not associated with increased costs. This is consistent with the results of the Fremantle Diabetes Study,17 but contradicts the findings of Yin et al18 who identified age, race, level of amputation, and comorbidities as predictors of increased cost. In our hospital, such patients with comorbidities undergo an integrated medical care by various specialists and therefore, they are usually admitted in an elective or semi-elective form when medically optimized in an outpatient setup. This results in a cost-efficient service. Furthermore, patients with multiple chronic comorbidities are less likely to deny the need for NMLEA and delay consenting.
Limitations of the Study
This is a retrospective study with its inherent susceptibility to various forms of bias, and the possible influence of unmeasured confounders (such as prescribed medications). Our data was collected from patients who were treated in a single institution, with a relatively small sample size. In clinical practice, the cost of NMLEA is realistically subject to great variation among different providers, and therefore we cannot generalize costs to other health-care systems. Finally, the current study did not measure the indirect costs of amputation from lost work hours, residual disability, prosthesis, and rehabilitation.
Conclusion
This study demonstrates that delayed conduct of amputation and prolonged LOS remain the most important determinants of the peri-operative cost of NMLEA. When amputation is deemed inevitable, an expedited multidisciplinary approach that includes psychological and social support may possibly reduce delays and lead to an efficient and cost-effective amputation service. Our findings should be interpreted carefully and a differentiation between health-care costs and the real cost of post-amputation disability for the person and the society is essential.
Ethical Approval
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendment. Ethical approval was obtained from Research and Ethics Committee of Jordan University of Science and Technology, and King Abdullah University Hospital, Irbid, Jordan. The consent from patients was waived due to the retrospective nature of the study. We confirm that the privacy of the participants was saved, and the data was anonymized and maintained with confidentiality.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no financial ties or conflicts of interest to disclose.
References
1. Jordan RW, Marks A, Higman D. The cost of major lower limb amputation: a 12-year experience. Prosthet Orthot Int. 2012;36(4):430–434. doi:10.1177/0309364612441489
2. Boulton AJM, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi:10.1016/S0140-6736(05)67698-2
3. Frykberg RG, Cook JJ, Simonson DC. Epidemiology and health care cost of diabetic foot problems. In: Veves A, Giurini JM, Guzman RJ, editors. The Diabetic Foot: Medical and Surgical Management.
4. Peacock JM, Keo HH, Duval S, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005–2008. Prev Chronic Dis. 2011;8(6):A141.
5. Elixhauser A, Andrews RM. Profile of inpatient operating room procedures in US hospitals in 2007. Arch Surg. 2010;145(12):1201–1208. doi:10.1001/archsurg.2010.269
6. Ponticello M, Andersen CA, Marmolejo VL. Limb salvage versus amputation: a closer look at the evidence, costs and long-term outcomes. Podiatry Today. 2016;29(3):30–39.
7. Barshes NR, Chambers JD, Cohen J, Belkin M. Model to optimize healthcare value in ischemic extremities 1 (MOVIE) study collaborators. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg. 2012;56(4):1015–24 e1. doi:10.1016/j.jvs.2012.02.069
8. Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil. 2005;86(3):480–486. doi:10.1016/j.apmr.2004.06.072
9. Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3 Suppl):17S–22S. doi:10.1016/j.jvs.2010.06.003
10. O′ Brien J, Ward A, Salas M. Managing diabetic foot ulcers: economic consequences in the USA. Expert Rev Pharmacoecon Outcomes Res. 2003;3(1):25–32. doi:10.1586/14737167.3.1.25
11. NHS England/Policy/Analytical Service. Programme budgeting finance manual 2012/13. Available from: https://www.networks.nhs.uk/nhs-networks/health-investment-network/documents/2012-13%20Programme%20Budgeting%20Guidance%20documents.zip.
12. Kerr M; for Diabetes UK. Diabetic foot care in England, an economic case study. 2017. Available from: https://www.evidence.nhs.uk/document?id=1915227&returnUrl=Search%3Fq%3DAmputation%2Bcost&q=Amputation+cost.
13. Al-Thani H, Sathian B, El-Menyar A. Assessment of healthcare costs of amputation and prosthesis for upper and lower extremities in a Qatari healthcare institution: a retrospective cohort study. BMJ Open. 2019;9:e024963. doi:10.1136/bmjopen-2018-024963
14. Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–429. doi:10.1016/j.apmr.2007.11.005
15. Moxey P, Gogalniceanu P, Hinchliffe R, et al. Lower extremity amputations—a review of global variability in incidence. Diabet Med. 2011;28(10):1144–1153. doi:10.1111/dme.2011.28.issue-10
16. Tan JH, Hong CC, Shen L, Tay EY, Lee JK, Nather A. Costs of patients admitted for diabetic foot problems. Ann Acad Med Singapore. 2015;44(12):567–570.
17. Davis W, Norman P, Bruce D, Davis T. Predictors, consequences and costs of diabetes-related lower extremity amputation complicating type 2 diabetes: the Fremantle diabetes study. Diabetologia. 2006;49(11):2634–2641. doi:10.1007/s00125-006-0431-0
18. Yin H, Radican L, Kong SX. A study of regional variation in the inpatient cost of lower extremity amputation among patients with diabetes in the United States. J Med Econ. 2013;16(6):820–827.
19. Yost ML. Cost-benefit analysis of critical limb ischemia in the era of the affordable care act. Endovascular Today. 2014;5:29–36.
20. World Health Organization. 29th World health assembly. Ninth revision of the International Classification of Diseases; 1976. Available from: https://apps.who.int/iris/handle/10665/93061.
21. Jeffcoate W, Macfarlane R, Fletcher E. The description and classification of diabetic foot lesions. Diabet Med. 1993;10(7):676–679. doi:10.1111/dme.1993.10.issue-7
22. Turner HC, Lauer JA, Tran BX, Teerawattananon Y, Jit M. Adjusting for inflation and currency changes within health economic studies. Value Health. 2019;22(9):1026–1032. doi:10.1016/j.jval.2019.03.021
23. Lombardo FL, Maggini M, De Bellis A, Seghieri G, Anichini R. Lower extremity amputations in persons with and without diabetes in Italy: 2001–2010. PLoS One. 2014;9(1):e86405. doi:10.1371/journal.pone.0086405
24. Jensen PS, Petersen J, Kirketerp-Møller K, Poulsen I, Andersen O. Progression of disease preceding lower extremity amputation in Denmark: a longitudinal registry study of diagnoses, use of medication and healthcare services 14 years prior to amputation. BMJ Open. 2017;7(11):e016030. doi:10.1136/bmjopen-2017-016030
25. Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi:10.1016/S0140-6736(13)61249-0
26. Ajlouni K, Khader YS, Batieha A, Ajlouni H, El-Khateeb M. An increase in prevalence of diabetes mellitus in Jordan over 10 years. J Diabetes Complications. 2008;22(5):317–324. doi:10.1016/j.jdiacomp.2007.01.004
27. Cho N, Shaw J, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
28. Gerdtham U-G, Clarke P, Hayes A, Gudbjornsdottir S. Estimating the cost of diabetes mellitus-related events from inpatient admissions in Sweden using administrative hospitalization data. Pharmacoeconomics. 2009;27(1):81–90. doi:10.2165/00019053-200927010-00008
29. Kurichi JE, Vogel WB, Kwong PL, Xie D, Bates BE, Stineman MG. Factors associated with total inpatient costs and length of stay during surgical hospitalization among veterans who underwent lower extremity amputation. Am J Phys Med Rehabil. 2013;92(3):203–214. doi:10.1097/PHM.0b013e31827446eb
30. The Vascular Society of Great Britain and Ireland. A best practice clinical care pathway for major amputation surgery; April 2010. Available from: https://www.vascularsociety.org.uk/_userfiles/pages/files/Resources/Vasc_Soc_Amputation_Paper_V2.pdf.
31. The National Diabetes Foot Care Audit, 2014–2018. The fourth annual report; May, 2019. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-footcare-audit/2014-2018.
32. NICE guidelines: diabetic foot problems: prevention and management. NICE Guidelines; January 2016. Available from: https://www.nice.org.uk/guidance/ng19/resources/diabetic-foot-problems-prevention-and-management-pdf-1837279828933.
33. National Confidential Enquiry into Patient Outcome and Death. Lower limb amputation: working together; 2014. Available from: https://www.ncepod.org.uk/2014report2/downloads/WorkingTogetherFullReport.pdf.
34. Singh S, Evans L, Datta D, Gaines P, Beard J. The costs of managing lower limb-threatening ischaemia. Eur J Vasc Endovasc Surg. 1996;12(3):359–362. doi:10.1016/S1078-5884(96)80257-7
35. Johnson B, Evans L, Drury R, Datta D, Morris-Jones W, Beard J. Surgery for limb threatening ischaemia: a reappraisal of the costs and benefits. Eur J Vasc Endovasc Surg. 1995;9(2):181–188. doi:10.1016/S1078-5884(05)80088-7
36. Chery J, Semaan E, Darji S, Briggs WT, Yarmush J, D’Ayala M. Impact of regional versus general anesthesia on the clinical outcomes of patients undergoing major lower extremity amputation. Ann Vasc Surg. 2014;28(5):1149–1156. doi:10.1016/j.avsg.2013.07.033
37. Moreira CC, Farber A, Kalish JA, et al. The effect of anesthesia type on major lower extremity amputation in functionally impaired elderly patients. J Vasc Surg. 2016;63(3):696–701. doi:10.1016/j.jvs.2015.09.050
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
