Back to Journals » Drug Design, Development and Therapy » Volume 14
Analysis of the Doxorubicin and Doxorubicinol in the Plasma of Breast Cancer Patients for Monitoring the Toxicity of Doxorubicin
Authors Harahap Y , Ardiningsih P, Corintias Winarti A, Purwanto DJ
Received 24 February 2020
Accepted for publication 29 July 2020
Published 25 August 2020 Volume 2020:14 Pages 3469—3475
DOI https://doi.org/10.2147/DDDT.S251144
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Yahdiana Harahap,1 Puspitasari Ardiningsih,1 Agatha Corintias Winarti,1 Denni Joko Purwanto2
1Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia; 2Functional Medical Staff of Surgical Oncology, “Dharmais” Cancer Hospital, Jakarta, Indonesia
Correspondence: Yahdiana Harahap Email [email protected]
Introduction: Doxorubicin is an anthracycline antibiotic used as an anticancer agent. Long-term use of this anticancer agent could accumulate its metabolite, doxorubicinol, and cause cardiomyopathy, due to its cardiotoxicity. This cardiotoxic effect depends on the amount of doxorubicin and doxorubicinol accumulated in the body. This study aimed to analyze doxorubicin and doxorubicinol levels in the blood plasma of breast cancer patients.
Methods: Participants of this study were 30 breast cancer patients who had received doxorubicin in their therapy regimen. The samples were analyzed using ultra-high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS), with the Acquity UPLC BEH C18 Waters chromatography column (2.1 x 100 mm : 1.7 μm). Plasma (250 μL) samples were prepared by protein precipitation, using methanol. The mobile phase consisted of 0.1% acetic acid (eluent A) and acetonitrile (eluent B), with gradient elution; the flow rate was 0.15 mL/min and runtime, 7 min.
Results and Discussion: This method was linear in the range of 1– 1000 ng/mL for doxorubicin and 0.5– 500 ng/mL for doxorubicinol. This method was successfully used to analyze doxorubicin and doxorubicinol, simultaneously, using hexamethylphosphoramide as the internal standard, in the plasma of breast cancer patients. Results showed that the measured concentrations of doxorubicin and doxorubicinol ranged between 12.54– 620.01 ng/mL and 1.10– 27.00 ng/mL, respectively. The measured cumulative doses of doxorubicin ranged between 48.76 and 319.01 mg/m2; thus, the risk of cardiomyopathy in the surveyed patients was under 4%, according to literature.
Keywords: analysis, breast cancer, cardiotoxic, doxorubicin, doxorubicinol, partial validation, plasma, LC-MS/MS
Introduction
Doxorubicin (DOX) or Adriamycin is an anthracycline antibiotic.1,2 DOX is indicated for a broad range of malignant neoplasms.3 DOX was one of the first-line anticancer therapies, with clinical activity in many types of cancer, including breast, endometrial, ovarian, testicular, liver, and lung cancers, neuroblastoma, Ewing’s sarcoma, Hodgkin and non-Hodgkin lymphomas.4 Long-term use of this anticancer agent could have side effects.5 Cardiomyopathy is one of the side effects of its cardiotoxicity.6 DOX is metabolized by CYP2D6, CYP3A4, and P-glycoprotein.7 DOX is metabolized in the body into its main metabolite, doxorubicinol (DOXol).
DOXol is a major metabolite of DOX.8 DOX is rapidly metabolized by the cytoplasmic NADPH-dependent aldo-keto reductase into DOXol.9 A previous study implicated DOXol in the cardiotoxicity of patients administered DOX therapy. It was due to the ability of DOXol to form free radicals and disrupt the function of the ion pump in the sarcoplasmic reticulum of cardiac cells. Long-term use of DOX could lead to DOXol accumulation in the body; thus, it could increase the risk of cardiotoxicity.6 DOXol has a more potent cardiotoxic action than DOX.10 DOX dosage is important. If the dose given to patients is too high, the cardiotoxic effects can occur.
Materials and Methods
Reference Standard Samples and Materials
DOX hydrochloride was purchased from Hisun Pharmaceutical (Zhejiang, China), DOXol from Toronto Research Chemical (Canada, USA), Hexamethylphosphoramide (HMPA) from Sigma-Aldrich (Singapore), acetic acid, acetonitrile (HPLC grade), and methanol for analysis from Merck (Darmstadt, Germany), and ultra-pure water.
Preparation of Solutions and Standards
DOX, DOXol, and HMPA stock solutions were prepared in methanol to obtain a concentration of 1000 ng/mL. The stock solutions were serially diluted to obtain working solutions of 10 ng/mL of DOX and DOXol, and 100 ng/mL of HMPA. All solutions were stored at 4ºC and brought to room temperature before use.
Sample Preparation
Sample preparation was conducted by protein precipitation using methanol. A 50 μL of IS solution (100 ng/mL) was added to 250 μL aliquot of plasma sample and vortex-mixed for 10 sec. Methanol (250 μL) was added to the mixture, vortex-mixed for 30 sec, and centrifuged at 14.000 rpm for 10 min. From the final mixture, 200 μL of the supernatant was transferred into a sample cup and evaporated to dryness, using nitrogen at 55ºC for 20 min. The residue was reconstituted in 100 μL of the mobile phase, 0.1% acetic acid-acetonitrile (10:90), vortex-mixed for 10 sec, then, sonicated for 2 min. The final mixture was transferred into a vial and 10 μL injected into the LC-MS/MS system for analysis. The research had received Ethical Clearance from The Committee of The Medical Research Ethics of the Dharmais Cancer Hospital; No. 031/KEPK/III/2019.
Method Validation
Full validation was performed according to the European Medicines Agency (EMEA) guidelines on bioanalytical method validation Committee for Medicinal Products for Human Use and Bioanalytical Method Validation Guidance for Industry by FDA.11,12 Full validation was conducted by validating LLOQ, linearity, selectivity, precision and accuracy, recovery, dilution integrity, matrix effects, stability, and carry over.
Linearity
The calibration curve consisted of at least six concentrations, including blank and zero samples. A 950 μL blank plasma was spiked with 50 μL of working solutions serially diluted into seven calibration levels of samples containing DOX (1; 3; 25; 50; 500; 800; and 1000 ng/mL) and DOXol (0,5; 1.5; 10; 25; 250; 400; and 500 ng/mL). The seven calibration levels of samples were prepared with the selected method. A 10 μL of solutions was injected into the LC-MS/MS system for analysis. Calibration curves were considered acceptable when the correlation coefficient (r) was greater than 0.98 for biological matrix and bias of calculated concentrations within ±15% of nominal concentrations, except the LLOQ was within ±20%.
Accuracy and Precision
DOX and DOXol working solutions were diluted with blank plasma to obtain four concentrations (LLOQ, QCL, QCM, and QCH). Each of these concentrations was prepared with the selected method and injected into the LC-MS/MS system for analysis. The validation was replicated five times. Accuracy and precision were considered acceptable when the bias of calculated concentrations was within ±15% of nominal concentrations, except LLOQ was within ±20%.
Application of the Method
This research was approved by the Research Ethics Committee of “Dharmais” Cancer Hospital (031/KEPK/III/2019). Participants of this study were 30 breast cancer patients who had received DOX in their therapy regimen. The procedure was explained to the participants in detail during sampling, and they signed informed consents before participating. The inclusion criteria were patients diagnosed with breast cancer, who had received DOX in their therapy regimen, and who had signed the informed consent. Whereas the exclusion criteria were patients who had not been diagnosed with breast cancer, for whom DOX is contraindicated, and who did not sign the informed consent.
This study used the venipuncture technique to draw blood from patients. About 2–3 mL of blood was collected into anticoagulant EDTA tubes, 20–90 min post DOX administration. The tube was centrifuged at 3000 rpm for 20 min to obtain blood plasma. The supernatant was transferred into a sample cup and stored at −80ºC until analysis.
Results and Discussion
Chromatography System
The analysis in this study was performed using LC-MS/MS, with the Acquity UPLC BEH C18 Waters chromatography column (2.1 x 100 mm x 1.7 μm). The column temperature was 45ºC. The mobile phase consisted of a combination of 0.1% acetic acid in water-acetonitrile, with gradient elution. The flow rate was 0.15 mL/min and runtime, 7 min. The injection volume was 10 μL. Mass detection was performed using ESI (+) ion source and Triple Quadrupole (TQD) mass analyzer in Multiple Reaction Monitoring (MRM) analysis mode. The capillary voltage was 3 kV, with 450ºC desolvation temperature and 500 L/hour gas flow rate. The cone voltage was 42 V. The m/z values for DOX was 544.22 > 397.06, DOXol, 546.22 > 363.06, and HMPA, 180.03 > 135.16.
Validation Assay
Full validation assay was conducted by validating LLOQ, linearity, selectivity, precision and accuracy, recovery, dilution integrity, matrix effects, stability, and carry over. The linearity of each calibration curve was determined by plotting the peak area ratio (y) of the analyte to IS versus nominal concentration (x) of DOX and DOXol. The calibration curves were linear in the range 1–1000 ng/mL for DOX (r = 0.9976) and 0.5–500 ng/mL for DOXol (r = 0.9986).
Precision and Accuracy
The precision and accuracy data can be seen in Table 1. The intra-batch accuracy and precision performed on LLOQ, QCL, QCM, and QCH fulfilled the guideline requirement. The accuracy (%bias value) of DOX and DOXol was less than 20%, and their precision (%CV value) were 7.39–10,07% and 3.53–9,16%, respectively.
 |
Table 1 Summary of Precision and Accuracy Validation |
Selectivity
The representative chromatograms from the LC-MS/MS analysis of blank plasma and spiked LLOQ of DOX, DOXol, and HMPA can be seen in Figures 1 and 2. There were no significantly interfering peaks, due to endogenous components or reagents, observed for doxorubicin, doxorubicinol, or hexamethylphosphoramide.
 |
Figure 1 Chromatogram of blank plasma. |
 |
Figure 2 Chromatogram of LLOQ. |
Recovery
The mean extraction recoveries of DOX were 93.47%, 96.88%, and 94.33% (n = 3) at QCL, QCM, and QCH concentrations, with %CV values of 2.48%, 1.58%, and 2.02%, respectively. The mean extraction recoveries of DOXol were 93.18%, 92.38%, and 93.35% (n = 3) at QCL, QCM, and QCH concentrations, with %CV values of 4.95%, 1.53%, and 2.52%, respectively. The mean extraction recovery of HMPA was 85.67% with %CV value of 1.00%.
Carryover
The measured peak area of the blank sample injected, after ULOQ calibration standard, was between 3.78–12.63% of the peak area of the analyte at LLOQ for DOX, 1.02–2.87% LLOQ for DOXol and 0.38–1.01% LLOQ for HMPA.
Dilution Integrity
The dilution integrity testing results were acceptable because the dilution fulfilled accuracy and precision requirements, with %diff and %CV not more than 15%, which was in the blank human plasma until concentrations QCH and half QCH.
Matrix Effects
The internal standard normalized matrix factor values of DOX were 0.92 and 0.95 at the concentrations QCL and QCH, with %CV of 4.16% and 2.62%, respectively. The internal standard normalized matrix factor values of doxorubicinol were 0.92 and 0.95 at concentrations QCL and QCH, with %CV of 4.16% and 2.62%, respectively. While for HMPA, the mean matrix effect was 95.00%, with %CV of 3.45%. These data indicate that the ME (ion suppression or enhancement) from human plasma was negligible, under the current conditions.
Stability
Storage of stock solutions of doxorubicin, doxorubicinol, and HMPA, in methanol, at room temperature for 24 hours, and in the refrigerator (−4°C) for 20 days, did not alter the analytes DOX, DOXol, or HMPA. The stability test results of DOX and DOXol in plasma were stable during sample preparation, storage conditions, autosampling, and after 3 freeze-thaw cycles.
Sample Analysis
There were two breast cancer therapy regimens, 5-Fluorouracil - Adriamycin-Cyclophosphamide (FAC) and Adriamycin-Cyclophosphamide (AC). The dosage of FAC regimen ranged from 68–90 mg and 81–100 mg for AC regimen. The chemotherapy cycle of the patients consisted of cycles 1, 2, 3, 4, 5, and 6. Each cycle was 3 weeks long. There were 27 patients receiving FAC regimen and 3 patients receiving AC regimen. All the patients were women with age ranging from 27–68 years old, diagnosed with invasive breast carcinoma of no special type (NST). Data on the breast cancer patients can be seen in Table 2.
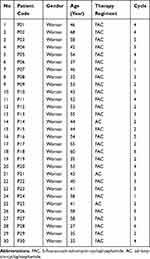 |
Table 2 The Data of Breast Cancer Patients |
This method was applied to determine the concentration of DOX and DOXol in breast cancer patients. The results showed that calculated DOX in the patients ranged from 12.54–620.01 ng/mL and DOXol, from 1.10–27.00 ng/mL. The calculated amount of DOX and DOXol gave a wide range of concentrations among patients (Figure 3). This variation might occur because of two probabilities. The first probability is the differences in sampling time after DOX administration. This relates to the duration of chemotherapy administration, which was not fixed in each patient. The shortest sampling time was found in sample P20 (21 min) and the longest in sample P13 (90 min), post administration. The significance of sampling time versus DOX and DOXol levels was determined using Pearson correlation. The result showed that there was a significant correlation between sampling time and DOX level (r = −0.515, p = 0.004), but not DOXol level (r = −0.161, p = 0.395). It can be concluded that the longer the sampling time, the lower DOX levels in patients. The data of chemotherapy can be seen in Table 3.
 |
Table 3 The Data of Chemotherapy |
 |
Figure 3 Graphic of Doxorubicin and Doxorubicinol measurement. |
The second probability is polymorphisms in patients using DOX for chemotherapy. According to a previous study, DOX yields high variance in pharmacokinetic and pharmacodynamic profiles, caused by a polymorphism in CBR1 and CBR3 proteins that convert DOX into DOXol.13 CBR1 and CBR3 are genes encoding carbonyl reductases. CBR1 correlates with significantly higher DOX exposure levels, suggesting the possibility of reduced intracellular conversion to DOXol in patients.14 This was shown in the DOX level results, which was much higher than DOXol levels in all patients. Thus, there is a possible polymorphism occurring in all patients in this study.
Furthermore, DOXol has been implicated in the cardiotoxicity in patients on DOX therapy. Long-term use of DOX could accumulate DOXol and cause cardiomyopathy.6 In this study, cumulative doses were determined by multiplying drug dosage by the body surface area (BSA) of patients. The BSA of patients were calculated using a simplified body surface area formula.15 The highest cumulative dose was found in sample P18 (319.01 mg/m2), after 6 cycles, and the lowest in sample P24 (48.76 mg/m2), after 1 cycle. According to a previous study, DOX accumulation could cause cardiomyopathy, with incidence rates of 4% at 500–550 mg/m2, 18% at 551–600 mg/m2, and 36% at >600 mg/m2. The results showed that the cumulative dose ranged from 48.76–319.01 mg/m2. Therefore, the risk of cardiomyopathy in the surveyed patients was under 4%, according to literature.6 The cumulative dose can be seen in Figure 4.
 |
Figure 4 Cumulative doses of patients. |
Conclusion
The method in this study was successfully used to analyze doxorubicin and doxorubicinol using hexamethylphosphoramide as the internal standard, in the plasma of breast cancer patients simultaneously. The results showed that the measured concentrations of doxorubicin and doxorubicinol ranged between 12.54–620.01 ng/mL and 1.10–27.00 ng/mL, respectively, and the measured cumulative doses of doxorubicin ranged between 48.76–319.01 mg/m2. It can be concluded that the risk of cardiomyopathy in the surveyed patients was under 4%, according to the previous literature.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mitry MA, Edwards JG. Doxorubicin induced heart failure: phenotype and molecular mechanisms. IJC Hear Vasc. 2016;10:17–24. doi:10.1016/j.ijcha.2015.11.004
2. Barpe DR, Rosa DD, Froehlich PE. Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur J Pharm Sci. 2010;41(3–4):458–463. doi:10.1016/j.ejps.2010.07.015
3. Schaupp CM, White CC, Merrill GF, Kavanagh TJ. Metabolism of doxorubicin to the cardiotoxic metabolite doxorubicinol is increased in a mouse model of chronic glutathione deficiency: a potential role for carbonyl reductase 3. Chem Biol Interact. 2015;234:154–161. doi:10.1016/j.cbi.2014.11.010
4. Katzung BG, Masters SB, Trevor AJ. Basic & Clinical Pharmacology.
5. Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. The Breast. 2015;24:S149–53. doi:10.1016/j.breast.2015.07.035
6. Luu AZ, Chowdhury B, Al-Omran M, Teoh H, Hess DA, Verma S Role of endothelium in doxorubicin-induced cardiomyopathy. JACC Basic to Transl Sci. 2018;(6):861–870 doi:10.1016/j.jacbts.2018.06.005
7. Drug BC. Doxorubicin; 2010;5(March):1–10. Available from: http://www.bccancer.bc.ca/drug-database-site/DrugIndex/Doxorubicin_monograph.pdf.
8. Zeng X, Cai H, Yang J, Qiu H, Cheng Y, Liu M. Pharmacokinetics and cardiotoxicity of doxorubicin and its secondary alcohol metabolite in rats. Biomed Pharmacother. 2019;116(April):108964. doi:10.1016/j.biopha.2019.108964
9. Ahmed S, Kishikawa N, Ohyama K, Wada M, Nakashima K, Kuroda N. Selective determination of doxorubicin and doxorubicinol in rat plasma by HPLC with photosensitization reaction followed by chemiluminescence detection. Talanta. 2009;78(1):94–100. doi:10.1016/j.talanta.2008.10.043
10. Sakai-Kato K, Saito E, Ishikura K, Kawanishi T. Analysis of intracellular doxorubicin and its metabolites by ultra-high-performance liquid chromatography. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878(19):1466–1470. doi:10.1016/j.jchromb.2010.03.040
11. EMEA. Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009 Rev 1 Corr 2** [Internet]; 2011;44(July 2011):1–23. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf.
12. FDA. Bioanalytical Method Validation Guidance. Cder; May 2018:25.
13. Marsh S, Liu G. Pharmacokinetics and pharmacogenomics in breast cancer chemotherapy. Adv Drug Deliv Rev. 2009;61(5):381–387. doi:10.1016/j.addr.2008.10.003
14. Lal S, Sandanaraj E, Wong ZW, et al. CBR1 and CBR3 pharmacogenetics and their influence on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008;99(10):2045–2054. doi:10.1111/j.1349-7006.2008.00903.x
15. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi:10.1056/NEJM198710223171717
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
