Back to Journals » Journal of Blood Medicine » Volume 13
Analysis of JAK2V617F Tyrosine Kinase Mutation in Blood Donors with Erythrocytosis – A Pilot Study in a Tertiary Care Teaching Hospital of South India
Authors Gaddam M, Prakash P, Devegowda D, Kumar R
Received 13 April 2022
Accepted for publication 14 July 2022
Published 13 August 2022 Volume 2022:13 Pages 439—446
DOI https://doi.org/10.2147/JBM.S370687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Manasa Gaddam,1 Pallavi Prakash,2 Devananda Devegowda,3 Ravindran Kumar4
1Department of Pathology, JSS Medical College, JSSAHER, Mysuru, Karnataka, India; 2Transfusion Medicine and Blood Center, Department of Pathology, JSS Medical College, JSSAHER, Mysuru, Karnataka, India; 3Department of Biochemistry, JSS Medical College, JSSAHER, Mysuru, Karnataka, India; 4Department of Microbiology, JSS Medical College, JSSAHER, Mysuru, Karnataka, India
Correspondence: Pallavi Prakash, Transfusion Medicine and Blood center, Department of Pathology, JSS Medical College, JSSAHER, Mysuru, Karnataka, 570007, India, Tel +91 9986649850, Fax +91 821-2548345 ; +91 821-2493819, Email [email protected]
Purpose: Transfusion services and blood centers provide immediate medical evaluation to blood donors by physical examination and hemoglobin (Hb) screening. Screening for Hb value before every blood donation is mainly aimed to rule out anemia. However, it is not uncommon to defer the donors for high Hb value which can be due to primary or secondary polycythemia. This study aimed to analyze the frequency of JAK2V617F mutation among blood donors with a high Hb of > 18 g/dl.
Patients and Methods: A prospective study was conducted over a period of 18 months involving blood donors with a persistently high Hb value of > 18 g/dl. Complete blood count (CBC), JAK2V617F gene mutation and Serum Erythropoietin (EPO) levels in study donors were analyzed. Descriptive statistical analysis was performed using SPSS, version 24 (IBM, USA).
Results: Of 13,798 screened donors, 48 donors (0.34%) had persistent erythrocytosis with a high Hb value of > 18 g/dl. Their age ranged between 20– 50 years with a mean of 31.2 ± 6.66. The CBC parameters including red blood cell (RBC) count, Hb%, hematocrit (Hct), white blood cell (WBC) count and the platelet count ranged from 4.35– 8.43 million/μL (6.2 ± 0.6), 18.6– 24.4 g/dl (19 ± 0.94), 51.9– 83.3% (58 ± 5.02), 3.99– 10.8 × 103/μL (7.8 ± 1.5), and 120– 450 × 103/μL (227 ± 57.2), respectively. Estimated mean EPO value was 8.29 mIU/± 0.04. JAK2V617F mutation was detected in 2 donors (4.1%).
Conclusion: The prevalence of persistent erythrocytosis among blood donors was 0.34% and among them, two donors (4.1%) harbored the JAK2V617F mutation. Thus, blood centers play an important role in the primary screening of donors with high hemoglobin leading to early detection and management of polycythemia vera (PV).
Keywords: blood donors, high hemoglobin, erythropoietin, polycythemia vera
Introduction
Blood donation is an important part of healthcare all over the world and is a life-sustaining and life-saving procedure. More than one hundred million units of blood are donated each year all over the world.1 Blood donors have to undergo their hemoglobin (Hb) values being checked before every blood donation to rule out anemia, which is the most frequent cause of a temporary deferral. However, it is not uncommon to observe Hb values above or near the upper limit of the normal reference range.2
Erythrocytosis is defined as an increase in the number of erythrocytes in the whole blood. Erythrocytosis refers to a red blood cell (RBC) count above the sex-specific normal range.3 It is suspected when the individual presents with a Hb >16.5 g/dl in men and >16.0 g/dl in women or hematocrit (Hct) >49% in men and >48% in women or an increase in red cell mass (RCM) of >25% above the mean predicted value.4 Erythrocytosis and polycythemia are frequently used synonymously. The term polycythemia refers to a group of disorders, typically characterized by persistent elevation in packed cell volume (PCV) due to an increase in the number of RBCs in the peripheral blood circulation.5
Erythrocytosis can be classified into relative or absolute. Relative erythrocytosis occurs due to a decrease in the plasma volume and absolute erythrocytosis is due to an increase in RCM.6 The etiology for an absolute erythrocytosis can be further subdivided into primary (low erythropoietin) and secondary causes (high erythropoietin). Primary causes are due to intrinsic problems in the bone marrow which leads to an increase in erythroid production. The primary causes can be further subdivided into congenital and acquired. Secondary causes are due to physiological or pathological processes, outside the bone marrow which leads to the accelerated process of erythropoiesis by means of increased erythropoietin secretion; these conditions can also be further subdivided into congenital and acquired.7
High Hb may be a sign of an underlying disease and can pose a risk for vascular accidents and investigations must be performed to rule out the primary disease.8 Studies suggest that some blood donors with erythrocytosis may have hidden pathologic conditions like polycythemia vera (PV). Studies have shown that the JAK2V617F mutation has been detected in about 65–97% of patients with PV.5 Based on these findings, the first step in the algorithm proposed for diagnosing PV in cases with erythrocytosis is the screening for the JAK2V617F mutation.2 Thus, this study aimed to analyze the frequency of the JAK2V617F mutation among blood donors with high Hb >18 g/dl. The study will contribute to the screening of blood donors, to detect or diagnose the medical problems associated with high Hb values. Since there is a paucity of data available on blood donors with erythrocytosis in India, it is hoped that the results of this study can be used as one of the referral data for future research.
Materials and Methods
Study Design and Setting
A prospective exploratory study to analyze the JAK2V617F mutation among blood donors with high Hb was conducted in the department of Transfusion Medicine and Blood Center of an 1800 bedded university teaching hospital of southern India between November 2019 to May 2021 (18 months). The study was reviewed and approved by the institutional ethical committee of JSS Medical College, JSS Academy of Higher Education and Research in accordance with the Declaration of Helsinki. (Reference:- IEC-419/2019). Written informed consent was obtained from all individual participants included in the study.
Inclusion and Exclusion Criteria
Blood donors with persistent erythrocytosis of Hb >18 g/dl were included in the study. Donors with erythrocytosis who did not have Hb >18 g/dl following repeat blood count after 3 months or those who did not follow up were excluded from the study.
Study Methods
All the donors coming to the blood center during the study period were subjected to pre-donation counseling and screening tests for fitness as per the departmental standard operating procedure (SOP) for donor selection. This SOP is written with reference to the Drugs and Cosmetics Act (the rules thereunder) and supplemented by the Directorate General of Health Services and National AIDS (Acquired Immune Deficiency Syndrome) Control Organization (NACO) guidelines, including their amendments.7,9 During the screening process the donors were evaluated by entering details in donor registration cum consent forms which included a questionnaire, a brief physical examination by a medical officer followed by Hb estimation. Hb estimation was performed by an automated hematology analyzer (Sysmex XN-1000) and recorded.
As per the SOP of donor deferral, donors with a high Hb value (>18 g/dl) were temporarily deferred and counselled. A detailed history was taken from donors with emphasis on their history of smoking, alcohol consumption and travel to high altitude. All donors deferred due to high Hb were followed up for repeat complete blood count (CBC) after 3 months. During the follow up, donors with Hb >18 g/dl were enrolled in the study (Figure 1) and written informed consent was obtained from the donors by the investigators. Venous blood samples (3 mL in ethylenediamine tetraacetic acid (EDTA) vacutainer and 3 mL in plain vacutainer) were collected for the JAK2V617F mutation study and erythropoietin (EPO) assay from the donors meeting the inclusion criteria.
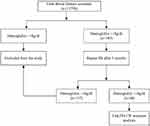 |
Figure 1 Algorithm showing the recruitment of study population. |
The EDTA samples from the donors were centrifuged and the buffy coat was separated. Deoxyribonucleic acid (DNA) was extracted from the buffy coat sample using the DNA extraction kit manufactured by Spinstar Total DNA kit (ADT Biotech Sdn Bhd, Selangor DE, Malaysia) as per the kit instructions and stored at −80 °C till further use. A blood sample from a known PV patient with JAK2V617F mutation was selected as a positive control for the RT-PCR study. Serum was separated by centrifugation of clotted blood sample and stored at −80 °C for EPO estimation. The donor DNA samples were subjected to JAK2V617F mutation testing by the OncoscreenT JAK2V617F Mutation Detection Kit from the manufacturer Mylab Discovery Solutions (Maharashtra, India). EPO level was detected using the ELISA method from the manufacturer Biomerica (California, United States of America) according to the kit instructions. The EPO normal range was taken to be 3.22–31.9 mIU/mL. The CBC of randomly selected 200 donors not meeting the inclusion criteria were analyzed as controls.
Statistical Analysis
The obtained data was entered into MS Excel. SPSS computer program version 24 was used for statistical analysis. Descriptive statistical analysis was used for frequency of the CBC parameters of donors with persistent erythrocytosis. Independent sample t-test was used to compare the CBC parameters between patients with a sustained erythrocytosis and randomly selected normal donors. A p-value of less than 0.05 was considered statistically significant.
Results
A total of 13,798 blood donors were screened during the study period and among them a majority (98.8%) were men. Of the screened donors, 962 (6.9%) donors were deferred from donating blood for various reasons like low Hb, high Hb, consumption of alcohol, hypertension and medical reasons like cold, fever, dengue, asthma in decreasing order of frequency. High hemoglobin value (Hb >18 g/dl) was noted in 185 (1.3%) deferred donors and a majority of them (99.4%) were male donors. Among 185 donors, their history of smoking, alcohol intake and decreased intake of water throughout the day was noted in 27%, 25%, and 13% respectively. None of the donors had a history of heart or respiratory diseases, abnormal bleeding tendencies, sleep disorders or any drug intake.
Among the 185 deferred donors, 48 (25.9%) had persistent erythrocytosis on follow up and their samples were analyzed for the JAK2V617F mutation. The descriptive statistics of the 48 subjects with persistent erythrocytosis is shown in Table 1. Of the 48 cases, two cases (4.1%) harbored the JAK2V617F mutation (Figure 2). Their serum EPO levels ranged from 1.29–22.46 mIU/mL and the mean EPO was 8.75 mIU/mL with a standard deviation (SD) of 2.67. Comparison of hematological parameters between donors with persistent erythrocytosis (48 cases) and randomly selected healthy donors showed statistically significant differences in Hb, packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelet count values (Table 2). The details of the two donors with JAK2V617F mutation are as shown in the table (Table 3).
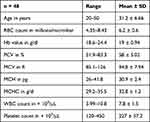 |
Table 1 Descriptive Statistics of Donors with Persistent Erythrocytosis |
 |
Table 2 Complete Blood Count Parameters of Donors with Persistent Erythrocytosis and Healthy Blood Donors |
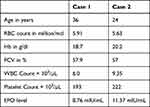 |
Table 3 Details of Donors with Positive JAK2V617F Mutation |
Discussion
Donor screening at blood centers is conducted in accordance with the Drug and Cosmetic Act (D&C act) regulations, as well as national standards, to protect the safety of the donor, and to ensure the safety, purity and potency of the blood collection.9 Strict donor selection and deferral criteria are followed at the time of screening. The deferral rate of blood donors during pre-donation screening in the study period was estimated as 6.9%. Studies on donor deferrals conducted all over the world showed a deferral rate ranging from 5.19–35.6%.10 The blood donors during pre-donation screening were deferred due to various reasons and the most common cause for the donor deferral is low Hb as noted in various studies.11–14 A high percentage of donor deferrals are due to high Hb (0.5%) as noted in our previous study, which prompted us to study the JAK2V617F mutation among the blood donors with high Hct levels to diagnose the underlying disease.11
With the change in the criteria to diagnose PV as per “The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms” the two important major criteria are high Hb value (Hb of >16.5 g/dl in men and Hb >16.0 g/dl in women) and presence of the JAK2V617F mutation.4 As per the guidelines bone marrow biopsy may not be required in cases with sustained absolute erythrocytosis of Hb levels of 18.5 g/dl in men or 16.5 g/dl in women.4 As per the institutional SOP, the Hb cut off of more than 18 g/dl was the criteria to defer the donors. Accordingly, 1.3% of the total screened donors were deferred due to high Hb (>18 g/dl). The donor deferral rate due to high Hb in the present study was comparable to the studies done by Tagariello et al2 (0.9%), Hatami et al15 (1.23%) and Al-Nuri et al16 (1.6%). In contrast to our study, studies done by Arslan et al17 (0.4%), Kandasamy et al12 (0.39%), and by Magnussen et al18 (0.1%) had a lower rate of donor deferrals due to High Hb. Studies by Kamaruzzaman et al5 (7.8%) and Birjandi et al19 (2.4%) had a higher deferral rate due to a high Hb value. Although there is a precise cut-off for low Hb value (<12.5 g/dl), as per WHO and American Association of Blood Banking (AABB) for donor deferral, there is a lack of precise upper limit cut-off for Hb. Every blood center has to set its own ranges for blood donation parameters.20 The wide variation in donor deferral due to high Hb can be attributed to the different methods used in the estimation of Hb value. Many blood centers in India use the copper sulfate method for Hb detection which does not have the competency to distinguish between normal and high Hb levels.12 Hence use of the automated hematology analyzers which gives an accurate Hb value has to be encouraged in blood centers.
It is important to distinguish between PV and secondary polycythemia which is a complicated task due to various clinical profiles, evolution of the disease, and therapeutic implications.21 PV should be worked up by subjecting the patients to JAK2V617F mutation.22 JAK2V617F mutation is one of the major criteria as per “The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms”.4 In the present study, persistent erythrocytosis was noted in 48 donors and among them 2 cases (4.1%) tested positive for the JAK2V617F mutation. The prevalence of JAK2V617F mutation in donors with a Hct level above the upper limit of normal was estimated to be 1% and 2.1% in the studies performed by Tagariello et al,2 and Magnussen et al,18 respectively. Kamaruzzaman et al5 did not find any donors positive for mutation test among the donors with erythrocytosis. Rubaie et al23 noted a higher prevalence of 21.3% blood donors positive for JAK2V617F mutation.
The JAK2V617F negative cases also have to be evaluated extensively for causes of secondary erythrocytosis. One of the common causes of relative erythrocytosis is dehydration due to decreased intake of water or hot weather.5 Most of our blood donors claimed adequate intake of water, but this may not be true because India is a tropical country with a high daytime temperature, during which most of the blood donors were screened. Patients, who do not fall into the category of PV or secondary polycythemia, are grouped into idiopathic erythrocytosis, which is a diagnosis of exclusion and a substantial number of cases fall into this category.24
In the present study, the 2 cases with JAK2V617F mutation had serum EPO levels in the normal range. Subnormal EPO is a minor criterion in the diagnosis of PV.4 Studies have documented subnormal EPO levels in patients with PV, but a low EPO level is not pathognomonic of PV.25 Normal EPO levels were estimated in 20% and 32% of polycythemia patients by Vannucchi et al26 and Lupak et al27 respectively. Thus, the normal serum EPO level does not exclude PV and a subnormal serum erythropoietin level is not specific for the diagnosis of PV.26,27
Blood donors with erythrocytosis have to be subjected to further investigations after ruling out causes of secondary polycythemia. PV has to be ruled out in donors with persistent erythrocytosis. Diagnosing PV via JAK2V617F mutation testing directs the donors in need to access proper treatment before the onset of thrombosis and other complications which can decrease their lifespan.
Limitations
The sample size was small hence results must be validated in a larger scale study. Exon 12 mutation analysis could not be performed; hence we could not rule out PV in the 46 cases. Bone marrow examination could not be performed on the JAK2V67F mutation-positive cases.
Conclusion
Blood donation is a valuable and indispensable component saving millions of lives all over the world. Hematological evaluation of all blood donors is a mandatory process in blood centers to ensure donor safety. Donors with persistent polycythemia could have hidden pathologic conditions like PV. These donors should be thoroughly evaluated and tested for JAK2V617F mutation along with serum EPO levels and bone marrow examination. The prevalence of persistent erythrocytosis among blood donors was 0.34% and among them, two donors (4.1%) harbored JAK2V617F mutation. This study highlights the role of the blood centers in the identification of potential PV cases, refer them for further evaluation and thus play an important role in advancing preventive medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Myers DJ, Collins RA. Blood donation. StatPearls; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525967/.
2. Tagariello G, Di Gaetano R, Sartori R, et al. The JAK2(V617F) tyrosine kinase mutation in blood donors with upper-limit haematocrit levels. Blood Transfus. 2009;7(2):111–116. doi:10.2450/2008.0049-08
3. Mithoowani S, Laureano M, Crowther MA, Hillis CM. Investigation and management of erythrocytosis. CMAJ. 2020;192(32):E913–E918. doi:10.1503/cmaj.191587
4. Barbui T, Thiele J, Gisslinger H, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15. doi:10.1038/s41408-018-0054-y
5. Kamaruzzaman SF, Noor NH, Hassan MN, Yusoff SM, Abdullah WZ. Detection of JAK2 V617F mutation among donors with erythrocytosis. Am J Med Sci. 2018;3(2):19–25. doi:10.24191/jchs.v3i2.7056
6. Kumar V, Abbas AK, Aster JC. Red blood cell and bleeding disorders. Polycythemia. In: Robbins Basic Pathology.
7. Messinezy M, Pearson TC. The classification and diagnostic criteria of the erythrocytoses (polycythaemias). Clin Lab Haematol. 1999;21(5):309–316. doi:10.1046/j.1365-2257.1999.00246.x
8. Wouters HJ, Mulder R, van Zeventer IA, et al. Erythrocytosis in the general population: clinical characteristics and association with clonal hematopoiesis. Blood Adv. 2020;4(24):6353–6363. doi:10.1182/bloodadvances.2020003323
9. Ministry of Health and Family Welfare, Government of India, New Delhi. Guidelines for blood donor selection & referral –NACO; 2017. Available from: http://naco.gov.in/sites/default/files/Letter%20reg.%20%20guidelines%20for%20blood%20donor%20selection%20%26%20referral%20-2017.pdf.
10. Pisudde PM, Shyam S, Rekha D, Gon S. Evaluation of pre-donation deferral reason among the blood donors visiting ESIC hospital in Eastern India. J Blood Disord Transfus. 2015;6(250):2.
11. Bobati S, Basavraj V, Prakash P. Analysis of predonation loss of blood donors due to deferrals-in a tertiary care hospital set up. J Health Allied Sci. 2016;5(1):15–18. doi:10.4103/2278-344X.173874
12. Kandasamy D, Shastry S, Chenna D, Mohan G. Blood donor deferral analysis in relation to the screening process: a single-center study from southern India with emphasis on high hemoglobin prevalence. J Blood Med. 2020;11:327–334. doi:10.2147/JBM.S265461
13. Vyas N, Prakash Sapre J, Maheshbhai Maru A, Ramkrishna Shah A. Donor deferral criteria-one-year study at a tertiary care hospital. J Diagn Pathol Oncol. 2021;6(2):90–93. doi:10.18231/j.jdpo.2021.020
14. Sabari Priya E. Retrospective analysis of patterns of donor deferral among blood donors in a tertiary care hospital. Int J Contemp Med Res. 2019;6(1):A6–A9.
15. Hatami H, Maghsoodlu M, Salehifar P, Karimian M, Ferdowsi S. Analyzing the causes of blood donor deferrals and characteristics of deferred individuals in Kurdistan Province, Iran. Int J Basic Sci Med. 2018;3(3):114–119. doi:10.15171/ijbsm.2018.21
16. Al-Nuri RN. The frequency of blood donors and polycythemia among general population in Nineveh Governorate, Iraq. Ann Coll Med Mosul. 2021;42(2):141–147. doi:10.33899/mmed.2020.128424.1049
17. Arslan O. Whole blood donor deferral rate and characteristics of the Turkish population. Transfus Med. 2007;17(5):379–383. doi:10.1111/j.1365-3148.2007.00738.x
18. Magnussen K, Hasselbalch HC, Ullum H, Bjerrum OW. Characterization of blood donors with high haemoglobin concentration. Vox Sang. 2013;104(2):110–114. doi:10.1111/j.1423-0410.2012.01644.x
19. Birjandi F, Gharehbaghian A, Delavari A, Rezaie N, Maghsudlu M. Blood donor deferral pattern in Iran. Arch Iran Med. 2013;16(11):657–660.
20. Sultan S, Irfan SM, Baig MA, Usman SM, Shirazi UA. Insight into donor deferral pattern based on peripheral blood counts: an experience from South Pakistan. Asian J Transfus Sci. 2017;11(2):151–155. doi:10.4103/0973-6247.214357
21. Ibrahim IK, Hassan R, Ali EW, Omer A. Polycythaemia vera among sudanese patients with special emphasis on JAK2 mutations. Asian Pac J Cancer Prev. 2019;20(1):41–44. doi:10.31557/APJCP.2019.20.1.41
22. Djulbegovic M, Dugdale LS, Lee AI. Evaluation of polycythemia: a teachable moment. JAMA Intern Med. 2018;178(1):128–130. doi:10.1001/jamainternmed.2017.6213
23. AL-Rubaie HA, Khudeir MA, Al-Bayaa IM. Detection of JAK2V617F tyrosine kinase mutation and estimation of serum erythropoietin in blood donors who have high hematocrit. J Fac Med Baghdad. 2014;56(4):395–400. doi:10.32007/med.1936/jfacmedbagdad.v56i4.12
24. Gangat N, Szuber N, Pardanani A, Tefferi A. JAK2 unmutated erythrocytosis: current diagnostic approach and therapeutic views. Leukemia. 2021;35(8):2166–2181. doi:10.1038/s41375-021-01290-6
25. Prchal JF, Prchal JT, Polycythemia V, et al. Williams Hematology.
26. Vannucchi AM. From leeches to personalized medicine: evolving concepts in the management of polycythemia vera. Haematologica. 2017;102(1):18–29. doi:10.3324/haematol.2015.129155
27. Lupak O, Han X, Xie P, Mahmood S, Mohammed H, Donthireddy V. The role of a low erythropoietin level for the polycythemia vera diagnosis. Blood Cells Mol Dis. 2020;80:102355. doi:10.1016/j.bcmd.2019.102355
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

