Back to Journals » Journal of Pain Research » Volume 8
Analysis of opioid-mediated analgesia in Phase III studies of methylnaltrexone for opioid-induced constipation in patients with chronic noncancer pain
Authors Webster LR , Brenner DM, Barrett AC, Paterson C, Bortey E, Forbes WP
Received 8 May 2015
Accepted for publication 24 August 2015
Published 30 October 2015 Volume 2015:8 Pages 771—780
DOI https://doi.org/10.2147/JPR.S88203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Michael Schatman
Lynn R Webster,1 Darren M Brenner,2 Andrew C Barrett,3 Craig Paterson,3 Enoch Bortey,3 William P Forbes3
1PRA Health Sciences, Salt Lake City, UT, 2Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, 3Salix, a Division of Valeant Pharmaceuticals North America LLC, Bridgewater, NJ, USA
Background: Subcutaneous methylnaltrexone is efficacious and well tolerated for opioid-induced constipation (OIC) but may theoretically disrupt opioid-mediated analgesia.
Methods: Opioid use, pain intensity, and opioid withdrawal (Objective Opioid Withdrawal Scale [OOWS] and Subjective Opiate Withdrawal Scale [SOWS] scores) were reported in a randomized, double-blind trial with an open-label extension (RCT) and an open-label trial (OLT) evaluating safety in adults with chronic noncancer pain. In the RCT, patients taking ≥50 mg of oral morphine equivalents daily with <3 rescue-free bowel movements weekly received methylnaltrexone 12 mg once daily (n=150), every other day (n=148), or placebo (n=162) for 4 weeks, followed by open-label methylnaltrexone 12 mg (as needed [prn]; n=364) for 8 weeks. In the OLT, patients (n=1,034) on stable opioid doses with OIC received methylnaltrexone 12 mg prn for up to 48 weeks.
Results: Minimal fluctuations of median morphine equivalent dose from baseline (BL) were observed in the RCT double-blind period (BL, 154.8–161.0 mg/d; range, 137.1–168.0 mg/d), RCT open-label period (BL, 156.3–174.6; range, 144.0–180.0) and OLT (BL, 120 mg/d; range, 117.3–121.1 mg/d). No significant change from BL in pain intensity score occurred in any group at weeks 2 or 4 (both P≥0.1) of the RCT double-blind period, and scores remained stable during the open-label period and in the OLT (mean change, —0.2 to 0.1). Changes from BL in OOWS and SOWS scores during the double-blind period were not significantly impacted by methylnaltrexone exposure at weeks 2 or 4 (P>0.05 for all).
Conclusion: Methylnaltrexone did not affect opioid-mediated analgesia in patients with chronic noncancer pain and OIC.
Keywords: Relistor, mu-opioid receptor, antagonist, opioids, tolerance, withdrawal
Corrigendum for this paper has been published.
Introduction
It has long been recognized that opioid analgesics are effective for treating moderate-to-severe chronic noncancer pain (CNCP) and are among the most commonly prescribed medications for this indication.1,2 Opioid-induced constipation (OIC) is a common, often problematic adverse effect of opioid therapy and, unlike other adverse effects (eg, nausea), patients rarely develop tolerance to OIC.3,4 The reported prevalence of OIC in patients with CNCP varies based on the definition used and the specific patient population, but has typically been reported to be >40%.1,4–6 A systematic review of eleven studies of opioid therapy (administered from 4 days to 8 weeks) reported that for every three patients treated with opioids, one would be more constipated than a patient who received placebo (relative risk, 3.6 [95% confidence interval {CI}, 2.7–4.7]).1
OIC can compromise pain management, with gastrointestinal (GI) adverse effects causing patients to skip or reduce their opioid doses, resulting in inadequate pain control.4,5,7 Real-world clinical data also indicate a negative impact of OIC on activities of daily living and work productivity (ie, presenteeism).8 Over-the-counter agents (eg, laxatives) are generally unsatisfactory for relieving OIC4–6,8 because they do not target the underlying cause – μ-opioid receptor activation in the GI tract.9,10
Methylnaltrexone, a derivative of naltrexone, is a selective, peripherally acting μ-opioid receptor antagonist that inhibits opioid-induced increases in orocecal transit time and gastric emptying.11–13 The efficacy and safety of methylnaltrexone for the treatment of OIC have been demonstrated in patients with advanced illness receiving palliative care.14–19 Additionally, in a randomized, controlled study in patients with CNCP, a rescue-free bowel movement (RFBM) within 4 hours of the first dose (the primary efficacy endpoint) was achieved in 34.2% of patients who received methylnaltrexone 12 mg once daily (qd) or every other day (qod) versus 9.9% of patients who received placebo (P<0.001);13 similar results were observed during an open-label extension of the trial, during which patients received methylnaltrexone 12 mg as needed (prn).13
Methylnaltrexone at therapeutic doses does not appear to interfere with the central analgesic effects of opioid pain medications,20 and it has not been known to precipitate withdrawal when concomitantly administered with opioids.14–17 However, based on data from studies in animals, it is theoretically possible that analgesia could be affected by feedback to the central nervous system from peripheral neurons.21 Indeed, preliminary indirect data suggest a potential influence of supratherapeutic doses of methylnaltrexone (≥45 mg/kg) on psychopharmacologic actions of morphine.22
The objective of this study was to examine the potential effects of methylnaltrexone (in a dose of 12 mg) on opioid medication use, opioid analgesia, and opioid withdrawal symptoms in patients with CNCP and OIC, who participated in a Phase III, randomized, placebo-controlled trial (RCT)13 and a Phase III, open-label trial (OLT) of methylnaltrexone.
Methods
Patient population
Randomized, placebo-controlled trial
The patient population and the study design of the RCT have been previously published.13 Briefly, adults who had a history of chronic noncancer-related pain of ≥2 months, who also had received oral, transdermal, or subcutaneous opioids (average daily dose of ≥50 mg oral morphine equivalents for ≥2 weeks) for at least 1 month’s duration, were screened. Patients were eligible for study inclusion if they had <3 RFBMs per week and ≥1 of the following: hard or lumpy stools, straining during bowel movements (BMs), or sensation of incomplete evacuation. An RFBM was defined as any BM occurring without laxative use within the previous 24 hours. Patients with inflammatory bowel disease, evidence of bowel obstruction or impaction, and those with a history of rectal bleeding, malignancy (within the previous 5 years), or chronic constipation occurring before beginning opioid therapy were excluded. Patients who had received subcutaneous methylnaltrexone in the past, those who were breast-feeding or pregnant, and those who had a history of drug or alcohol abuse (within the previous year) were also ineligible for participation.
Open-label trial
Key patient inclusion and exclusion criteria were similar to those of the RCT.13 Ambulatory patients ≥18 years of age who had experienced pain unrelated to a malignant condition for >2 months, who were receiving daily oral, transdermal, intravenous, or subcutaneous opioids, and who had OIC ≥1 month prior were screened. OIC was defined as having ≥2 of the following: hard or lumpy stools for ≥25% of BMs; straining during ≥25% of BMs; a sensation of incomplete evacuation after ≥25% of BMs; facilitation of a BM by manual maneuvers ≥25% of the time; or <3 RFBMs per week. Patients who were pregnant or breast-feeding and those who had a diagnosis of clinically significant GI disorder (eg, bowel obstruction, fecal incontinence, rectal prolapse) were not allowed to participate. Patients were also ineligible if they had a history of drug or alcohol abuse during the previous year or had a history of inflammatory bowel disease, irritable bowel syndrome, megacolon, rectal bleeding, malignancy, or chronic constipation occurring before the onset of opioid therapy.
Study design
Both trials were conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and the ethical principles according to the Declaration of Helsinki. All patients provided written informed consent.
Randomized, placebo-controlled trial
The randomized, double-blind, multicenter, Phase III trial was conducted at 91 sites in the USA and Canada between August 2007 and November 2008 (ClinicalTrials.gov identifier: NCT00529087). The study consisted of a 2-week screening period, two sequential treatment phases (4-week double-blind period and 8-week open-label extension period), and a 2-week, posttreatment follow-up. During the double-blind period, eligible patients were randomized according to computer-generated, block design, randomization schedule in a 1:1:1 ratio to receive subcutaneous methylnaltrexone 12 mg qd, methylnaltrexone 12 mg qod, or placebo for 4 weeks. All patients, investigators, and study personnel were blinded to treatment. Patients who completed the double-blind period were eligible for treatment with subcutaneous methylnaltrexone 12 mg prn (max, 1 dose/d) during the 8-week open-label phase.
Open-label trial
In a separate, multicenter, Phase III, open-label safety trial conducted at 120 sites worldwide from December 3, 2008 to September 20, 2010 (ClinicalTrials.gov identifier: NCT00804141), patients received subcutaneous methylnaltrexone 12 mg prn (minimum, 1 dose/wk; maximum, 1 dose/d) for up to 48 weeks and were then followed for 2 weeks posttreatment.
Efficacy endpoints and safety assessments
Daily morphine equivalent dose
Dose adjustments and addition of opioid agents for breakthrough pain were permitted and recorded throughout the RCT and OLT. The average of daily oral morphine equivalent dose (MED) was calculated as the sum of the total oral MED in a given interval of time divided by the number of days within that interval. If no dose was recorded by the patient on a certain day, it was assumed that the opioid dose administered was zero.
Pain intensity
In the RCT and OLT, average pain ratings during the previous 24 hours were assessed using the 11-point pain intensity scale (score: 0= no pain, 10= worst possible pain).23 Assessments were performed at baseline (BL), day 1, weeks 2 and 4 (double-blind period), and at weeks 6, 8, and 12 (open-label period) during the RCT. In the OLT, average pain rating was performed at BL, at weeks 4, 8, 12, 16, 24, 32, 40, 48, and either at the time of study discontinuation or during a follow-up visit approximately 2 weeks after the end of the treatment phase.
Opioid withdrawal symptoms
Opioid withdrawal was evaluated by the Objective Opioid Withdrawal Scale (OOWS) and the Subjective Opiate Withdrawal Scale (SOWS) scoring systems.24 The OOWS consists of 13 items that assess common motor and autonomic signs of opiate withdrawal, and scoring (range, 0–13) is conducted by an observer. The SOWS is a 16-item, patient-reported scoring system that rates the severity of common motor, autonomic, GI, musculoskeletal, and psychic symptoms associated with opiate withdrawal.24 Each item is scored from 0 (not at all) to 4 (extremely), with a maximum total score of 64.24 Because abdominal cramping, which is a withdrawal symptom typically included in the total scores of the OOWS and SOWS and may be associated with constipation, is also common with administration of methylnaltrexone, it was considered a potentially confounding factor. Therefore, OOWS and SOWS scores in the RCT and in the OLT were calculated both with and without items related to abdominal cramping.
In the RCT, OOWS and SOWS scores were determined at BL before administration of methylnaltrexone, 1 hour after drug administration, and during the double-blind period at weeks 2 and 4. The SOWS score was also determined during weeks 6, 8, and 12 of the open-label period. In the OLT, OOWS and SOWS were performed on day 1 before administration of treatment and approximately 1 hour after administration.
Statistical analyses
In the double-blind period of the RCT, analyses involved the modified intention-to-treat population, which included all patients who were randomly assigned to treatment (ie, the intent-to-treat population) and received ≥1 dose of study drug. During the open-label period of the RCT, analyses included all patients who received ≥1 dose of study drug. Analyses were conducted using observed data (ie, no imputation). An analysis of covariance model with treatment as a factor and BL as covariate was used to compare morphine dose equivalent, pain intensity, and OOWS and SOWS between-treatment groups in the double-blind period. All variables ascertained during the open-label period were reported as descriptive statistics. Analyses of the change from BL pain intensity score during the open-label period were conducted using a paired t-test. Sample size for this study was based on primary and secondary endpoints and has been previously reported.13 Briefly, to achieve a statistical power of 95% to detect a treatment effect of 15% in the number of patients who had an RFBM within 4 hours after the first dose of study medication, using a two-sided chi-square test with a significance level of 0.05, it was determined that 314 and 157 patients were required in the methylnaltrexone and placebo groups, respectively.
In the OLT, analyses included all patients who received ≥1 dose of methylnaltrexone, and the analyses were performed on observed data. A formal power calculation was not performed for the OLT; however, it was anticipated that at least 1,000 patients would be required to obtain 300 patients for 6-month exposure and 100 patients for the 1-year exposure groups.
Results
Patient disposition and demographics
In the RCT, 469 of the 1,037 (45.2%) patients screened were included in the study and randomly assigned to treatment with methylnaltrexone 12 mg qd, methylnaltrexone 12 mg qod, or placebo for 4 weeks (Figure 1A).13 Of these, 460 patients received ≥1 dose of study medication; most (n=388; 84.3%) of the 460 patients completed the double-blind phase.13 The majority of patients (95.9%) who completed the double-blind phase were then enrolled in the open-label phase, but only 364 patients (93.8%) received ≥1 dose of study medication in the open-label phase, and of these, 303 (83.2%) completed the trial (Figure 1A). The most common reason for discontinuation in the RCT and open-label phase was adverse events (AEs), of which abdominal pain was the most common, causing discontinuations in 2.0% and 3.4% of patients who received methylnaltrexone qd and qod, respectively,13 and in 1.1% of patients in the open-label phase. In the OLT, 1,034 of 1,673 (61.8%) patients screened were enrolled and received ≥1 dose of methylnaltrexone (Figure 1B). Of those, 477 patients (46.1%) completed the study; experiencing an AE was the most common reason for study discontinuation. The most common AEs causing discontinuation were abdominal pain (4.7%), nausea (2.5%), and diarrhea (2.3%).
  | Figure 1 Patient disposition in the RCT (A) and OLT (B). |
Demographics and BL characteristics were similar across the three treatment groups in the RCT13 and between the RCT and OLT studies (Table 1).13 However, the total median BL MED across the three treatment groups (n=460) in the double-blind phase of the RCT was higher (160.0 mg/d) compared with the OLT (120.0 mg/d). In both phases of the RCT and in the OLT, most patients were white and female, with back pain reported as the primary pain condition (Table 1). The most commonly prescribed opioids during the RCT double-blind period across the three treatment groups (n=460) were oxycodone (n=93, 20.2%), methadone (n=86, 18.7%), and hydrocodone (n=66, 14.3%); these medications were also the opioids most commonly used by patients (n=364) during the open-label phase (oxycodone: n=85, 23.4%; methadone: n=71, 19.5%; hydrocodone: n=58, 15.9%). In the double-blind period of the RCT, use of hydrocodone (range, 12.7%–15.5%) and oxycodone (range, 18.2%–23.3%) was similar among the treatment groups. Methadone use was greater in the methylnaltrexone 12 mg qd (20.7%) and placebo (22.2%) groups versus the methylnaltrexone 12 mg qod group (12.8%). The most commonly prescribed opioids during the OLT (n=1,034) were morphine sulfate (n=232, 22.4%), oxycodone (n=214, 20.7%), and methadone (n=153, 14.8%).
  | Table 1 Demographics and baseline characteristics |
Median daily morphine equivalent dose
In the RCT, the median daily MED (assessed weekly) showed minimal fluctuations during both the double-blind and open-label periods (Figure 2A); however, a significant increase from BL in median daily MED was observed with methylnaltrexone 12 mg qod during week 1 of the double-blind period versus placebo (P<0.02). In the open-label period, median daily MED remained constant (methylnaltrexone 12 mg qd: range, 150.0–180.0 mg/d; methylnaltrexone qod: range, 144.0–162.6 mg/d; placebo: range, 160.0–180.0 mg/d) and was not impacted by treatment assignment during the double-blind phase. In the OLT, median daily MED (assessed monthly) also remained unchanged from BL, ranging from 117.3 to 121.1 mg/d (Figure 2B).
Pain intensity
In the RCT, mean pain intensity scores for methylnaltrexone qd and qod exhibited no significant changes from BL at weeks 2 and 4 of the double-blind period compared with placebo (Table 2). Mean pain intensity scores also remained stable with methylnaltrexone 12 mg during the open-label period (mean change from BL at week 12, −0.2; P=0.1 versus BL). In addition, there were no differences in pain intensity scores during the open-label treatment when evaluated by prior treatment during the RCT. Consistent with results observed during the RCT, mean pain intensity scores were unchanged from BL up to 48 weeks of treatment in the OLT (Table 3).
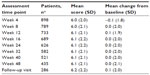  | Table 3 Change from baseline in pain intensity during OLT |
Opioid withdrawal symptoms
During the RCT double-blind phase, minimal changes in OOWS scores were observed (Table 4). Similar trends in OOWS scores were observed when scores were calculated with and without abdominal-cramping-related items. At BL (day 1), when the OOWS score was determined prior to and 1 hour after initial drug exposure, patients who received methylnaltrexone 12 mg qod had slightly greater withdrawal symptoms 1 hour after drug exposure compared with placebo (mean score difference [95% CI], 0.4 [0.2–0.6] and 0.3 [0.1–0.5] with and without abdominal-cramping-related items, respectively; P<0.001 for both). No other statistically significant differences in OOWS score were observed in other treatment groups or throughout the RCT.
Changes from BL in SOWS scores were small and tended to slightly fluctuate throughout the RCT double-blind period (Table 5). As observed with OOWS, overall trends were similar with SOWS scores calculated with and without abdominal-cramping-related items. At BL, 1 hour after first drug treatment, a significant difference in SOWS score versus placebo was observed in patients who received methylnaltrexone 12 mg qod (difference [95% CI]: 2.1 [0.6–3.7], P<0.01 and 1.7 [0.2–3.1], P<0.03, with and without abdominal-cramping-related items, respectively); such differences versus placebo were not observed at any other time during the RCT double-blind period for methylnaltrexone 12 mg qod.
During the OLT, the mean change from BL in OOWS score (mean difference [standard deviation {SD}], 0.2 [1.1] and 0.1 [0.9], with and without abdominal-cramping-related items, respectively) and SOWS score (mean difference [SD], −2.5 [7.1] and −2.8 [6.6], respectively) was small.
Discussion
The stability of median daily MED and pain intensity scores and the lack of any clinically meaningful central opioid withdrawal effects with methylnaltrexone in both RCT and OLT indicate that methylnaltrexone treatment for OIC in patients with CNCP does not reduce opioid-mediated analgesia or precipitate opioid withdrawal. These results are consistent with previous reports that demonstrated a lack of effect on analgesia and central withdrawal with intravenous11 and subcutaneous14–17,25 methylnaltrexone in patients receiving opioids.
Opioid withdrawal syndrome is a multifaceted symptom complex including central (altered heart rate, anxiety, or irritability) and peripheral (bone or joint aches, GI upset) withdrawal symptoms. Methylnaltrexone antagonizes μ-opioid receptors in the GI tract (periphery), but has limited effects on central opioid receptors because of its polarity and lipid solubility, which restricts its ability to cross the blood–brain barrier. In the current study, opioid dosage required for pain relief, pain intensity scores, and objective and subjective measures of pain were generally unaffected by methylnaltrexone, supporting its lack of centrally mediated effects. A single significant between-group difference in OOWS and SOWS scores was reported at week 1 in the methylnaltrexone qod group versus placebo, but, because no difference was observed in the methylnaltrexone qd group (ie, patients who had a higher cumulative methylnaltrexone exposure) and the difference was not apparent after week 1, this was likely an artifact. Observable effects on central manifestations of withdrawal (eg, anxiety) were minimal in the RCT13 and OLT,26 but other effects linked to opioid withdrawal (abdominal pain) were reported. However, abdominal pain may be related to the normal, propulsive effects of a BM; therefore, it is a potential confounder when attempting to ascertain whether such GI AEs might be related to opioid withdrawal or merely to methylnaltrexone’s activity of BM induction. Interestingly, a post hoc analysis18 of two randomized, placebo-controlled studies of OIC in patients with advanced illness14,17 showed that the incidence of abdominal pain decreased after the first dose of methylnaltrexone, while response to methylnaltrexone treatment was maintained. The similarity between OOWS and SOWS scores calculated with and without abdominal-cramping-related items in the current study suggests that abdominal symptoms that may be associated with methylnaltrexone did not significantly alter overall patient pain perception.
The RCT double-blind period was of relatively short duration (ie, 6 weeks), thereby limiting conclusions regarding long-term effects of methylnaltrexone on opioid analgesia compared with placebo; however, the similarity of the results from the RCT double-blind (ie, 4-week duration) and open-label (8-week duration) periods and those from the OLT (48-week duration) supports the conclusion that analgesia would not be affected with long-term methylnaltrexone use. The current study also did not assess OOWS and SOWS throughout the OLT. Again, this limits the long-term conclusions that may be drawn from the study. However, the impact of methylnaltrexone administration on OOWS and SOWS scores would be anticipated to be observable within the short, postinjection time frame in which the OOWS and SOWS were administered in the OLT. The lack of any alterations in OOWS and SOWS supports the longer term data reported in the RCT.
Conclusion
Results indicated no demonstrable effects of the peripherally acting μ-opioid receptor antagonist methylnaltrexone on opioid-mediated analgesia. Thus, methylnaltrexone may be considered as an option for the treatment of OIC, without significant concerns of compromising pain management strategies in patients with CNCP.
Acknowledgments
Technical editorial and medical writing assistance, under the direction of the authors, was provided by Mary Beth Moncrief, PhD, and Jillian R Gee, PhD, Synchrony Medical Communications, LLC, West Chester, PA, USA. Funding for this support was provided by Salix, a Division of Valeant Pharmaceuticals North America LLC, Bridgewater, NJ, USA.
The trial was funded by Wyeth Research, which was acquired by Pfizer Inc. in October 2009. The current analyses were supported by Salix.
Author Contributions
All authors contributed toward data analysis, drafting and critically revising the paper. The authors all had complete access to the data and approved the final draft of the manuscript. They agree to be accountable for all aspects of the work.
Disclosure
Lynn R Webster has received financial support from BioDelivery Sciences International, Raleigh, NC, USA; Cara Therapeutics, Shelton, CT, USA; Charleston Labs, Jupiter, FL, USA; Collegium Pharmaceutical, Canton, MA, USA; Egalet Corporation, Wayne, PA, USA; Grünenthal USA, Bedminster Township, NJ, USA; Inspirion, Basking Ridge, NJ, USA; Insys Therapeutics, Chandler, AZ, USA; Jazz Pharmaceuticals, Palo Alto, CA, USA; Kaléo Pharmaceuticals, Richmond, VA, USA; Mallinckrodt Pharmaceuticals, St. Louis, MO, USA; Nektar Therapeutics, San Francisco, CA, USA; Orexo, Morristown, NJ, USA; Pfizer, New York, NY, USA; Signature Therapeutics, Palo Alto, CA, USA; Teva, Malvern, PA, USA; Trevena, Exton, PA, USA; and Zogenix, San Diego, CA, USA.
Darren M Brenner is a consultant and/or speaker for Salix, a Division of Valeant Pharmaceuticals North America LLC, Bridgewater, NJ, USA; Forest Laboratories (acquired by Actavis), New York, NY, USA; Ironwood Pharmaceuticals, Cambridge, MA, USA; Procter & Gamble, Cincinnati, OH, USA; AstraZeneca, Wilmington, DE, USA; and the Gi Health Foundation Clark, NJ, USA.
Andrew C Barrett, Craig Paterson, Enoch Bortey, and William P Forbes are former employees of Salix. The authors report no other conflicts of interest in this work.
References
Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380. | |
Bader S, Jaroslawski K, Blum HE, Becker G. Opioid-induced constipation in advanced illness: safety and efficacy of methylnaltrexone bromide. Clin Med Insights Oncol. 2011;5:201–211. | |
Clemens KE, Klaschik E. Managing opioid-induced constipation in advanced illness: focus on methylnaltrexone bromide. Ther Clin Risk Manag. 2010;6:77–82. | |
Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European patient survey (PROBE 1). Pain Med. 2009;10(1):35–42. | |
Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27(12):1224–1232. | |
Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182(5A Suppl):11S–18S. | |
Noble M, Tregear SJ, Treadwell JR, Schoelles K. Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J Pain Symptom Manage. 2008;35(2):214–228. | |
Coyne KS, LoCasale RJ, Datto CJ, Sexton CC, Yeomans K, Tack J. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res. 2014;6:269–281. | |
Tavani A, Bianchi G, Ferretti P, Manara L. Morphine is most effective on gastrointestinal propulsion in rats by intraperitoneal route: evidence for local action. Life Sci. 1980;27(23):2211–2217. | |
Manara L, Bianchi G, Ferretti P, Tavani A. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J Pharmacol Exp Ther. 1986;237(3):945–949. | |
Yuan CS, Foss JF, O’Connor M, Toledano A, Roizen MF, Moss J. Methylnaltrexone prevents morphine-induced delay in oral-cecal transit time without affecting analgesia: a double-blind randomized placebo-controlled trial. Clin Pharmacol Ther. 1996;59(4):469–475. | |
Murphy DB, Sutton JA, Prescott LF, Murphy MB. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology. 1997;87(4):765–770. | |
Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain. 2011; 12(5):554–562. | |
Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008; 358(22):2332–2343. | |
Lipman AG, Karver S, Cooney GA, Stambler N, Israel RJ. Methylnaltrexone for opioid-induced constipation in patients with advanced illness: a 3-month open-label treatment extension study. J Pain Palliat Care Pharmacother. 2011;25(2):136–145. | |
Chamberlain BH, Cross K, Winston JL, et al. Methylnaltrexone treatment of opioid-induced constipation in patients with advanced illness. J Pain Symptom Manage. 2009;38(5):683–690. | |
Slatkin N, Thomas J, Lipman AG, et al. Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J Support Oncol. 2009;7(1):39–46. | |
Slatkin NE, Lynn R, Su C, Wang W, Israel RJ. Characterization of abdominal pain during methylnaltrexone treatment of opioid-induced constipation in advanced illness: a post hoc analysis of two clinical trials. J Pain Symptom Manage. 2011;42(5):754–760. | |
Nalamachu SR, Pergolizzi J, Taylor R Jr, et al. Efficacy and tolerability of subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: a responder analysis of 2 randomized, placebo-controlled trials. Pain Pract. 2015;15(6):564–571. | |
Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther. 2007;82(1):48–53. | |
Yuan CS, Foss JF. Gastric effects of methylnaltrexone on μ, κ, and δ opioid agonists induced brainstem unitary responses. Neuropharmacology. 1999;38(3):425–432. | |
Zacny JP, Wroblewski K, Coalson DW. Methylnaltrexone: its pharmacological effects alone and effects on morphine in healthy volunteers. Psychopharmacology (Berl). 2015;232(1):63–73. | |
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. | |
Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13(3):293–308. | |
Yu CS, Chun HK, Stambler N, et al. Safety and efficacy of methylnaltrexone in shortening the duration of postoperative ileus following segmental colectomy: results of two randomized, placebo-controlled phase 3 trials. Dis Colon Rectum. 2011;54(5):570–578. | |
Webster L, Michna E, Khan A, et al. The long-term efficacy of subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with chronic nonmalignant pain. J Pain. 2011; 12(Suppl 4):P70. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.