Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Analysis of the Outcomes of 73 Patients with Exogenous Positive Insulin Anti-Body
Authors Yao D, Chu JP , Chen ZY
Received 16 August 2022
Accepted for publication 26 October 2022
Published 15 November 2022 Volume 2022:15 Pages 3543—3553
DOI https://doi.org/10.2147/DMSO.S386436
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Dan Yao,1 Jian-Ping Chu,2 Zhi-Yan Chen1
1Department of Endocrinology, Xiangshan Hospital of TCM Medical and Health Group, Ningbo, 315700, People’s Republic of China; 2Department of Endocrinology, Ningbo City First Hospital, Ningbo, 315000, People’s Republic of China
Correspondence: Jian-Ping Chu, Department of Endocrinology, Ningbo first hospital, No.59 Liuting Street, Ningbo, Zhejiang Province, 315000, People’s Republic of China, Tel/Fax +86-574-87089141, Email [email protected]
Objective: This study aimed to discuss adjusting the treatment plan for patients with type 2 diabetes mellitus (T2DM) who are positive for exogenous insulin antibody (IA). The outcome of patients who are IA-positive with an adjusted treatment plan was considered.
Methods: The treatment plan for patients with IA-positive T2DM was adjusted to oral medication or long-acting insulin + oral medication. Insulin antibody, C-peptide, and insulin were re-examined before treatment and at 1, 3, 6, 12, 18, and 24 months after treatment. The time of IA-negative seroconversion and its indexes, including blood glucose, C-peptide, and insulin, were recorded and analyzed.
Results: After adjusting the treatment plan for 2 years, in 73 patients, 57 had IA-negative seroconversion, and 16 had positive IA. The blood glucose, C-peptide, insulin, glycosylated hemoglobin (HbA1c), and the daily dose of insulin in the seroconversion group and the non-seroconversion group decreased compared with before the adjustment of the treatment plan (P < 0.05). The negative seroconversion rate within 2 years was related to the insulin concentration before treatment.
Conclusion: Patients with IA-positive T2DM need to adjust their treatment plans in time. Even if IA does not turn negative within 2 years after adjusting the treatment plan, the levels of blood glucose, C-peptide, insulin, and HbA1c along with the insulin dosage would be significantly improved, which can benefit patients. The higher the fasting insulin and 2-hour insulin values before adjusting the treatment plan, the longer the time required for IA to turn negative.
Keywords: insulin antibody, diabetes, type 2, negative seroconversion rate
Introduction
Positive insulin antibody (IA), which is mainly observed at the early phase of type 1 diabetes mellitus, can also be found in people using mercaptans or in patients with type 2 diabetes mellitus (T2DM) and treated with exogenous insulin. Positive IA associated with sulfhydryl-containing drugs is called insulin autoimmune syndrome (IAS), manifested by spontaneous hypoglycemia and endogenous hyperinsulinemia. Positive IA induced by exogenous insulin in patients with T2DM is termed exogenous insulin antibody syndrome (EIAS). The two major differences between IAS EIAS are: (1) EIAS is induced by exogenous insulin; (2) Patients with IAS have a high incidence of hypoglycemia and more severe symptoms. EIAS may induce immune reactions in some people. There are various immune responses induced by exogenous insulin, which may include insulin allergy, lipodystrophy, insulin resistance, insulin kinetic changes, hypoglycemia, and microvascular disease.1–3 Hypoglycemia is induced by irregular release of insulin after insulin autoantibody binding to insulin. Various adverse reactions can be attributed to insulin antibody (IA).4 Cheng reported that in patients with diabetes with a total treatment course of insulin of fewer than 2 weeks, the positive rate of IA was significantly increased over the course of more than 1 year,5 and the positive rate showed an increasing trend with the increase in use time.5–7 Therefore, the incidence of positive IA is both high and harmful. Our previous study8 showed that the IA-positive rate in T2DM patients treated with insulin was as high as 40.2% in Xiangshan, Ningbo, Zhejiang, China. Accordingly, we need to adjust the treatment plans in time to improve the prognosis of patients, including controlled blood glucose, alleviated hyperinsulinemia, and delayed development of diabetes-related complications.
To this end, we enrolled 73 patients who were IA positive. We adjusted the treatment plan and observed seroconversion-related factors and the changes in blood glucose, C-peptide, insulin, and glycosylated hemoglobin (HbA1c) before and after the negative seroconversion to provide a reference for the adjustment of the treatment plan of such patients in the future.
Methods
Subjects
Patients who were diagnosed with T2DM, treated with insulin therapy in our hospital between May 1st 2018 and April 31st 2019, and tested as IA-positive in anti-IA qualitative test were screened. The inclusion criteria were as follows: (1) The diagnosis of T2DM was in accordance with the WHO Diagnostic Criteria of T2DM in 1999:9 diabetic symptoms (eg, polyuria, irritable thirst, unexplained weight loss); or fasting glucose (no caloric intake for at least 8 hours) ≥ 7.0 mmol/L (≥ 126 mg/dl); or 2-hour glucose ≥ 11.1 mmol/L (≥ 200 mg/dl) in oral glucose tolerance test; (2) Patients with continuous use of insulin for ≥ 2 weeks.10,11 Patients who met the following exclusion criteria were excluded: (1) History of sulfhydryl-containing drug therapy in the past six months; (2) Change of insulin type in the past two years; (3) Moderate to severe abnormalities in liver function and kidney function; (4) Myocardial infarction, cerebral infarction, trauma, surgery and stressful conditions in the past six months; (5) Presence of acute complications of diabetes (6) Body mass index ≥ 28 kg/m2 (due to small sample population). This study was approved by the local ethics committee and all patients provided written informed consent.
Treatments and Tests
All patients with positive IA had their treatment changed to oral antidiabetic drugs (OAD). After 2 weeks of treatment, the patients were divided into groups. Those whose blood glucose reached the standard (fasting blood glucose 4.4–7.0 mmol/L and 2-h postprandial blood glucose ≤ 10.0mmol/L) continued to take OAD. Recombinant insulin glargine was prescribed to those whose blood glucose did not meet the standard.
Fifty patients were treated twice daily with subcutaneous injections of premixed human insulin (including arginine biosynthetic human insulin injection (premixed 30R), arginine biosynthetic human insulin injection (premixed 50R), arginine zinc recombinant human insulin mix injection 70/30, 50/50 mix recombinant human insulin injection).Twenty-two patients were treated twice daily with subcutaneous injections of premixed insulin analogs (including arginine zinc recombinant lysergic insulin mix 25R, arginine zinc recombinant lysergic insulin mix 50R, menadione insulin 30 injection, menadione insulin 50 injection). One patient was treated once daily with subcutaneous injection of a long-acting insulin analogue combined with three pre-meal ultra-short-acting insulin analogues - dettaglin + menadione insulin.
The patients in the two groups were re-examined before treatment and at 1, 3, 6, 12, 18, and 24 months after treatment. After getting up in the morning (after fasting for 10–12 hours), a steamed bread meal test (100 g) was conducted, during which blood samples were taken to measure blood glucose, insulin, C-peptide (fasting and 2 hours after a steamed bread meal), IA, and HbA1c. The time of IA-negative seroconversion and indexes including blood glucose, C-peptide, and insulin were recorded and analyzed.
Test methods, reagents, and instruments: C-peptide and insulin were detected by the immune electrochemiluminescence method using a C-peptide/insulin detection reagent and a Cobas e601 analyzer (F. Hoffmann–La Roche [Roche]). Blood glucose was detected by the hexokinase method using a glucose detection reagent and a Beckmann AU5800 analyzer (MedicalSystem Biotechnology Co. Ltd.). HbA1c was detected by high-performance liquid chromatography using an HbA1c detection reagent and an MQ-2000PTHbA1c analyzer (Shanghai Medconn Biotechnology Co. Ltd.). Finally, IA was detected by radioimmunoassay using 125I insulin antibody analytical reagent and an sn-6105 gamma counter (Beijing North Institute of Biotechnology Co. Ltd.).
Statistical Analysis
The data were analyzed using SPSS 22.0 (IBM) statistical software. Count data were compared using a Chi-squared test, and measurement data were expressed as mean ± standard deviation or mean (25th percentile, 75th percentile). When the measurement data were normally distributed, they were compared between two groups using an independent-sample t-test and among multiple groups using a univariate analysis of variance. If the measurement data were not normally distributed, they were compared using nonparametric tests. Data were compared between two groups using two independent-sample t-tests, and data before and after treatment were compared within one group using a paired-sample t-test. A value of P < 0.05 was considered statistically significant. The charts were tabulated using Graph Pad 8.0 (Dotmatics) and Sigma Plot 14.0 (Systat), and post-editing was conducted using Adobe Illustrator CC 2017 (Adobe) and Photoshop CS6 (Adobe).
Results
From May 1, 2018, to April 30, 2019, there were 90 patients with IA-positive T2DM in the Endocrinology Department of our hospital. Of these, 83 met the enrollment conditions. Ten patients refused to change their treatment plans, and 73 patients met the enrollment conditions (all agreed to participate in this study). Therefore, 81.1% of the total patients enrolled in this study. Of the patients participating in the study, 38 were male, and 35 were female. The ratio of males to females was 1.09:1. The patients’ ages ranged from 38 to 76 years, with an average age of 59.8 ± 9.6 years.
Negative Seroconversion of Insulin Antibody
All 73 patients with an adjusted treatment plan were followed up for 2 years. A total of 57 patients had IA-negative seroconversion. Among these, 44 patients had IA-negative seroconversion within 1 year, and 13 patients had it within 2 years. Two patients had IA-negative seroconversion within 1 month. Fasting insulin as well as 2-h insulin concentrations were higher in the IA non-negative seroconversion than in the IA negative seroconversion group (both within 1 year and 2 years) (Figure 1). There was no significant difference in fasting insulin and 2-h insulin between the IA negative seroconversion within 1 year and IA negative seroconversion within 2 years groups. There was no difference in pre-treatment BMI between patients with or without IA negative seroconversion (P=0.848 > 0.05).
Comparison Before and After Negative Seroconversion
The fasting blood glucose (FBG), 2-hour blood glucose, HbA1c, fasting C-peptide, 2-hour C-peptide, fasting insulin, 2-hour insulin, and the daily doses of insulin in the IA-negative seroconversion group and the IA non-negative seroconversion group after 2 years of treatment were lower than before treatment (P < 0.05, Table 1).
 |
Table 1 Comparison of Conditions Before and After Treatment in Negative Converted Group and Non-Negative Converted Group |
After treatment, there were no differences between the two groups in gender, age, BMI, course of the disease, insulin use time, FBG before treatment, 2-hour blood glucose, HbA1c, fasting C-peptide, and the 2-h insulin (P > 0.05). The 2-h C-peptide, fasting insulin, and 2-h insulin were greater in the non-negative seroconversion group than in the negative seroconversion group. The difference in fasting insulin was statistically significant (P < 0.05), while that in 2-h insulin was not statistically significant (P = 0.055 > 0.05) (Table 2).
 |
Table 2 Comparison Between Negative Converted Group and Non-Negative Converted Group After Treatment |
After changing the treatment plan, the daily dose of insulin, insulin level, and 2-hour insulin level decreased in those patients who failed in oral medication and turned to insulin treatment regardless of whether IA changed to negative (P < 0.05, Figure 2).
After treatment, fasting C-peptide, 2-hour C-peptide, insulin, and 2-hour insulin concentration in the negative seroconversion group at both 0 (Figure 3A) and 120 minutes (Figure 3B) decreased fastest in the first 3 months. The decrease slowed down after 3 months.
Comparison Between the Oral Drug Group and Insulin Group
Of the 73 patients, 29 turned to oral medication (ie, pro-secretory agents, biguanides, alpha glucosidase inhibitors, and/or SGLT-2 inhibitors), and their blood glucose reached the standard. Of these, 26 had IA-negative seroconversion, and the negative seroconversion rate was 89.66%; 44 patients turned to long-acting insulin + oral medication (ie, biguanides and/or alpha glucosidase inhibitors) due to a failure in oral medication, and 31 had IA-negative seroconversion. The negative seroconversion rate was 70.46%.
There was no difference in the time of IA seroconversion between patients using insulin and those not using insulin (P = 0.195). Twenty-nine patients who changed to oral medication were not found to have hypoglycemia during the observation period. Only one of the 44 patients who adjusted to insulin treatment had fasting hypoglycemia one time, and no further hypoglycemia occurred after adjusting insulin dose and diet preaching.
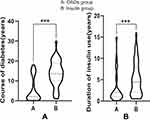
The fasting C-peptide and 2-hour C-peptide results in the oral drug group were higher than those in the insulin group (Figure 4). The duration of T2DM and insulin use time in the oral drug group were significantly lower than in the insulin group (Figure 5). In addition, HbA1c in the oral drug group was higher than in the insulin group before and after treatment. The duration of diabetes in the oral drug group was shorter than that in the insulin treatment group, and the fasting C-peptide and 2h C-peptide before and after treatment were higher than those in the insulin treatment group (P < 0.05, Table 3).
 |
Table 3 Comparison Between OADs Group and Insulin Group Before and After Treatment |
Discussion
With the widespread use of purified human insulin preparations, the incidence of IA positivity has gradually decreased. However, in patients with diabetes, especially in Asian populations, EIAS continues, which caused by exogenous insulin.12 This may be related to the genes of Asian people.9,10,13 In view of the many hazards caused by IA, we still need to pay attention to the adjustment of treatment plans for such groups. There have been reports on IA-negative seroconversion, but the relevant descriptions are vague. Therefore, this study was performed.
In formulating a treatment plan, we tried to avoid the use of exogenous insulin. For patients with poor islet function who must be treated with insulin, we used the lowest possible dose of insulin or insulin with relatively low immunogenicity. A previous report revealed that the positive rate of IA was related to the dosage of insulin.5 Insulin analog replacement is an effective method for the treatment of EIAS.10,14 Insulin glargine has achieved good efficacy in treating patients with positive IA or alleviating local and systemic insulin allergy in patients without anti-allergic drugs.15–17 Therefore, in patients who were IA positive, if oral drug treatment failed, we shifted to insulin glargine + oral drug for hypoglycemic treatment to reduce the possibility of IA positivity.
Of the 73 patients in this study, 2.73% had negative seroconversion within 1 month, 59.46% of the patients had IA-negative seroconversion within 1 year, and 78.08% of the patients had IA-negative seroconversion within 2 years. A previous study conducted by Ionescu-Tîrgovişte et al18 revealed that among 42 patients who were IA positive, the antibody disappeared in 24% of patients within 100 days after adjusting the treatment plan. The positivity disappeared when the initial level of IA was low. Sometimes, it lasted more than 1 year18–20 and even more than 2 years.18 These findings were consistent with the present study’s conclusion. Because the IA test was a qualitative detection in the present study, it is impossible to predict the correlation between the negative seroconversion rate and the IA titer. However, the present study revealed that the negative seroconversion rate was negatively correlated with the insulin concentration before treatment. There was no significant correlation between insulin concentrations in patients with IA 1-year turnaround and 2-year turnaround, which may be due to small sample size. Therefore, these results may not be generalized to the correlation between time to turnaround and insulin concentrations before adjustment of treatment regimen.
After adjusting the treatment plan, the blood glucose, HbA1c, C-peptide, insulin, and the daily doses of insulin in the negative seroconversion group and the non-negative seroconversion group were lower than the values before treatment. The fasting insulin, 2-hour insulin, and insulin doses in the negative seroconversion group were lower than in the non-negative seroconversion group after 2 years. However, the differences between the two groups were not statistically significant. Therefore, whether patients had IA-negative seroconversion or not within 2 years, it was necessary to adjust the treatment plan, and patients benefitted from it.
There is a case report2 stating that the insulin dose decreased from 300 units/day to 58 units/day after the replacement of human insulin therapy with insulin lispro. In the present study, in the insulin treatment group, the insulin dose decreased from 42.57 ± 16.25 (units/day) before treatment to 21.16 ± 6.97 (units/day) after treatment. Therefore, patients’ insulin doses can be significantly reduced after switching to insulin that does not cause an immune response in the treated individual. This reduces hyperinsulinemia in patients, allows the blood glucose to reach a desirable standard, and controls it better. On the other hand, the long-term benefit for patients may be reflected in the control of diabetes complications, the improvement of life therapy, and survival rates.
After treatment, the fasting C-peptide, 2-hour C-peptide, insulin, and 2-hour insulin concentration values in the negative seroconversion group decreased fastest in the first 3 months, and the decrease slowed down after 3 months. The rapid decrease in insulin concentration may be related to the half-life of IA. After IA metabolism, the insulin binding to IA decreases, reducing insulin concentration in the body. Therefore, the hyperinsulinemia of patients can be relieved rapidly after switching the treatment plan. The decrease in C-peptide levels may be related to the improvement of hyperglycemia and chronic hyperinsulinemia in vivo, as follows: a. Blood glucose level is the most important factor in stimulating insulin secretion; with a decrease in blood glucose, insulin secretion can be reduced. b. Chronic hyperinsulinemia may lead to decreased insulin sensitivity in vivo,21 causing insulin resistance. Therefore, after hyperinsulinemia is relieved, insulin sensitivity will improve, and insulin secretion in the body will decrease. This manifests as decreased C-peptide and improved islet β-cell hypersecretion.
In the oral drug group, 89.66% of patients had IA-negative seroconversion within 2 years. In the insulin treatment group, 70.46% of patients had IA-negative seroconversion within 2 years. The negative seroconversion rate in the oral drug group was higher than that in the insulin treatment group, and the difference between the two groups was not statistically significant (P = 0.052 > 0.05). One possible reason the negative seroconversion rate of patients with oral drugs was higher than that of the insulin treatment group is that some patients in the insulin treatment group may produce IA again during the treatment with long-acting insulin analogs.
With the prolongation of the course of T2DM, the islet function of patients shows a downward trend. Therefore, in patients with a long course of the disease, the probability of failure in hypoglycemic treatment with oral drugs, such as secretagogues, increases. In this study, insulin glargine was added for treatment after oral drug failure. This grouping meant significant differences in the C-peptide and course of the disease between the two groups, as follows: a. The fasting C-peptide and 2-hour C-peptide values in the oral drug group were higher than in the insulin treatment group. b. The course of diabetes in the oral drug group was significantly lower than in the insulin treatment group. The possible reasons for the higher C-peptide in the oral drug group than in the insulin treatment group are: (1) the use of prohormone therapy may affect C-peptide; (2) better in the oral drug group had better islet function than the insulin treatment group.
When selecting treatment plans for patients with IA-positive T2DM, this trial mainly focused on oral drugs or oral drugs + insulin glargine. However, in clinical practice, in addition to the above drugs, we can more flexibly select individualized treatment plans suitable for patients according to their conditions. Our previous study showed that the IA-positive rate after long-acting insulin analogs was lower than after premixed human insulin or premixed insulin analogs.8 Therefore, the patients with IA positivity in whom oral treatment was unsuccessful were additionally treated with long-acting insulin analogs.
However, in clinical practice, there are case reports that the daily dose of insulin gradually decreased after exogenous IA-positive patients replaced human insulin with insulin lispro and finally stopped using insulin after 2 months.22 Although it is a case report, this situation is relatively rare, but it also gave us an idea about when to change the treatment plan. This may be due to the difference in molecular structure between exogenous insulin and endogenous insulin or the difference in purity, excipients, storage methods, or administration methods.23–27 Therefore, different insulin preparations have different immunogenic effects on different patients. A patient may use one insulin preparation to induce IA positivity, but it may not have the same immune response when using other insulin preparations. This is why some patients who are IA-positive had IA-negative seroconversion after switching to non-similar insulin preparations.
For patients who are overweight or obese with acceptable islet functions, GLP-1 analog, metformin, SGLT-2I, and other drugs can be considered for replacement. One case report stated that the anti-insulin antibody disappeared completely after treatment with liraglutide for 1 year.28 Treatment with GLP-1 analogs was not included in the present study, and it needs to be further researched.
This trial has the following limitations: a. The IA test was a qualitative detection; therefore, it is impossible to monitor the change in IA titer after adjusting the treatment plan. b. The research population was patients in primary hospitals in China, and the sample size was small. Therefore, it does not represent the overall population or reflect inter-ethnic situations. c. The follow-up time of patients who were IA-positive after the adjustment of the treatment plan was 2 years, and the clinical observation time was short. It is expected that such patients can be followed up in the future to further understand IA-negative seroconversion, the occurrence and development of diabetes complications, survival rates, and other related situations.
Conclusion
Therefore, for patients with exogenous IA positivity, the treatment plan should be adjusted according to each patient’s conditions. Most patients can have IA-negative seroconversion within 2 years after replacing the treatment plan. If the patient’s islet function is acceptable, and the course of the disease is short, insulin can be stopped and oral hypoglycemic drugs used. In particular, metformin and α glycosidase inhibitors can significantly reduce plasma IA concentrations.12,29 Patients with IA positivity and obesity can be treated with GLP-1 analog and SGLT-2I. For patients with a long course of the disease and poor islet function for whom oral medication is unsuccessful, appropriate insulin preparations can be used instead, and the minimum effective dose can be applied.24 After adjusting the treatment regimen, patients can benefit from a decrease in insulin dose and insulin and 2-h insulin, regardless of whether the IA is converted to negative.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Medical Health Group of Xiangshan County Traditional Chinese Medicine Hospital (No.P2021-K04). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
This work was supported by the Xiangshan County Science and Technology Bureau (No.2021C6015).
Disclosure
The authors declare that they have no competing interests.
References
1. Home P, Derwahl KM, Ziemen M, et al. Anti-insulin antibodies and adverse events with biosimilar insulin lispro compared with humalog insulin lispro in people with diabetes. Diabetes Technol Ther. 2018;20:160–170. doi:10.1089/dia.2017.0373
2. Lahtela JT, Knip M, Paul R, Antonen J, Salmi J. Severe antibody-mediated human insulin resistance successful treatment with the insulin analog lispro. Diabetes Care. 1997;20:71–73. doi:10.2337/diacare.20.1.71
3. Timon W, Van Haeften M. Clinical significance of insulin antibodies in insulin-treated diabetic patients. Diabetes Care. 1989;12:641–648. doi:10.2337/diacare.12.9.641
4. Brange J, Ribel U, Hansen JF, et al. Monomeric insulins obtained by protein engineering and their medical implications. Nature. 1988;333:679–682. doi:10.1038/333679a0
5. Cheng M, Yang X, Zhu X, Yao L. Preliminary study on determination of serum insulin antibody in diabetic patients. Chin J Endocrinol Metabol. 1994;10:47–48.
6. Yao D, Chu J, Cheng S. Clinical analysis 64 cases of T2DM with positive insulin autoantibody induced by exogenous insulin in primary hospital. Mod Pract Med. 2019;31:360–362.
7. Li F, Wu Z, Chi Q, Qiao X, Wang S. Immunogenicity of recombinant human insulin in treatment of type 2 diabetes mellitus. Chin J Clin. 2013;7:9081–9084.
8. Yao D, Zhu ZQ, Chen ZY, et al. Insulin antibody as a biomarker to monitor the development of type 2 diabetes in county hospitals in China. Int J Diabetes Dev Ctries. 2022. doi:10.1007/s13410-022-01110-2
9. Matsuyoshi A, Shimoda S, Tsuruzoe K, et al. A case of slowly progressive type 1 diabetes with unstable glycemic control caused by unusual insulin antibody and successfully treated with steroid therapy. Diabetes Res Clin Pract. 2006;72(3):238–243. doi:10.1016/j.diabres.2005.10.018
10. Uchigata Y, Kuwata S, Tokunaga K, et al. Strong association of insulin autoimmune syndrome with HLA-DR4. Lancet. 1992;339:393–394. doi:10.1016/0140-6736(92)90080-M
11. Xia LM, Zhang AP, Zheng Q, et al. Quercetin-3-O-β-D-glucuronide inhibits mitochondria pathway-mediated platelet apoptosis via the phosphatidylinositol-3-kinase/ AKT pathway in immunological bone marrow failure. World J Tradit Chin Med. 2022;8:115–122. doi:10.4103/wjtcm.wjtcm_44_21
12. Hu X, Chen F. Exogenous insulin antibody syndrome (EIAS): a clinical syndrome associated with insulin antibodies induced by exogenous insulin in diabetic patients. Endocr Connect. 2018;7:R47–R55. doi:10.1530/EC-17-0309
13. Tamura Y, Kimbara Y, Funatsuki S, et al. A case of insulin antibody-induced glucose instability in an elderly woman with type 2 diabetes on hemodialysis, successfully ameliorated with liraglutide. Diabetol Int. 2013;4(1):71–75. doi:10.1007/s13340-012-0100-0
14. Airaghi L, Lorini M, Tedeschi A. The insulin analog aspart a safe alternative in insulin allergy. Diabetes Care. 2001;24:2000. doi:10.2337/diacare.24.11.2000
15. Yasuda H, Nagata M, Moriyama H, et al. Human insulin analog insulin aspart does not cause insulin allergy. Diabetes Care. 2001;24:2009. doi:10.2337/diacare.24.11.2008
16. Moriyama H, Nagata M, Fujihira K, et al. Treatment with human analog insulin glargine resolves a generalized allergy to human insulin in type 1 diabetes. Diabetes Care. 2001;24:411–412. doi:10.2337/diacare.24.2.411
17. Yoshida M, Asai M, Miyata M, Ogawa K, Maeda H, Oiso Y. Combination therapy with liraglutide and sulfonylurea for a type 2 diabetic patient with high titer of anti-insulin antibodies produced by insulin therapy. Diabetes Res Clin Pract. 2012;96:e55–56. doi:10.1016/j.diabres.2011.12.024
18. Ionescu-Tirgoviste C, Mincu I, Simionescu L, et al. Disappearance rate of insulin antibodies after discontinuing insulin treatment in 42 type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1984;27:592–595. doi:10.1007/BF00276974
19. Koyama R, Nakanishi K, Kato M, Yamashita S, Kuwahara H, Katori H. Hypoglycemia and hyperglycemia due to insulin antibodies against therapeutic human insulin. Am J Med Sci. 2005;329:259–264. doi:10.1097/00000441-200505000-00007
20. Haeften TWV, Krom BA, Gerich JE. Prolonged fasting hypoglycemia due to insulin antibodies in patient with non-insulin-dependent diabetes mellitus effect of insulin withdrawal on insulin-antibody-binding kinetics. Diabetes Care. 1987;10:160–163. doi:10.2337/diacare.10.2.160
21. Rajan S, Shankar K, Beg M, et al. Chronic hyperinsulinemia reduces insulin sensitivity and metabolic functions of brown adipocyte. J Endocrinol. 2016;230:275–290. doi:10.1530/JOE-16-0099
22. Asai M, Kodera T, Ishizeki K, et al. Insulin lispro reduces insulin antibodies in a patient with type 2 diabetes with immunological insulin resistance. Diabetes Res Clin Pract. 2003;61:89–92. doi:10.1016/S0168-8227(03)00105-0
23. Zhou F, Tian H. Clinical significance of exogenous insulin induced insulin antibody production in diabetic patients. J Pract Hosp Clin. 2014;11:18–21.
24. Zhu L, Zhu Y, Sun K, et al. Diagnosis and treatment of insulin autoimmune syndrome induced by exogenous insulin: a case report and literature review. Chin J Diabetes. 2017;25:743–747.
25. Sauerborn M, Brinks V, Jiskoot W, Schellekens H. Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci. 2010;31:53–59. doi:10.1016/j.tips.2009.11.001
26. Asai M, Yoshida M, Miura Y. Immunologic tolerance to intravenously injected insulin. N Engl J Med. 2006;354:307–309. doi:10.1056/NEJMc052463
27. Galloway JA, Root MA, Bergstrom R, et al. Clinical pharmacologic studies with human insulin (recombinant DNA). Diabetes Care. 1982;5:13–22. doi:10.2337/diacare.5.2.S13
28. Hirai H, Ogata E, Kikuchi N, et al. The effects of liraglutide on both hypereosinophilic insulin allergy and the characteristics of anti-insulin antibodies in type 2 diabetes mellitus: a case report. J Med Case Rep. 2016;10:202. doi:10.1186/s13256-016-0994-4
29. Ouyang X, Bian R, Gu L, et al. Analysis of insulin antibody production in patients with type 2 diabetes mellitus.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.