Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Analysis of the Financial Impact of Using Cangrelor on the Safety and Efficacy Outcomes in Patients Undergoing Percutaneous Coronary Intervention in Whom Oral Therapy with P2Y12 Inhibitors is Not Feasible or Desirable, in Spain
Authors Lizano-Díez I, Paz Ruiz S
Received 4 November 2020
Accepted for publication 1 January 2021
Published 27 January 2021 Volume 2021:13 Pages 77—87
DOI https://doi.org/10.2147/CEOR.S290377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Samer Hamidi
Irene Lizano-Díez,1 Silvia Paz Ruiz2
1Ferrer, Barcelona, Spain; 2SmartWorking4U SLU, Benicàssim, Spain
Correspondence: Irene Lizano-Díez
Ferrer, Av Diagonal 549, Barcelona 08029, Spain
Tel +34 93 600 37 00
Email [email protected]
Purpose: Cangrelor is an intravenous, direct-acting, reversible P2Y12 inhibitor indicated for the reduction of thrombotic cardiovascular events in patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) in whom oral P2Y12 inhibitors are not feasible or desirable. The objective was to assess the financial impact of introducing cangrelor into the hospital formulary in Spain.
Patients and Methods: A budget impact model was developed to calculate the cost difference between two scenarios (without and with cangrelor) to treat CAD patients undergoing PCI in whom oral P2Y12 inhibitors are not feasible or desirable, over 3 years. Intravenous P2Y12 inhibitor (cangrelor), oral P2Y12 inhibitors (clopidogrel, prasugrel, and ticagrelor), and glycoprotein IIb-IIIa inhibitors (GPIs) for bail-out use were considered. Epidemiological, efficacy (thrombotic events including cardiac death), safety (bleeding events), and costs (€, 2019) data were based on Spanish registries, clinical trials, and meta-analyses. One-way sensitivity analysis established the effect of uncertainty on results.
Results: For years 1, 2, and 3, the target population to receive cangrelor was 607, 1,822, and 3,340 patients, and cangrelor uptake was 23.70%, 58.30%, and 51.30%, respectively. The 3-year budget impact was 1,021,717€ varying from 50,245€ in year 1 to 599,272€ in year 3. The results were sensitive to the number of patients treated with GPIs in Spanish hospitals.
Conclusion: Based on our results, the financial effort needed to introduce the use of cangrelor in patients undergoing PCI in whom antiplatelet therapy with oral P2Y12 inhibitors is not feasible or desirable barely exceeds one million € over three years, in Spain.
Keywords: P2Y12 inhibitors, cangrelor, percutaneous coronary intervention, budget impact
Introduction
In Spain, acute coronary syndromes (ACS), including ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI), and unstable angina are the leading cause of morbidity and mortality, and of elevated healthcare costs.1 Percutaneous coronary intervention (PCI) is the recommended reperfusion strategy for both STEMI (primary PCI) and high-risk NSTEMI patients.2 In 2018, 72,520 PCIs were performed in 109 hospitals in Spain; 21,261 were done in acute myocardial infarction (91% primary PCI), exceeding the estimates of 400 primary PCIs per million population.3
Platelet inhibition is a key component of the periprocedural adjunctive therapy.4,5 Oral P2Y12 inhibitors (clopidogrel, prasugrel, or ticagrelor) with concomitant acetylsalicylic acid (ASA) are the standard of care.6 However, and despite the significant advances made in the use of adjunctive antiplatelet treatment and in the PCI procedure, 12% of STEMI and 4.3% of NSTEMI patients remain at risk for periprocedural thrombotic complications (including myocardial infarction and stent thrombosis), as well as at risk for major bleedings.7–10 In 2018, the budget impact of treating patients with ACS undergoing PCI with oral prasugrel, ticagrelor, or clopidogrel was calculated to reach 76 million € in Spain.11 Pharmacy, myocardial infarction, urgent revascularization, minor and major bleeding, and stroke were the major cost components.11 In patients with ACS presenting with cardiogenic shock (5–10% of STEMI and 2–3% of NSTEMI12,13), or with active vomiting (30% of STEMI patients14) in whom treatment with oral P2Y12 inhibitors may not be feasible, glycoprotein IIb-IIIa inhibitors (GPIs) are parenteral options.10,15 However, GPIs are recommended for bail-out use only, provided their narrow therapeutic window.4,5
Cangrelor is an intravenous (iv) P2Y12 receptor inhibitor that prevents adenosine diphosphate (ADP) signaling and platelet activation in a direct and reversible way within two minutes of administering a bolus followed by continuous infusion. The antiplatelet effect is consistently maintained along the duration of the infusion, and platelet function returns to normal within one hour following the cessation of it.16–18 Co-administered with ASA, cangrelor is indicated for the reduction of thrombotic cardiovascular events in adults with coronary disease undergoing PCI who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable.18 The pooled analysis of 3 pivotal trials (CHAMPION program)7,19,20 evaluating the efficacy and safety of cangrelor vs clopidogrel reported that cangrelor significantly reduced the odds of the primary efficacy composite of all-cause death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis at 48 h by 19% (p = 0.0007 vs clopidogrel), and stent thrombosis (key secondary endpoint) by 41% (p = 0.0008).18 As it might be expected for a potent and immediate antiplatelet strategy, cangrelor increased periprocedural GUSTO mild bleeding events (16.8% vs 13.0%, cangrelor vs clopidogrel; p<0.0001).18 Based on the evidence on the efficacy and safety of cangrelor provided by the CHAMPION program,7,18–20 the ESC guidelines recommend that cangrelor may be considered as an iv option to achieve an immediate inhibition of the ADP-induced platelet aggregation after iv bolus plus perfusion, and to allow the restoration of the normal platelet function within 1 h after the cessation of the perfusion.4,5
This study was undertaken to assess the financial impact of introducing cangrelor into the drug formulary of hospital pharmacies in Spain as adjunctive treatment to reduce the risk of periprocedural thrombotic events in candidate patients for PCI, in whom antiplatelet therapy with oral P2Y12 inhibitors is not feasible or desirable.
Patients and Methods
Budget Impact Model
A budget impact model was built in Excel for Microsoft® to calculate the difference in costs of two hypothetical scenarios from the perspective of the National Health System, over a three-year time horizon (2019–2021), in Spain:
1. Current scenario, without cangrelor assuming patients receive antiplatelet treatment with oral P2Y12 inhibitors (pre-treatment) or GPIs (bail-out).
2. Alternative scenario, with cangrelor assuming patients receive oral P2Y12 inhibitors (pre-treatment) or cangrelor or GPIs (bail-out).
Inputs and Data Sources
First, a conceptual budget impact model was defined to set the appropriate PCI scenarios to be compared. Second, the medical literature and diverse sources of pharmacoeconomic information were reviewed to identify the most relevant and consistent data which may reflect as best as possible usual clinical practice in Spain. The pivotal clinical trial of cangrelor was used to estimate the efficacy and safety of cangrelor compared with clopidogrel;18 in the absence of indirect comparisons, a meta-analysis was utilized to calculate the efficacy and safety of cangrelor compared with prasugrel and ticagrelor;21 Spanish interventional cardiology registries were searched to identify population data and typical use of GPIs;22–24 market research information was gathered to infer market share over three years.
Third, assumptions were made to solve data inconsistencies, and the opinion of clinical experts was sought for validation. Three interventional cardiologists working for more than ten years in reference PCI Cath-Labs in Spain gave their expert opinion and validated sources, inputs, assumptions, and calculations. For validation, a semi-structured questionnaire was distributed by email. Experts were asked to choose the most appropriate group of data from the entire set extracted from the literature. In case of discrepancies, an external expert was required for the final decision.
The model was finally nourished with a set of endorsed epidemiological, clinical, and direct healthcare costs data, as follows (Tables 1–3):
 |
Table 1 Size and Distribution of the PCI Population in Spain Over Three Years |
 |
Table 2 Distribution (in Percentage) of the Use of GPIs and Oral P2Y12 Inhibitors without and with Cangrelor in the Hospital Formulary in the PCI Population in Spain |
 |
Table 3 Efficacy and Safety Outcome Rates of P2Y12 Inhibitors in the PCI Population in Spain |
1. Epidemiological data: total PCI population3,22,23 primary PCI population23,25 and population of PCI patients in whom the use of oral P2Y12 inhibitors is not feasible or desirable.13,26,27
2. Clinical data: diagnosis at entry (STEMI, NSTEMI, stable CAD and other, that included any cardiovascular event that according to the criteria of the responsible physician deserved a PCI);11,23 pharmacological treatment (before and during PCI);11,18,22,28,29 risk of ischemic and bleeding events with each antiplatelet strategy.18,21
3. Direct healthcare costs data: ischemic and bleeding events unitary costs;30 drugs unitary costs.31
The size, growth, and percentage distribution (primary PCI/total PCI) of the PCI population were estimated from the Spanish registry of interventional cardiology for the 2016 to 2018 period.3,22,23,25 A 2% steady growth of the total PCI population over the three years of analysis was assumed.
In a face-to-face interaction, the clinical experts validated the final version of the model and the budget impact results.
Target Population
The target population included STEMI and NSTEMI patients candidate for PCI, in whom antiplatelet therapy with oral P2Y12 inhibitors was not feasible or desirable.12–14 Its size was derived from the total PCI population in Spain (74,217 individuals in year 1, 75,978 in year 2, and 77,768 in year 3) (Table 1).3,22,23
The percentage of patients in whom antiplatelet therapy with oral P2Y12 inhibitors was not feasible or desirable was based on data from the national registry of interventional cardiology on the 2016 to 2018 period and PCI population growth, on the estimated incidence of cardiogenic shock in PCI candidates, and on the opinion of clinical experts on drugs usage. It was calculated in 1.02% of the total PCI population in year 1, and to grow to 5.37% in year 313,26,27 (Table 1). It reached a total of 7,220 patients over three years (Figure 1). It was forecasted that adjuvant antiplatelet treatment with oral P2Y12 inhibitors would be needed in 607, 1,822, and 3,340 of these patients, respectively. They totalized 5,769 patients over three years (Table 1).
 |
Figure 1 Three years distribution of the total, primary PCI and PCI population in whom antiplatelet therapy with oral P2Y12 inhibitors is not feasible or desirable, in Spain. |
Use of Antiplatelet Therapy in the PCI Population
In the scenario without cangrelor in the hospital formulary, it was considered that 80% of the PCI patients received oral P2Y12 inhibitors (pre-treatment) and 6.50% of the patients received GPIs (bail-out), based on data taken from national registries,24 the literature,11 and on the opinion of clinical experts (Table 2).
In the scenario with cangrelor in the hospital formulary, the population receiving P2Y12 inhibitors was distributed amongst those receiving oral P2Y12 inhibitors and those receiving cangrelor. The proportion of patients in whom the use of oral P2Y12 inhibitors would not be feasible or desirable and could therefore be treated with cangrelor was 23.70% in year 1, 58.30% in year 2, and 51.30% in year 3 of the total PCI population (Table 2). The proportion of patients treated with GPIs (bail-out) slightly decreased with cangrelor in the hospital formulary, based on the opinion of clinical experts.
Clinical Outcomes
Periprocedural clinical outcomes (up to 48h of PCI) were ischemic events (stent thrombosis, myocardial infarction, ischemia-driven revascularization, cardiac death) (efficacy),18 and major and minor bleeding events according to the Thrombolysis in Myocardial Infarction (TIMI) criteria21 (safety) with P2Y12 inhibitors (Table 3). The pooled analysis of the CHAMPION PROGRAM published by Steg et al (2013)18 that compared cangrelor to clopidogrel and the network metanalysis by Westman et al (2017)21 that allowed to compare cangrelor, ticagrelor, and prasugrel to clopidogrel were used to derive ischemic and bleeding data.
Costs Data
Costs data are shown in supplementary Table 1. Pharmacological costs included P2Y12 inhibitors and GPIs (bail-out) and were driven from official databases.31 A 7.5% discount was applied to the cost of ticagrelor and cangrelor, and a 15% discount was considered for abciximab and eptifibatide following national drug price regulations (Real Decreto Ley (RDL) 8/2010).32 No discounts were applied to generic antiplatelet drugs. Clinical events costs were estimated from the literature.30
Costs were updated to 2019 € based on the Consumer Price Index (CPI) percentage changes for Spain33 and it was assumed that they would remain unchanged from 2020 onwards.
Assumptions
Assumptions were made when data were unavailable or inconsistent. They are summarized in Table 4. Assumptions were validated by clinical experts to better represent the usual clinical practice in Spain.
 |
Table 4 Assumptions Considered in the Budget Impact Model (Base Case) |
Sensitivity Analysis
A one-way sensitivity analysis was conducted to assess the impact of different parameters on the budget impact results. The parameters included in the sensitivity analysis were suggested by the clinical experts. Percentage use of GPIs [current scenario: mean, 6.50% (range: 3.00% - 12.70%); alternative scenario: mean, 6.35% (range: 2.60% - 12.40%)]; percentage use of generic clopidogrel [mean: 62% (range: 50% - 100%)]; and costs of clinical events (±20%) were considered to highly influence budget impact according to usual practice in Spain.18,25.
Ethical Considerations
This was a pharmacoeconomic, non-interventional study, based on the collection of data from the literature and from public health reports and registries without involvement of human subjects. Ethical approval was not required to conduct this study.
Results
In the base case scenario, PCI patients in whom oral P2Y12 inhibitors are not feasible or desirable reaches 7,220 over three years (Figure 1); 5,769 patients would benefit from cangrelor as shown in Tables 1 and 2 and would benefit from achieving the corresponding efficacy and safety outcomes as described in Table 3. In this scenario, it was calculated that adding cangrelor at a tentative ex-factory price of 336.70€ per vial (legal VAT and a 7.5% mandatory discount included) into the hospital formulary, for the acute care of PCI patients in whom the antiplatelet therapy with oral P2Y12 inhibitors is not feasible or desirable would represent 1,021,717€ over 3 years, in Spain (Table 5). The budget impact would be 50,245€ in year one, 372,200€ in year two, and 599,272€ in year three.
 |
Table 5 Budget Impact Results for Cangrelor Amongst PCI Patients in Whom Antiplatelet Therapy with Oral P2Y12 Inhibitors is Not Feasible or Desirable in Spain (Three-Year Time Horizon) |
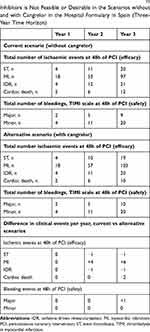
The introduction of cangrelor would modify the costs associated with the use of P2Y12 inhibitors in the hospital (Figure 2). It may increase the hospital pharmacy costs associated to the use of P2Y12 inhibitors totalizing 976,802 € over three years. Patients will remain on their long-term oral antiplatelets after receiving cangrelor during PCI. The costs of GPIs use may be −4,705€ lower. There would also be differences in the number and costs of clinical events between the scenario without cangrelor compared with the scenario with cangrelor in the hospital formulary (Table 6). The costs of ischemic and bleeding events may represent 47,119€ and 2,500€ over three years, respectively. Greater differences may appear in the incidence of thrombotic events compared to the incidence of bleeding episodes. The budget impact would be sensitive to the size of the population using GPIs (bail-out) and to the number of clinical events over three years (Figure 3). The cost of clinical events or the percentage of usage of clopidogrel would have minor influence in the budget impact.
 |
Figure 3 Tornado diagram: influence of key inputs on the budget impact of cangrelor for managing PCI patients in whom oral P2Y12 inhibitors are not feasible or desirable, in Spain. |
Discussion
The results of the present economic analysis demonstrate that the financial impact of introducing cangrelor into the drug formulary of hospital pharmacies for the adjunctive treatment of approximately 6,000 PCI patients in whom the use of oral P2Y12 inhibitors is not feasible or desirable, slightly exceeds one million € over three years, in Spain. Compared to the budget impact of adjunctive oral antiplatelet drugs, it was reported that increasing by 5% to 10% the use of oral prasugrel only, or prasugrel and ticagrelor, versus clopidogrel in ACS patients undergoing PCI reached 239 million € to 252 million € over 3 years in Spain (2017 €).11
Likewise, two previous economic studies conducted in Spain (in 2010 and 2012, respectively) analyzed the cost implications of replacing clopidogrel by prasugrel or ticagrelor in patients undergoing PCI.34,35 The first study anticipated a budget impact of 8,245 million € in one year if 5% of the patients treated with clopidogrel were given prasugrel instead.35 The second study showed a budget impact in the range of 63,000€ to 86,000€ per each avoided death due to thrombotic events after replacing clopidogrel by ticagrelor, without considering bleeding costs.34 Overall, these data revealed that an important financial effort was made to introduce the two potent oral P2Y12 inhibitors, prasugrel and ticagrelor, into the drug formulary in Spain. Introducing cangrelor into the formulary of hospital pharmacies in Spain implies higher pharmacological costs. However, compared to drug costs, revascularization procedures and length of hospital stay are greater contributors of direct medical costs during the first year following an acute coronary event. They may reach up to one billon € in Spain.36
Taking into account the frame of the present analysis, meaning early PCI (<2 h)4,5 under clinical conditions in which the adjuvant antiplatelet treatment with oral P2Y12 inhibitors may not be feasible or desirable, it should also be considered that the antiplatelet effect of crushed ticagrelor37 and chewed prasugrel38 were also investigated as potential alternatives to improve their oral bioavailability. Although the results showed that time to maximum plasma concentration was shorter in the crushed ticagrelor group versus the integral tablets group, the median time of 2 h vs 4 h, respectively, still represents the delayed onset of the ticagrelor antiplatelet effect.37 In line with these results, the recently published FABOLUS FASTER pharmacodynamic study compared the antiplatelet effect of chewed prasugrel vs a loading dose of integral prasugrel tablet and demonstrated that chewed prasugrel led to higher active metabolite concentration but not to a greater platelet aggregation inhibition.38 The results of these pharmacodynamic studies emphasize that even the faster oral P2Y12 alternatives may still not be desirable. In this context, cangrelor represents an affordable iv alternative for immediate P2Y12 inhibition.
The therapeutic value of cangrelor is well aligned to the expected for a highly effective antiplatelet strategy to be used in the acute setting of PCI.39,40 Antiplatelet drugs with a favorable benefit/risk profile are needed to improve the overall clinical and economic outcomes of PCI patients in Spain, including the reduction of the length of hospital stay and the decrease in the number of periprocedural clinical events.41 In this regard, cangrelor is an innovative iv antiplatelet adjunctive strategy to guarantee an immediate, potent, and rapidly reversible effect in patients undergoing PCI, in whom an oral P2Y12 inhibition is not feasible or desirable.42
There are limitations in the present budget impact model that should be considered. Although all the inputs used in the analysis were taken from published registries, clinical trials and metanalysis and were further validated by external clinical experts, it was not always possible to identify the precise factors that matched the model definitions and represented the acute hospital setting of ACS, in Spain. For instance, PCI population growth and percentage distributions for P2Y12 inhibitors usage may differ from the model assumptions in forthcoming years implying changes in the budget impact estimates. The same cost for both minor and major bleeding events were considered. As a consequence, bleeding costs may have been overestimated. Likewise, other cost assumptions may further influence these results, both positively and negatively. Adverse events associated to GPIs were not modelled and may have influenced the budget impact results. Differences in cases characterization, studies design and patients’ representativeness amongst publications made data selection difficult to carry out. For this reason, assumptions were made to solve this lack of available evidence.
Despite these limitations, this budget impact analysis provides an estimate of introducing a novel antiplatelet iv strategy for PCI patients in whom the use of oral P2Y12 inhibitors is not feasible or desirable. Further research should focus on determining the most cost-effective scenarios for using cangrelor in the clinical practice.
Conclusions
Cangrelor represents an innovative iv strategy for the adjunctive antiplatelet therapy in PCI patients in whom an oral P2Y12 inhibition is not feasible or desirable. The economic effort that would be needed to incorporate cangrelor into the hospital pharmacy formulary seems to be within reasonable margins in Spain, barely exceeding one million € over 3 years. However, further research with a more robust design is needed to confirm these findings.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Preliminary results of this budget impact analysis were presented at the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) Europe Congress, 2-6 November 2019, Copenhagen.
Funding
Ferrer funded the development of the study and the writing of the manuscript.
Disclosure
ILD is an employee at Ferrer. SPR reports personal fees from Ferrer, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Dégano IR, Elosua R, Marrugat J. Epidemiology of acute coronary syndromes in spain: estimation of the number of cases and trends from 2005 to 2049. Rev Esp Cardiol. 2013;66(6):472–481. doi:10.1016/j.recesp.2013.01.019
2. Weintraub WS, Mandel L, Weiss SA. Antiplatelet therapy in patients undergoing percutaneous. coronary intervention: economic considerations. Pharmacoeconomics. 2013;31(11):959–970. doi:10.1007/s40273-013-0088-8.Antiplatelet
3. Cid Alvarez AB, Rodriguez Leor O, Moreno R, Peez de Prado A. Registro Español de Hemodinámica y Cardiología Intervencionista. XXVIII Informe Oficial de la Sección de Hemodinámica y Cardiología Intervencionista de la Sociedad Española de Cardiología (1990-2018) [Spanish Cardiac Catheterization and Coronary Intervent. Rev Esp Cardiol. 2019;72(12):1043–1053. doi:10.1016/j.recesp.2019.07.023
4. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39(2):119–177. doi:10.1093/eurheartj/ehx393
5. Collet J-P, Thiele H, Barbato E, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;2020:1–79.
6. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi:10.1093/eurheartj/ehy394
7. Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with Cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303–1313. doi:10.1056/NEJMoa1300815
8. Lipinski MJ, Satler LF. Intraprocedural thrombotic events: what’s the real cost? Catheter Cardiovasc Interv. 2015;86(1):40–41. doi:10.1002/ccd.26064
9. Capodanno D, Ferreiro JL, Angiolillo DJ. Antiplatelet therapy: new pharmacological agents and changing paradigms. J Thromb Haemost. 2013;11:316–329. doi:10.1111/jth.12219
10. Hanna EB, Rao SV, Manoukian SV, Saucedo JF. The evolving role of glycoprotein IIb/IIIa inhibitors in the setting of percutaneous coronary intervention: strategies to minimize bleeding risk and optimize outcomes. JACC Cardiovasc Interv. 2010;3(12):1209–1219. doi:10.1016/j.jcin.2010.09.015
11. Talegon Albariño I, García García J, Gross Kasztanovits E;, Martín Conde JA, Crespo C, Rodríguez Barrios JM. Análisis del Impacto Presupuestario de los nuevos antiagregantes plaquetarios prasugrel (Efient) y ticagrelor (Brilique) frente a la terapia estándar con clopidogrel en el tratamiento de pacientes con Síndrome Coronario Agudo sometidos a Intervención Co. 2018;13(5):882–893.
12. Ruiz-Bailén M, Rucabado-Aguilar L, Expósito-Ruiz M, et al. Cardiogenic shock in acute coronary syndrome. Med Sci Monit. 2009;15(3):57–66. doi:10.5005/jp/books/12337_8
13. Kataja A, Harjola V-P. Cardiogenic shock: current epidemiology and management. Contin Cardiol Educ. 2017;3(3):121–124. doi:10.1002/cce2.62
14. Park CB, Hwang HJ, Cho JM, Jo BH, Kim CJ. Acute myocardial infarction patient with recurrent vomiting: what is the best treatment? Int J Cardiol. 2013;162(3):E56–E57. doi:10.1016/j.ijcard.2012.05.117
15. Bhatt DL, Topol EJ. Current role of platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. J Am Med Assoc. 2000;284(12):1549–1558. doi:10.1001/jama.284.12.1549
16. European Medicines Agency. Kengrexal. Summary of product characteristics. 2020. Available from: https://www.ema.europa.eu/en/documents/product-information/kengrexal-epar-product-information_en.pdf.
17. European Medicines Agency-Committee for Medicinal Products for Human Use. Kengrexal-Assessment report. CHMP Summary of Positive Opinion for Kengrexal. 2015. Available from: https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-kengrexal_en.pdf.
18. Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013;382(9909):1981–1992. doi:10.1016/S0140-6736(13)61615-3
19. Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361(24):2330–2341. doi:10.1056/NEJMoa0908629
20. Harrington RA, Stone GW, McNulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361(24):2318–2329. doi:10.1056/NEJMoa0908628
21. Westman PC, Lipinski MJ, Torguson R, Waksman R. A comparison of cangrelor, prasugrel, ticagrelor, and clopidogrel in patients undergoing percutaneous coronary intervention: A network meta-analysis. Cardiovasc Revascularization Med. 2017;18(2):79–85. doi:10.1016/j.carrev.2016.10.005
22. Serrador, Frutos AM, Jiménez-Quevedo P, Pérez de Prado A, Pan Álvarez-ossorio M. Spanish Cardiac Catheterization and Coronary Intervention Registry. 26th Official Report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990-2016). Rev Española Cardiol (English Ed. 2017;70(12):1110–1120. doi:10.1016/j.rec.2017.10.017
23. Cid Álvarez AB, Rodríguez, Leor O, Moreno R, Pérez de Prado A. Spanish Cardiac Catheterization and Coronary Intervention Registry. 27th Official Report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990-2017). Rev Española Cardiol (English Ed. 2018;71(12):1036–1046. doi:10.1016/j.rec.2018.09.009
24. Sociedad Española de Cardiología. Registro Nacional De Actividad En Cardiología Intervencionista - Sección Hemodinámica y Cardiología Intervencionista. Registro de Actividad; 2016. Available from: https://www.hemodinamica.com/cientifico/registro-de-actividad/.
25. Sociedad Española de Cardiología. Registro Nacional de Actividad de Cardiología Intervencionista - Sección Hemodinámica. Registro de Actividad; 2017. https://www.hemodinamica.com/cientifico/registro-de-actividad/.
26. Anderson ML, Peterson ED, Peng SA, et al. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification a report from NCDR. Circ Cardiovasc Qual Outcomes. 2013;6(6):708–715. doi:10.1161/CIRCOUTCOMES.113.000262
27. Expert Consensus. Ferrer Galenica. 2019.
28. Hamon M, Nienaber CA, Galli S, et al. Bivalirudin in percutaneous coronary intervention: the EUROpean BiValIrudin UtiliSatION in Practice (EUROVISION) Registry. Int J Cardiol. 2014;173(2):290–294. doi:10.1016/j.ijcard.2014.03.003
29. Plosker G, Ibbotson T. Eptifibatide: a pharmacoeconomic review of its use in percutaneous coronary intervention and acute coronary syndromes. Pharmacoeconomics. 2003;21(12):885–912. doi:10.2165/00019053-200321120-00005
30. Latour-Perez J, De-Miguel-Balsa E. Cost effectiveness of fondaparinux in non-ST-elevation acute coronary syndrome. Pharmacoeconomics. 2009;27(7):585–595. doi:10.2165/11310120-000000000-00000
31. PortalFarma. BotPlus. BotPlus. 2013. https://botplusweb.portalfarma.com/.
32. Agencia Estatal Boletin Oficial del Estado. Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. 2010 Available from: BOE-A-2010-8228. https://www.boe.es/buscar/doc.php?id=BOE-A-2010-8228..
33. Instituto Nacional de Estadística (INE). Calculation of the Consumer Price Index percentage changes (CPI system base 2016). How much has the CPI varied since …? 2019. Available from: https://www.ine.es/varipc/index.do?L=1.
34. Sociedad Española de Farmacia Hospitalaria - Grupo GENESIS. Ticagrelor en síndromes coronarios agudos. Informes de evaluación de medicamentos publicados por hospitales. Available from: https://gruposdetrabajo.sefh.es/genesis/genesis/Enlaces/InformesHosp_abc.htm?mL=1#T.
35. Sociedad Española de Farmacia Hospitalaria - Grupo GENESIS. Prasugrel en profilaxis antitrombotica en pacientes con SCA sometidos a ICP. Informes de evaluación de medicamentos publicados por hospitales. 2010. Available from: https://gruposdetrabajo.sefh.es/genesis/genesis/Enlaces/InformesHosp_abc.htm?mL=1#P.
36. Taylor MJ, Scuffham PA, McCollam PL, Newby DE. Acute coronary syndromes in Europe: 1-Year costs and outcomes. Curr Med Res Opin. 2007;23(3):495–503. doi:10.1185/030079906X167462
37. Alexopoulos D, Barampoutis N, Gkizas V, et al. Integral tablets of Ticagrelor in ST-segment elevation myocardial infarction patients: a randomized pharmacokinetic/pharmacodynamic study. Clin Pharmacokinet. 2016;55(3):359–367. doi:10.1007/s40262-015-0320-0
38. Gargiulo G, Esposito G, Avvedimento M, et al. Cangrelor, tirofiban, and chewed or standard prasugrel regimens in patients with ST-segment-elevation myocardial infarction: primary results of the FABOLUS-FASTER Trial. Circulation. 2020;142(5):441–454. doi:10.1161/CIRCULATIONAHA.120.046928
39. Davies A, Sculpher M, Barrett A, Huete T, Sacristán J, Dilla T. Prasugrel compared to clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a Spanish model-based cost effectiveness analysis. Farm Hosp. 2013;37(4):307–316. doi:10.7399/FH.2013.37.4.687
40. Molina-Cuadrado E, Mateo-Carrasco H, Nieto-Guindo P, Rodríguez-Gómez P. Long-term cost-effectiveness of ticagrelor versus clopidogrel in acute coronary syndrome in Spain. Farm Hosp. 2014;38(4):266–275. doi:10.7399/fh.2014.38.4.1132
41. de Lorenzo-pinto A, Herranz-Alonso A, Cuéllar-Basterrechea B, et al Clinical and economic impact of a multidisciplinary intervention to reduce bleeding risk in patients with acute coronary syndrome. Rev Esp Cardiol (Engl Ed). 2017;70(10):825–831. doi:10.1016/j.rec.2016.12.043
42. European Medicines Agency. Kengrexal Public Assessment Report. Assessment report. 2015. Available from: https://www.ema.europa.eu/en/documents/assessment-report/kengrexal-epar-public-assessment-report_en.pdf.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.