Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Analysis of Risk Factors for Postoperative Delirium After Liver Transplantation
Authors Chen J, Wang H, He Z, Li T
Received 21 March 2020
Accepted for publication 17 June 2020
Published 3 July 2020 Volume 2020:16 Pages 1645—1652
DOI https://doi.org/10.2147/NDT.S254920
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Junguo Chen, Hao Wang, Zhijun He, Ting Li
Department of Organ Transplantation, The Second Xiangya Hospital, Central South University, Changsha, People’s Republic of China
Correspondence: Ting Li
Liver Transplantation, The Second Xiangya Hospital, No. 139 Middle Renmin Road, Changsha 410011, People’s Republic of China
Tel +86 15084909000
Email [email protected]
Purpose: The study aimed to analyze the incidence of postoperative delirium (POD) and associated risk factors after liver transplantation (LT).
Patients and Methods: We identified and enrolled patients undergoing LT at the Second Xiangya Hospital, Central South University between August 2018 and May 2019. We abstracted their relevant clinical information and assigned the patients into a POD group and non-POD group to compare differences in clinical information. Risk factors of POD were analyzed using logistic regression.
Results: A total of 159 LT patients were enrolled. Forty-two patients exhibited delirium (26.4%). Of the 42 with delirium, 33 (78.6%) had delirium within 3 days postoperatively and a median duration of 5 days (quartile 3– 7 days). The results of binary logistic regression are as follows: preoperative ammonia (≥ 46 vs < 46 μmol/L; OR 3.51, 95% CI [1.31– 9.46], P< 0.05), Model for End-Stage Liver Disease (MELD) score (≥ 15 vs.< 15; OR 3.33, 95% CI [1.27– 8.79], P< 0.05), presence of hepatic encephalopathy (OR 3.30, 95% CI [1.20– 9.07], P< 0.05), aspartate aminotransferase (AST) on day 1 postoperatively (OR 1.33, 95% CI [1.06 − 1.68], P< 0.05), anhepatic period (OR 1.04, 95% CI [1.02 − 1.06], P< 0.01). The POD group had a longer intubation time (2925.0 vs 1410.0 min, P< 0.01), ICU length of stay (6 vs 4 d, P< 0.01) and increased medical costs (43.96 vs 33.74 ten thousand yuan, P< 0.01).
Conclusion: The incidence of POD in LT patients is a significant clinical feature. Ammonia ≥ 46 μmol/l, MELD score ≥ 15, hepatic encephalopathy, anhepatic period, and AST at 1 day postoperatively were independent risk factors for POD.
Keywords: liver transplantation, postoperative delirium, ammonia, hepatic encephalopathy, risk factors
A Letter to the Editor has been published for this article.
Introduction
Delirium is an acute reversible neurocognitive disorder status, manifesting as disturbance of consciousness, attention deficit, change in cognitive ability, and disturbed sleep cycle.1 The incidence of delirium in society is about 1–2%.2,3 Postoperative delirium (POD) is a delirium state arising after surgery. The incidence of POD differs due to surgical type and risk, with fluctuation between 12%-51%.4 The reports on the incidence of LT POD are substantially different in the literature. The incidence of LT POD has been reported to be 10.0%-26.7%,5,7 but Wang et al reported an incidence of up to 47.4%.8
There are few studies on the risk factors of LT POD. Previous studies have shown that a history of alcohol abuse, hepatic encephalopathy, acute physiology and chronic health evaluation II (APACHE) score ≥16, Child-Pugh level C, Model for End-Stage Liver Disease (MELD) score ≥15, preoperative hemodialysis, and liver donor type are related with the occurrence of LT POD.6,8 LT POD increases medical cost, prolongs the duration of mechanical ventilation, ICU length of stay, hospital length of stay, increases the incidence of other complications, and short-term and long-term mortality.5,7
This retrospective study analyzed the clinical information of 159 LT patients from August 2018 to May 2019 in the Second Xiangya Hospital, Central South University, to confirm the incidence and identify relevant risk factors of LT POD.
Patients and Methods
Patients
LT patients in the Second Xiangya Hospital, Central South University from Aug. 2018 to May 2019 were enrolled. Inclusion criteria included: (1) age ≥18 years old, (2) liver donor after brain death, (3) whole orthotopic liver transplantation. Exclusion criteria included: (1) multi-organ transplantation, (2) postoperative diagnosis of organic brain lesions, (3) primary liver graft malfunction, (4) congenital or genetic diseases, (5) a Richmond agitation sedation scale (RASS) ≤ −4.
We guarantee that there were not organs of prisoners, coerced, or paid individuals used in this study. We confirm all organs were from voluntary donation with written informed consent. This study was reviewed and approved by the Ethics Committee of the Second Xiang-ya Hospital. They were conducted in accordance with the Declaration of Istanbul (in addition to the Declaration of Helsinki).
Study Methods
We collected the following variables from the clinical records: sex, age, weight, previous medical history (ie, diabetes, hypertension, history of abdominal surgery, history of neuropsychosis), smoking history, alcohol abuse history, emergency surgery (preoperative hospitalization ≤3d), preoperative diagnosis (eg, liver cancer, acute liver failure, pre-transplant hepatic encephalopathy), liver function staging (Child-Pugh staging, MELD score), laboratory results for 3 days preoperatively (hepatic and renal function, electrolytes, coagulation function, blood ammonia and lactic acid). We also collected intraoperative information: time of operation, anhepatic period, intraoperative blood transfusion (eg, red blood cell, plasma, cryoprecipitate), intraoperative lactic acid, hypotension measured as systolic pressure <30% of baseline value, surgical procedure (eg, classic, piggyback). Postoperative information included: laboratory results on day one postoperatively (hepatic and renal function, electrolytes, coagulation function, blood ammonia and lactic acid), intubation duration, ICU length of stay, delirium duration, and postoperative hospital length of stay. We also collected information on the donor: age, liver weight, cold ischemia time, liver function on either the day before or day of harvest, electrolyte, donor liver pathological before infusion.
Two doctors independently diagnosed POD using the CAM-ICU scale. In there was no agreement, a third doctor made the diagnosis. Patients were assigned to either the POD or non-POD group (control group) to analyze the clinical information.
In our center, we used livers that were donated after brain death and obtained by in situ cold perfusions with liver and kidney together. The surgical procedure was either classic liver transplantation or modified piggyback liver transplantation with or without ligation of the splenic artery. After surgery, the patients were transferred into ICU with tracheal intubation. Basiliximab 20 mg was administered at 2 hours preoperatively and 4 days postoperatively. Methylprednisolone 500 mg was administered during the anhepatic period. Tacrolimus and mycophenolate mofetil were used for anti-rejection at 3 days postoperatively to maintain the concentration of tacrolimus at 8–12 ng/mL.
Statistical Analysis
SPSS 24.0 software was used to analyze the data. P<0.05 was defined as statistical significance. Normally distributed data were expressed as mean ± SD and analyzed by two-sample t-test. Non-normal measurement data were expressed as medians (Q25, Q75), and analyzed with the Mann–Whitney U-test. Dichotomous data were analyzed with a chi-square test. The univariate factors with a P<0.1 were included in the binary logistic regression analysis.
Results
General Information
A total of 159 patients were enrolled (Figure 1). Forty-two were diagnosed with a POD (26.4%) with an average age of 47.38 ± 9.29 years. Our sample included 33 men and nine women (Table 1). A total of 117 patients were classified as non-POD (a control group), including 101 men and 16 women. The average age of the control group was 49.19 ± 8.98 years (Table 1).
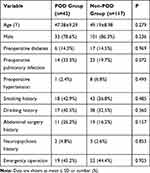 |
Table 1 Patients’ Characteristics |
 |
Figure 1 Flow chart of patients with LT. |
Comparison of Clinical Information Between POD and Non-POD Groups
There was no statistical difference found with patient characteristics, such as age and sex (P>0.05) (Table 1). We analyzed the preoperative information and found the following variables were statistically elevated in the POD group: acute hepatic failure, hepatic encephalopathy, Child-Pugh level C, MELD score ≥15, white blood cells (WBC), percentage of neutrophils (N%), total bilirubin (TBIL), direct bilirubin (DBIL), international normalized ratio (INR), plasma fibrinogen (FIB), and ammonia. Liver cancer in the POD group was significantly lower than in controls (Table 2). Among the intraoperative variables, the following, time of operation, anhepatic period, intraoperative red blood cells and cryoprecipitate, lactic acid, and hypotension, were significant. These variables were significantly higher in the POD group compared to the non-POD group (P<0.05) (Table 3). Postoperative measurements of alanine aminotransferase (ALT), aspartate aminotransferase (AST), TBIL, DBIL, INR, and FIB in the POD group were all significantly higher than those in the non-POD group (P<0.05) (Table 3). Donor characteristics (eg, age, sex) were not significantly different between POD and controls (Table 4).
 |
Table 2 Preoperative Information of LT Patients |
 |
Table 3 Intraoperative and Postoperative Information of LT Patients |
 |
Table 4 Information of Donated Liver |
Risk Factor of POD in LT Patients
We defined POD occurrence as the dependent variable of interest. Independent variables were selected from the univariate tests when the criteria of P<0.1 were met. Findings from the logistic regression were: preoperative ammonia (≥46 vs.<46 μmol/L; OR 3.51, 95% CI [1.31–9.46], P<0.05), MELD score (≥15 vs.<15; OR 3.33, 95% CI [1.27–8.79], P<0.05), presence of hepatic encephalopathy (OR 3.30, 95% CI [1.20–9.07], P<0.05), AST on day one postoperatively (OR 1.33, 95% CI [1.06 −1.68], P<0.05), anhepatic period (OR 1.04, 95% CI [1.02 −1.06], P<0.01). There was no collinearity among those five factors, and the test of goodness for fit for the equation was 84.3% (Table 5).
 |
Table 5 Multivariate Logistic Regression Analysis of Risk of Postoperative Delirium After Liver Transplantation |
Influence of Delirium
Of the patients diagnosed with POD, 78.6% (33/42) showed delirium 3 days postoperatively. The median duration was 5 (3–7) days. Intubation time, ICU length of stay, and medical cost in the POD group were more than those in the non-POD group, but no statistical difference was found in postoperative hospital length of stay (Table 6).
 |
Table 6 Influence of LT POD |
Discussion
POD is a prevalent clinical feature in LT patients postoperatively with a reported incidence of 10.0%-26.7%.5,7 The incidence of LT POD in this study was 26.4%, which was consistent with other studies. Previous studies have shown that alcohol abuse history, hepatic encephalopathy, APACHE II score ≥16, Child-pugh level C, MELD score ≥15, preoperative hemodialysis, and deceased liver donor are potentially related with the occurrence of LT POD.6,8 In this study, we found that hepatic encephalopathy, preoperative ammonia, MELD score, postoperative AST, and anhepatic period were the risk factors for POD via multivariate regression.
Currently, the pathogenesis of POD remains unclear as has been reported to be the result of multiple factors.9 The main physiopathologic mechanisms include blood-brain barrier injury, vascular endothelial cell injury, reduced cholinergic inhibitory receptor, neuroinflammation response, and disordered neurotransmitter.10 A systematic review on neuroimaging reported that white matter atrophy, edema, ischemic lesions, and inflammation in the frontal lobe, limbic system, parietal lobe and, temporal lobe were highly associated with POD.11 The cause for POD in LT patients postoperatively is complicated, which often suggests multiple factors.
Hepatic encephalopathy is reported to be one of the independent risk factors for LT POD.7,8 The physiopathologic mechanism of hepatic encephalopathy is complex and involves hyperammonemia, neuroinflammation reactions, neurotransmitter disorders, brain energy metabolism, and other factors.12 A neuroimaging study has demonstrated that during the development and progression of hepatic encephalopathy, patients will show interstitial edema,13 brain microstructural destruction,14 and white matter atrophy,15 all of which are related to attention deficit and cognitive dysfunction.16 The sites affected are located in the frontal lobe and basal ganglia17 and have similarities with the physiopathologic mechanism of POD, suggesting a role in high POD postoperative incidence in patients with hepatic encephalopathy.
Accumulation of ammonia is one of the main factors for the development and progression of hepatic encephalopathy. When hepatic encephalopathy happens, ammonia is not always increased, and patients with ammonia do not necessarily have hepatic encephalopathy.18 A recent study reported that ammonia had influences on the signaling pathway, gene expression and post-transcription protein modification,19,22 leading to damaged astrocytes, and manifesting as abnormal proliferation,23,24 the release of neurotransmitter22 or even apoptosis.23 As a part of the blood-brain barrier, astrocytes protect neuron from the toxicity of ammonia.25 Damage on this barrier will lead to brain edema.26 The sites of brain edema include the frontal lobe, temporal lobe, parietal lobe, and insular lobe,17,25 which may be related to the occurrence of delirium occurring in patients with hyperammonemia. In this study, we found that there was no correlation between ammonia and hepatic encephalopathy, and that ammonia was an independent risk factor for POD.
In anhepatic period, the whole liver of the recipient is resected. The portal vein, hepatic artery, and inferior vena cava above and below the liver are cut off, leading to hemodynamic changes, such as a decrease in venous return, cardiac output, and systemic circulation arterial pressure. These changes have an influence on cerebral blood perfusion and result in insufficient blood supply in the brain tissue. Meanwhile, metabolites are accumulated in the anhepatic period, resulting in metabolic disorders, such as acidosis, causing ischemia hypoxia injury and delirium.
ALT and AST are often used to assess the extent of damage in hepatocytes.27 In this study, AST was an independent risk factor for POD and AST increasing 1000u/l from the base level, the risk for the patient to suffer delirium increases by 1.33 times. Elevated postoperative AST and ALT possibly are related to the surgical operation and ischemia-reperfusion injury, suggesting the occurrence of delirium is closely related to donated liver injury and postoperative liver function recovery.
The MELD score is used to evaluate the severity of liver disease and acts as a general index for the distribution of liver and predicting mortality of patients waiting for transplant.28 In this study, a MELD score of ≥15 suggested an elevated risk of delirium.
According to related reports, drinking history is considered as one of the risk factors causing POD.8 However, in this study, alcoholic cirrhosis was not a risk factor for LT POD, possibly because there were few patients with alcoholic cirrhosis. The donor data in our center indicated that age of the donor, weight of donated liver, liver function, Na+ concentration, and coagulation function were not statistically different between the POD group and non-POD group.
In this study, the period of 3 days postoperatively has a higher incidence for delirium, during which period 78.6% of patients had developed POD. However, Lee et al,6 reported that 88% of LT POD had the onset on the first day, which differs from our report. The reason was that there were more hepatic encephalopathy patients among LT POD patients (50%, 21/42), and the anesthesia resuscitation time was relatively long, influencing initial delirium evaluation. Wang et al8 reported that the median duration of delirium onset was 5.5 days, which was consistent with our study. In this study, intubation time, ICU length of stay, and medical cost in the group were more than those in the non-POD group.
There is a little study in LT POD of prevention and treatment. Prevention with multicomponent nonpharmacological approaches remains important, such as The Hospital Elder Life Program (HELP).29 There is little evidence on the effects of nonpharmacological prevention. The pharmacological treatment of delirium in the ICU is still mostly based on clinical experience, as there is little high-quality evidence for its efficacy.30
There are some limitations to our study. First, the study is retrospective, and the data are not comprehensive. Some potential information were not collected such as anaesthesia drug. Secondly, CAM-ICU has been widely applied in the clinic, and the experience from doctors and nurses influences the diagnosis of delirium, leading to bias.
Conclusion
Hepatic encephalopathy, high preoperative ammonia, high MELD score, high postoperative AST, and long anhepatic period are the independent risk factors for LT POD occurrence. Enhancing the screening on LT POD patients, carefully monitoring patients with high MELD score or hepatic encephalopathy, and taking effective intervention in a timely manner will be helpful to reduce the occurrence of LT POD.
Acknowledgment
This research was supported by the Natural Science Foundation of Hunan province project (No. 2016JJ3165).
Disclosure
All authors declare no conflicts of interest related to this article.
References
1. Setters B, Solberg LM. Delirium. Prim Care. 2017;44:541–559. doi:10.1016/j.pop.2017.04.010
2. Reddy S, Irkal J, Srinivasamurthy A. Postoperative delirium in elderly citizens and current practice. Anaesthesiol Clin Pharmacol. 2017;33:291–299. doi:10.4103/joacp.JOACP_180_16
3. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5:210–220. doi:10.1038/nrneurol.2009.24
4. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26:277–287. doi:10.1016/j.bpa.2012.07.003
5. Lescot T, Karvellas CJ, Chaudlhury P, et al. Postoperative delirium in the intensive care unit predicts worse outcomes in liver transplant recipients. Can J Gastroenterol. 2013;27:207–212. doi:10.1155/2013/289185
6. Lee H, Oh SY, Yu JH, et al. Risk factors of postoperative delirium in the intensive care unit after liver transplantation. World J Surg. 2018;42:2992–2999. doi:10.1007/s00268-018-4563-4
7. Oliver N, Bohorquez H, Anders S, et al. Post-liver transplant delirium increases mortality and length of stay. Ochsner J. 2017;17:25–30.
8. Wang SH, Wang JY, Lin PY, et al. Predisposing risk factors for delirium in living donor liver transplantation patients in intensive care units. PLoS One. 2014;9:e96676. doi:10.1371/journal.pone.0096676
9. Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11:367–374. doi:10.1513/AnnalsATS.201306-210OC
10. Rengel KF, Pandharipande PP, Hughes CG. Postoperative delirium. Presse Med. 2018;47:e53e64. doi:10.1016/j.lpm.2018.03.012
11. Kalvas LB, Monroe TB. Structural brain changes in delirium: an integrative review. Biol Res Nurs. 2019;21:355–365. doi:10.1177/1099800419849489
12. Butterworth RF. Hepatic encephalopathy in cirrhosis: pathology and pathophysiology. Drugs. 2019;79:17–29. doi:10.1007/s40265-018-1017-0
13. Rovira A, Mínguez B, Aymerich FX, et al. Decreased white matter lesion volume and improved cognitive function after liver transplantation. Hepatology. 2007;46:1485–1490. doi:10.1002/hep.21911
14. Kumar R, Gupta RK, Elderkin-Thompson V, et al. Voxel-based diffusion tensor magnetic resonance imaging evaluation of low-grade hepatic encephalopathy. J Magn Reson Imaging. 2008;27:1061–1068. doi:10.1002/jmri.21342
15. Guevara M, Baccaro ME, Gómez-Ansón B, et al. Cerebral magnetic resonance imaging reveals marked abnormalities of brain tissue density in patients with cirrhosis without overt hepatic encephalopathy. J Hepatol. 2011;55:564–573. doi:10.1016/j.jhep.2010.12.008
16. Montoliu C, Felipo V. Current state of knowledge of hepatic encephalopathy (part II): changes in brain white matter tracts integrity are associated with cognitive deficits in minimal hepatic encephalopathy. Meta Brain Dis. 2016;31:1359–1360. doi:10.1007/s11011-016-9909-8
17. Grover VP, Crossey MM, Fitzpatrick JA, et al. Quantitative magnetic resonance imaging in patients with cirrhosis: a cross-sectional study. Metab Brain Dis. 2016;31:1315–1325. doi:10.1007/s11011-015-9716-7
18. Patel VC, White H, Stoy S, et al. Clinical science workshop: targeting the gut-liver-brain axis. Metab Brain Dis. 2016;31:1327–1337. doi:10.1007/s11011-015-9743-4
19. Wang F, Du T, Liang C, et al. Ammonium increases Ca (2+) signaling and upregulates expression of Cav1.2 gene in astrocytes in primary cultures and in the in vivo brain. Acta Physiol. 2015;214:261–274. doi:10.1111/apha.12500
20. Karababa A, Görg B, Schliess F, et al. O-GlcNAcylation as a novel ammonia-induced posttranslational protein modification cation in cultured rat astrocytes. Metab Brain Dis. 2014;29:975–982. doi:10.1007/s11011-013-9454-7
21. Haack N, DubIin P, Rose CR. Dysbalance of astrocyte calcium under hyperammonemic conditions. PLoS 0ne. 2014;9:e105832. doi:10.1371/journal.pone.0105832
22. Sobczyk K, Jördens MS, Karababa A, et al. Ephrin/Ephrin receptor expression in ammonia-treated rat astrocytes and in human cerebral cortex in hepatic encephalopathy. Neurochem Res. 2015;40:274–283. doi:10.1007/s11064-014-1389-9
23. Görg B, Karababa A, ShafiguIlina A, et al. Ammonia-induced senescence in cultured rat astrocytes and in human cerebral cortex in hepatic encephalopathy. Glia. 2015;63:37–50. doi:10.1002/glia.22731
24. Oenarto J, Karababa A, Castoldi M, et al. Ammonia-induced miRNA expression changes in cultured rat astrocytes. Sci Rep. 2016;6:18493. doi:10.1038/srep18493
25. Oja SS, Saransaari P, Korpi ER. Neurotoxicity of ammonia. Neurochem Res. 2017;42:713–720. doi:10.1007/s11064-016-2014-x
26. Bemeur C, Cudalbu C, Dam G, et al. Brain edema: A valid endpoint for measuring hepatic encephalopathy? Metab Brain Dis. 2016;31:1249–1258. doi:10.1007/s11011-016-9843-9
27. Labayen I, Ruiz JR, Ortega FB, et al. Liver enzymes and clustering cardiometabolic risk factors in European adolescents: the HELENA study. Pediatr Obes. 2015;10:361–370. doi:10.1111/ijpo.273
28. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi:10.1053/gast.2003.50016
29. Hshieh TT, Inouye SK, Oh ES, et al. Delirium in the elderly. Psychiatr Clin North Am. 2018;41(1):1–17. doi:10.1016/j.psc.2017.10.001
30. Serafim RB, Bozza FA, Soares M, et al. Pharmacologic prevention and treatment of delirium in intensive care patients: a systematic review. J Crit Care. 2015;30(4):799–807. doi:10.1016/j.jcrc.2015.04.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
