Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Analysis of Factors That Influence the Prognosis of Swallowing Function Rehabilitation Therapy in Patients with Dysphagia After Medullary Infarction
Authors Zhang D , Li Y, Li H, Fu W, Zeng J, Zeng X
Received 27 September 2021
Accepted for publication 29 December 2021
Published 16 January 2022 Volume 2022:18 Pages 97—107
DOI https://doi.org/10.2147/NDT.S341353
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Di Zhang, Yi Li, Heping Li, Weifeng Fu, Jing Zeng, Xi Zeng
Department of Rehabilitation Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou City, Henan Province, People’s Republic of China
Correspondence: Xi Zeng, Department of Rehabilitation Medicine, The First Affiliated Hospital of Zhengzhou University, Huiji District, Zhengzhou City, Henan Province, People’s Republic of China, Tel +861 359 250 5588, Email [email protected]
Purpose: This study investigated the factors that influence the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction.
Patients and Methods: This retrospective study was conducted using the clinical data of 51 patients who were diagnosed with dysphagia after medullary infarction and hospitalized at our institution between January 2019 and January 2021. As per the water swallow test (WST) grade at 1 month after rehabilitation treatment, patients were classified into the good prognosis group and the poor prognosis group. Univariate analysis as well as univariate and multivariate logistic regression analysis were used to analyze factors that influence the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction. Receiver operating characteristic (ROC) curves were then used to test the predictive ability of the significant parameters to predict the prognosis of the rehabilitation therapy in these patients.
Results: Univariate analysis and univariate logistic regression analysis showed that previous stroke (odds ratio [OR] = 1.361), dysarthria (OR = 3.771), disease course (OR = 1.112), National Institutes of Health Stroke Scale (NIHSS) score at admission (OR = 2.596), and infarct site (OR = 11.071) were all significantly correlated with the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction (P < 0.05). Multivariate logistic regression analysis showed that dysarthria (OR = 5.519, 95% confidence interval (CI) 1.413– 21.566), infarct site (OR = 18.634, 95% CI 1.696– 204.73), and the NIHSS score (OR = 1.001, 95% CI 1.536– 4.820) were independent influencing factors of the prognosis of swallowing function rehabilitation therapy in these patients. The ROC curve showed that the area under the curve for the combined prediction of the three indicators was 0.943.
Conclusion: The NIHSS score, dysarthria, and infarct site are independent influencing factors for the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction.
Keywords: medullary infarction, dysphagia, rehabilitation, prognosis, influencing factors
Introduction
Dysphagia is a common complication of stroke, with an incidence rate of 65%–90%.1 Studies have shown that dysphagia after stroke causes malnutrition in patients and can easily lead to aspiration pneumonia and even asphyxiation in severe cases.2,3 Dysphagia further reduces the quality of life of these patients, increases the risk of death, slows the recovery of body function, and significantly prolongs hospitalization.4 Swallowing is a complex physiological process that involves approximately 50 muscles and 5 pairs of cranial nerves.5 It is regulated both by the medulla oblongata swallowing center in the brainstem network structure and the extensive bilateral neural networks in the cortex and subcortex.6 The incidence, symptoms, and mechanisms of dysphagia caused by different stroke sites vary. The central pattern generator (CPG) of swallowing is located in the medulla oblongata, including the solitary nucleus, the nucleus ambiguous, and the surrounding reticular structure.7 The hypoglossal nerve nucleus, which is located deep in the hypoglossal nerve triangle of the fourth ventricle of the medulla, emits the hypoglossal nerve that innervates the tongue muscle. Hence, the incidence of medullary infarction combined with dysphagia is high (51%–94%)8,9 and the symptoms of dysphagia are serious. The main manifestations include the paralysis of tongue muscles, weak movements of the tongue, abnormal transport of the food bolus,10 abnormal hyolaryngeal excursion time, abnormal hyolaryngeal excursion amplitude,11 incomplete or inoperative opening of the cricopharyngeal muscle,12 and loss of swallowing patterned sequential movements.13 Studies on dysphagia after medullary infarction focused mostly on the summary of the characteristics of dysphagia in unilateral medullary infarction or compared the characteristics of dysphagia in bilateral medullary infarction. No studies have analyzed factors that affect the prognosis of the swallowing function based on the comprehensive rehabilitation therapy of swallowing function and nutritional support. Also, some studies have shown that comprehensive swallowing rehabilitation therapy and early nutritional intervention are vital for improving the swallowing function of patients with dysphagia after stroke.13,14 Therefore, patients with dysphagia after medullary infarction included in our study underwent comprehensive swallowing function rehabilitation therapy and intermittent oral–esophageal (IOE) tube feeding nutritional support. To provide a reference for clinical and rehabilitation treatment and for the prognosis of swallowing function in such patients, the clinical data of patients with dysphagia after medullary infarction were retrospectively collected to analyze the factors that influence the prognosis of swallowing function rehabilitation therapy.
Patients and Methods
Study Population
This study retrospectively collected the clinical data of patients with dysphagia after medullary infarction who were hospitalized in the Department of Rehabilitation Medicine of the First Affiliated Hospital of Zhengzhou University from January 2019 to January 2021. For patients with multiple hospitalization records, only the first admission case was included. The inclusion criteria were as follows: (1) age ≥18 years; (2) complete electronic medical records of the patient; (3) cerebral infarction diagnosed as per the 2018 American Heart Association/American Stroke Association guidelines for acute stroke15 and simple medullary infarction confirmed via brain magnetic resonance imaging; (4) use of comprehensive rehabilitation therapy for the swallowing function and IOE tube feeding nutritional support during patient’s hospitalization; (5) complaint of choking and dysphagia by the patient, with abnormal swallowing function [water swallow test (WST)16 grades II–V], and confirmation of dysphagia via a videofluoroscopic swallow study;17 and (6) no history of dysphagia before the onset. The exclusion criteria were as follows: (1) infarctions in addition to the medullary infarction; (2) no comprehensive swallowing rehabilitation therapy or IOE nutritional support during hospitalization; (3) dysphagia caused by other reasons, including hemorrhagic stroke, craniocerebral trauma, myasthenia gravis, motor neuron disease, Parkinson’s disease, Alzheimer’s disease, gastroesophageal reflux disease, tumor, and others; (4) incomplete clinical and imaging data as well as scale information; and (5) poor prognosis of swallowing function owing to the recurrence of stroke or occurrence of other diseases during hospitalization.
This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (review number: 2021-KY-0609-003). Based on the study’s retrospective design, the requirement of informed consent was waived. The exemption of informed consent has no adverse effects on the rights or welfare of the patients. This study strictly abided by the Declaration of Helsinki and anonymized all patient data.
Treatment Method
The following strategies were employed for treatment. (1) The treatment of primary stroke included basic interventions such as anticoagulation, thrombolysis, nerve nutrition, and circulation improvement. (2) Comprehensive swallowing rehabilitation therapy3 included direct training via compensatory posture (the normal side and semi-sitting position, swallowing with head up, swallowing with head down, or swallowing with head turned) and appropriate bite size and appropriate food trait ingestion training; indirect training18,19 via swallowing organ function training, Mendelsohn maneuver,19 shaker training method, Masako training method, protective swallowing training method, respiratory function training method, Rood technique, and others; and other training methods, including neuromuscular electrical stimulation and biofeedback therapy.20 (3) The final strategy was nutritional support, in which IOE was used to ensure the adequate intake of nutrition and water for patients while promoting the recovery of swallowing function.14
Research Methods
Clinical Data
The basic information of the patients collected at the time of admission was as follows.
(1) General information
This included gender, age, body mass index (BMI), and disease course (number of days from the onset of dysphagia to the beginning of comprehensive rehabilitation therapy and nutritional support for swallowing function).
(2) Medical history
This included high blood pressure, diabetes, and previous stroke.
(3) Neurological function assessment
This included the National Institutes of Health Stroke Scale (NIHSS) score (the total score of the scale).
(4) Swallowing function assessment
(i) The WST classification scale is classified into five levels: level I, can drink one sip without choking; level II, needs more than two attempts to drink without choking; level III, can drink one sip but accompanied by coughing; level IV, needs more than two attempts to drink and accompanied by coughing; and level V, chokes and often finds it difficult to drink at all.
(ii) The Drooling Severity Scale (DSS)21 is also divided into five grades: grade 1, relatively dry with no drooling; grade 2, slight and only damp lips; grade 3, moderately damp lips and jaw; grade 4, severe drooling that wet the clothes; and grade 5, extremely heavy drooling that wet the clothes, hands, and the daily contact area. In this study, >grade 2 that required clinical treatment was included for the analysis of prognostic factors.
(5) Complications
(i) Aspiration-associated pneumonia. Cough and sputum are present, but there is no evidence of pneumonia due to other causes. After dysphagia, pulmonary auscultation with moist rales is observed, and the presence of inflammatory lesions is confirmed using lung computed tomography (CT).
(ii) Central facial paralysis (no other cause of facial paralysis prior to medullary infarction). Paralysis of facial expression muscles on the opposite side of the lesion (shallow nasolabial folds and drooping corners of the mouth), the frontal branch is intact, there is frowning, and eye-closing movements are unobstructed. Voluntary movements of the lesions on the opposite side are lost, but the wry smile is still preserved, which is often accompanied by contralateral hemiplegia and central hypoglossal nerve palsy.
(iii) Dysarthria. It is identified based on the Frenchay Dysarthria Assessment, Second Edition (FDA-2).22 The patient has the necessary language formation and receptive abilities for communication. The only manifestation is difficulty in the formation of spoken sounds, primarily dysphonia, ambiguity, or abnormal vocalization, intonation, and speech speed. In severe cases, the patient is totally unable to pronounce words.
(iv) Central respiratory failure. Delayed central respiratory failure during hospitalization after medullary infarction; blood gas analysis shows hypercapnia and hypoxia; and various examinations show that the patient’s infarct focus does not change, there is no subarachnoid hemorrhage, and there is no evidence of respiratory failure caused by changes in pneumonia.
(6) Infarct site.
Lateral medullary infarction (LMI), medial medullary infarction (MMI), and lateral and medial medullary infarction (LMI+MMI) (as shown in Figure 1). In previous studies, medullary infarctions were horizontally grouped into two levels, LMI and MMI, and vertically grouped into three levels, cephalic, middle, and caudal.23,24 All patients included in this study had medullary cephalic infarction; hence, vertical grouping was not performed.
Prognosis and Grouping
As per the WST grade at 1 month after rehabilitation treatment, patients were classified into the good prognosis group [improved or restored swallowing function; WST grade lower than that at admission] and the poor prognosis group [no improvement or aggravation in the swallowing function; WST grade unchanged or higher than that at admission].
Statistical Analyses
All statistical analyses were performed using SPSS version 26.0 (IBM, Armonk, NY, USA). The Kolmogorov–Smirnov test was used to test the normality of continuous data. Normally distributed continuous data are represented as means ± standard deviation, whereas non-normally distributed data are presented as medians (interquartile range). Disaggregated data are expressed as percentages. Normally distributed data were assessed using Student’s t test, and non-normally distributed data were analyzed using the Mann–Whitney U-test. The chi-squared test or Fisher’s exact test was used for the intergroup comparisons of categorical variables. Multivariate logistic regression analysis was performed to investigate the factors that influence the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction. The test level α = 0.05, P < 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were used to test the predictive ability of significant parameters to predict the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction.
Results
Clinical Features
A total of 59 patients were enrolled in this study; 5 patients were excluded owing to incomplete assessment data related to swallowing function and neurological function and 2 patients were excluded because they did not undergo comprehensive swallowing function rehabilitation therapy and did not used IOE to ensure adequate nutrition and water intake. In addition, one patient was ruled out because of secondary cerebral hemorrhage within 1 month of hospitalized rehabilitation treatment. Therefore, 51 patients were finally included in the analysis. Table 1 shows the demographic and general characteristics of the two patient groups. The good and poor prognosis groups included 32 and 19 patients, respectively, with an average age of 59.5 ± 9.0 years. There were 41 males (80.3%) and 10 females (19.6%). No significant differences were observed in age, gender, hypertension, diabetes, central facial paralysis, BMI, aspiration-associated pneumonia, and WST at admission (grade) between the two groups (P > 0.05). Compared with the good prognosis group, the poor prognosis group had higher proportions of patients with previous stroke (47.3% vs 18.7%), dysarthria (84.2% vs 34.3%), central respiratory failure (10.5% vs 3.1%), higher NIHSS score at admission [10 (9–11) vs 4.5 (3.25–6)], and longer disease course [21 (17–27) vs 15 (13–18)]. These differences, including that for the infarct site (P = 0.03), were all statistically significant (P < 0.05) (Table 1).
 |
Table 1 Clinical Data and Prognosis of the Two Groups of Patients |
Univariate and Multivariate Binary Logistic Regression Analyses Were Used to Investigate the Factors That Influence the Prognosis of Swallowing Function Rehabilitation in Patients with Dysphagia After Medullary Infarction
The good and poor prognoses of swallowing function at 1 month after rehabilitation therapy were classified as dependent variables; they were assigned the values of 0 and 1, respectively. The variables in Table 1 with statistically significant differences (previous stroke, disease course, dysarthria, central respiratory failure, NIHSS score, and infarct site) were included in the binary logistic regression analysis model as independent variables, and univariate and multivariate binary logistic regression analyses were performed.
Single-factor binary logistic regression analysis revealed that previous stroke (P = 0.035), disease course (P = 0.009), dysarthria (P = 0.029), central respiratory failure (P = 0.305), infarct site (P = 0.035), and the NIHSS score (P < 0.001) were significantly associated with the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction (P < 0.05) (Table 2).
 |
Table 2 Swallowing Function Rehabilitation Treatment Prognosis Related to Single-Factor and Multivariate Regression Analysis |
Multivariate binary logistic regression analyses showed that dysarthria (odds ratio [OR] 5.519, 95% confidence interval [CI] 1.413–21.566, P = 0.014), infarct site (OR 18.634, 95% CI 1.696–204.73, P = 0.017), and the NIHSS score (OR 1.001, 95% CI 1.536~4.820, P = 0.001) were independent risk factors for the prognosis of swallowing rehabilitation therapy in these patients (Table 2).
ROC Curve Analysis of Predictors
ROC curve analysis was used to evaluate the predictive ability of dysarthria, infarct site, and high NIHSS scores on the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction. The analysis was performed on three-factor single diagnosis, double-combination diagnosis, and three-factor combined diagnosis. As shown in Table 3 and Figure 2, the predictive ability of the three-factor combined diagnosis was optimal, and the area under the three-factor combined diagnosis curve was 0.943 (95% CI 0.86–1.00, standard error 0.043).
 |
Table 3 ROC Curve Analysis of Predictors |
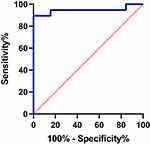 |
Figure 2 The ROC curve of the three-factor (A+B+C) combined predict the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction. |
Discussion
Comprehensive swallowing function rehabilitation therapy and early nutritional support have beneficial effects on the recovery of swallowing function in patients with dysphagia after stroke as well as on the overall prognosis following stroke.20,22 In practice, an active and individualized application of rehabilitation therapy is important for the effective recovery of the swallowing function, but the comprehensive application of rehabilitation therapy is the most complete and effective. IOE intermittent oral intubation is simple and safe to perform and can maintain the normal physiological functions of the digestive tract. Compared with traditional nutritional support methods (such as nasal feeding), the incidence of complications, including nasal mucosal injury, nasal and esophageal ulceration, reflux esophagitis, lung infection, gastrointestinal infection, aspiration, and other complications, is significantly reduced via IOE. While ensuring patient safety and the adequate intake of nutrients and water, IOE can also promote the recovery of swallowing function.14,25,26 Therefore, all patients included in this study underwent comprehensive swallowing rehabilitation therapy and IOE nutritional support based on the treatment of the primary disease of stroke. Based on this treatment strategy, the factors that influence the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction were explored.
A previous study showed that the probability of dysarthria to co-occur with dysphagia can reach up to 45.2% in patients with stroke and that dysarthria can be used as a predictor of dysphagia.27 The present study found that dysarthria is an independent risk factor for the poor prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction owing to the following reasons. Dysarthria caused by bilateral corticobulbar tract injury leads to throat muscle paralysis, which affects the pharyngeal phase of the swallowing process, timing of hyolaryngeal excursion, and range of hyolaryngeal excursion, thereby affecting the oropharyngeal sequential pattern or the swallowing process.28,29 Dysarthria after infarction was observed to involve the hypoglossal nerve nucleus, which mainly causes tongue muscle dyskinesia, accompanied by tongue muscle atrophy and tongue muscle tremor, all of which affect the pushing of the food bolus to the pharynx. McCullough et al30 found that dysarthria increases the risk of aspiration, which further increases this risk owing to dysphagia; this is not conducive to the prognosis of patients undergoing swallowing function rehabilitation therapy. Daniels et al31 reported that reduced communication effectiveness due to dysarthria affects the patient’s participation in rehabilitation treatment to varying degrees, thereby reducing the effectiveness of rehabilitation treatment for swallowing function. These results concluded a much closer relationship between swallowing and speech function in patients with dysarthria than is widely believed. The clinical management of patients with dysarthria should often include intervention aimed at dysphagia, and the clinical management of patients with dysphagia should often include intervention aimed at dysarthria.
In all previous studies,32,33 medullary infarctions were grouped horizontally into two types (LMI and MMI) and vertically into three types (cephalic, middle, and caudal), and analyses were performed on the types and incidence of dysphagia in different infarct positions. All patients included in the present study had medullary cephalic infarction; thus, only horizontal grouping into LMI, MMI, and LMI+MMI was performed. Additionally, in our study, multivariate logistic regression analysis showed that the infarct site is an independent risk factor for the prognosis of swallowing function rehabilitation in patients with dysphagia after medullary infarction. Tao et al34 concluded that the short-term prognosis of MMI is worse than LMI, but they used the modified Rankin Scale score as the short-term prognostic evaluation criteria. The purpose of the study by Tao et al was different from ours; however, their conclusions have a certain explanation for our findings. The clinical manifestations of LMI and MMI are heterogeneous, but both have different phases of dysphagia. MMI is primarily involved in the oral swallowing process, which causes tongue muscle dyskinesia, with tongue muscle atrophy and tremor; these affect the pushing of the food bolus to the pharynx. LMI is primarily involved in the swallowing process in the pharyngeal and oral stages. Miseon Kwon et al11 concluded that the most common problem in the LMI group was the range of hyolaryngeal excursion, whereas in the MMI group was the timing of hyolaryngeal excursion. That all can explain our result that the infarct site is an independent risk factor for the prognosis of swallowing function rehabilitation in patients with dysphagia after medullary infarction. The types and characteristics of dysphagia are different between patients with LMI and MMI, implicating a rationale for different treatment strategies and prognoses of swallowing function. LMI+MMI infarction may has a complicated stroke mechanism, which may aggravate dysphagia to a certain extent, wherein comprehensive obstacles in the oral and pharyngeal phases cause serious disorders in the sequential swallowing pattern and lead to a relatively slow recovery. Increasing the intensity and treatment rehabilitation time may be necessary, which can be investigated in future studies.
In the present study, the results revealed that the NIHSS scores at admission of patients with poor prognosis were higher than those of patients with good prognosis. The NIHSS score was an independent risk factor for the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction, which is consistent with previous studies.35,36 It was previously believed that the rehabilitation of swallowing function was related to the neuroplasticity of the unaffected functional areas. By promoting the plasticity of the viable functional areas and reorganizing the remaining functional areas and neural network structure, the swallowing function can be improved and restored.37 The NIHSS score is an important and commonly used stroke severity assessment scale and is a powerful predictor of stroke prognosis; it can fully represent the left and right brain functions at an earlier period.38 The higher the score, the more severe is the damage to the central control area of the relevant function; therefore, the more serious is the degree of neurological deficit and the degree of body function defect. Furthermore, the lower the degree of reorganization of the functional area and the neural network structure, the more difficult it is to recover the swallowing function.
In this study, univariate analysis and univariate binary logistic regression analysis showed that the disease course in the good prognosis group was significantly shorter than that in the poor prognosis group. This indicates that the disease course is associated with the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction. This suggests that the earlier the patient starts comprehensive swallowing function rehabilitation therapy and IOE nutritional support, the better is the recovery and improvement of the swallowing function, which is consistent with the study results of Jalal Bakhtiyari and Tianheng Zheng.39,40 Early enteral nutritional support can improve the nutritional status of patients with dysphagia after medullary infarction, thereby contributing to an improvement in the overall prognosis of the body and recovery of the swallowing function. Early comprehensive rehabilitation therapy for the swallowing function helped in the recovery of the swallowing function, which is consistent with the principle of brain neuroplasticity.41 There is a window in the early post-stroke period where brain neuroplasticity and the dynamic response of the central nervous system to the injury are enhanced. Comprehensive rehabilitation therapy is particularly effective for the recovery of related functions.42 Early functional rehabilitation therapy (starting 1–3 days after stroke) has been shown to reduce inflammatory cytokines,43 shrink the blood–brain barrier,44 inhibit cell apoptosis, increase brain-derived neurotrophic factor, and promote nerve remodeling.45
Univariate analysis showed that previous stroke is associated with the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction. Swallowing relies on CPG located on both sides of the medulla oblongata, which involves the brainstem motor nuclei, including the nucleus solitary and nucleus ambiguus, and the main interneuron groups and related network structures.14 Studies have shown that CPGs on both sides are widely connected to each other and that either side can coordinate the swallowing process of the pharynx and esophagus. The contraction of the pharynx muscle on the side of the lesion is weakened and the cricopharyngeal muscle fails to open. The food bolus generally passes through the pharynx smoothly via the compensation effect of CPG on the contralateral side.34 Previous stroke may involve the brain function area related to swallowing (including damage to pyramidal tract, insula, and basal ganglia due to unilateral hemispheric stroke.The brainstem stroke, pons stroke et al), but a good compensatory function of the uninvolved side prevents the patient from showing the clinical symptoms of dysphagia. However, part of irreversible damage to the functional areas of the brain directly affects the recovery of dysphagia after medullary infarction.
Delayed central respiratory failure in patients with medullary infarction during hospitalization has also been mentioned in previous studies. The diagnosis of central respiratory failure is based on the examination results showing that the patient’s infarct is unchanged, with no subarachnoid hemorrhage, and by excluding respiratory failure due to worsening pneumonia.46
Delayed central respiratory failure is often acute, blood gas analysis in such cases shows repeated hypercapnia and hypoxemia, and elderly patients with dysphagia medullary infarction are more likely to have respiratory failure in the subacute stage. In severe cases, tracheotomy and ventilator-assisted breathing are required immediately.47,48 The mechanical damage and stimulation of the oropharynx caused by intubation affect the patient’s swallowing function recovery and tracheotomy affects the patient’s normal swallowing function rehabilitation treatment measures, which directly affect the patient’s swallowing function recovery. In our study, three patients developed central respiratory failure: one patient in the good prognosis group and two patients in the poor prognosis group, the difference was statistically significant. However, in the multivariate binary logistic regression analyses, central respiratory failure did not show an independent association, which may be because of the small sample size in this study. However, for patients with dysphagia after medullary infarction, it is still necessary to closely monitor their subacute condition and pay attention to prevent delayed central respiratory failure to improve their prognosis.49
Limitations
This study was a single-center retrospective study, with a limited number of cases. At a later stage, a multi-center, large-sample, prospective clinical controlled trial study should be considered to better summarize the factors that influence the prognosis of swallowing function rehabilitation therapy in patients with post-medullary infarction dysphagia.
Conclusion
In summary, dysarthria, infarct site, and a high NIHSS score are independent risk factors for the prognosis of swallowing function rehabilitation therapy in patients with dysphagia after medullary infarction.
Acknowledgments
This work was supported by the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences [Item number 2020-PT310-01] and the Medical Science and Technique planning program of Henan province [Grant numbers 182102310153].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Souza J, Ribeiro P, de Paiva SAR, et al. Dysphagia and tube feeding after stroke are associated with poorer functional and mortality outcomes. Clin Nutr. 2020;39(9):2786–2792. doi:10.1016/j.clnu.2019.11.042
2. Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–2763. doi:10.1161/01.STR.0000190056.76543.eb
3. Zulin D, Tiecheng G, Zhiming T. Expert consensus on evaluation and Treatment of swallowing disorders in China (2017 edition). Chinese J Phys Med Rehabilit. 2017;39(12):881–892.
4. Altman K, Yu GP, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784–789. doi:10.1001/archoto.2010.129
5. De Felice F, de Vincentiis M, Luzzi V, et al. Late radiation-associated dysphagia in head and neck cancer patients: evidence, research and management. Oral Oncol. 2018;77:125–130. doi:10.1016/j.oraloncology.2017.12.021
6. Humbert I, Fitzgerald M, McLaren D, et al. Neurophysiology of swallowing: effects of age and bolus type. Neuroimage. 2009;44(3):982–991. doi:10.1016/j.neuroimage.2008.10.012
7. Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–969. doi:10.1152/physrev.2001.81.2.929
8. Norrving B, Cronqvist S. Lateral medullary infarction: prognosis in an unselected series. Neurology. 1991;41(2(Pt 1):244–248. doi:10.1212/WNL.41.2_Part_1.244
9. Chia L, Shen W. Wallenberg’s lateral medullary syndrome with loss of pain and temperature sensation on the contralateral face: clinical, MRI and electrophysiological studies. J Neurol. 1993;240(8):462–467. doi:10.1007/BF00874113
10. Kumral E, Afsar N, Kirbas D, et al. Spectrum of medial medullary infarction: clinical and magnetic resonance imaging findings. J Neurol. 2002;249(1):85–93. doi:10.1007/PL00007852
11. Kwon M, Lee J, Kim J. Dysphagia in unilateral medullary infarction: lateral vs medial lesions. Neurology. 2005;65(5):714–718. doi:10.1212/01.wnl.0000174441.39903.d8
12. Zhang J, Zhou Y, Zhao X-Q, et al. The characteristics and mechanisms of dysphagia in patients with dorsolateral medullary syndrome. Chin J Phys Med Rehabil. 2006;28(11):770–773.
13. Nakao M, Oshima F, Maeno Y, et al. Disruption of the obligatory swallowing sequence in patients with Wallenberg syndrome. Dysphagia. 2019;34(5):673–680.
14. Nakajima M, Kimura K, Inatomi Y, et al. Intermittent oro-esophageal tube feeding in acute stroke patients – a pilot study. Acta Neurol Scand. 2006;113(1):36–39. doi:10.1111/j.1600-0404.2005.00534.x
15. Powers W, Rabinstein A, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi:10.1161/STR.0000000000000158
16. Suiter D, Leder S. Clinical utility of the 3-ounce water swallow test. Dysphagia. 2008;23(3):244–250. doi:10.1007/s00455-007-9127-y
17. Costa M. Videofluoroscopy: the gold standard exam for studying swallowing and its dysfunction. Arq Gastroenterol. 2010;47(4):327–328. doi:10.1590/S0004-28032010000400001
18. Shaker R, Sanvanson P, Balasubramanian G, et al. Effects of laryngeal restriction on pharyngeal peristalsis and biomechanics: clinical implications. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G1036–G1043. doi:10.1152/ajpgi.00010.2016
19. Vose A, Nonnenmacher J, Singer M, et al. Dysphagia management in acute and sub-acute stroke. Curr Phys Med Rehabil Rep. 2014;2(4):197–206. doi:10.1007/s40141-014-0061-2
20. Bogaardt H, Grolman W, Fokkens W. The use of biofeedback in the treatment of chronic dysphagia in stroke patients. Folia Phoniatr Logop. 2009;61(4):200–205. doi:10.1159/000227997
21. Shimizu A, Maeda K, Koyanagi Y, et al. The global leadership initiative on malnutrition-defined malnutrition predicts prognosis in persons with stroke-related dysphagia. J Am Med Dir Assoc. 2019;20(12):1628–1633.
22. Ghio A, Giusti L, Blanc E, et al. French adaptation of the “Frenchay Dysarthria Assessment 2” speech intelligibility test. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137(2):111–116. doi:10.1016/j.anorl.2019.10.007
23. Kameda W, Kawanami T, Kurita K, et al. Lateral and medial medullary infarction: a comparative analysis of 214 patients. Stroke. 2004;35(3):694–699. doi:10.1161/01.STR.0000117570.41153.35
24. Oshima F, Yokozeki M, Hamanaka M, et al. Prediction of dysphagia severity: an investigation of the dysphagia patterns in patients with lateral medullary infarction. Intern Med. 2013;52(12):1325–1331. doi:10.2169/internalmedicine.52.0011
25. Juan W, Zhen H, Yan-Ying F, et al. A comparative study of two tube feeding methods in patients with dysphagia after stroke: a randomized controlled trial. J Stroke Cerebrovasc Dis. 2020;29(3):104602. doi:10.1016/j.jstrokecerebrovasdis.2019.104602
26. Wang Z, Chen J, Ni GX. Effect of an indwelling nasogastric tube on swallowing function in elderly post-stroke dysphagia patients with long-term nasal feeding. BMC Neurol. 2019;19(1):83. doi:10.1186/s12883-019-1314-6
27. Bahia M, Mourao L, Chun R. Dysarthria as a predictor of dysphagia following stroke. NeuroRehabilitation. 2016;38(2):155–162. doi:10.3233/NRE-161305
28. Nishio M, Niimi S. Relationship between speech and swallowing disorders in patients with neuromuscular disease. Folia Phoniatr Logop. 2004;56(5):291–304. doi:10.1159/000080066
29. Otapowicz D, Sobaniec W, Okurowska-Zawada B, et al. Dysphagia in children with infantile cerebral palsy. Adv Med Sci. 2010;55(2):222–227. doi:10.2478/v10039-010-0034-3
30. McCullough G, Wertz R, Rosenbek J. Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34(1–2):55–72. doi:10.1016/S0021-9924(00)00041-1
31. Daniels S, Schroeder M, DeGeorge P, et al. Defining and measuring dysphagia following stroke. Am J Speech Lang Pathol. 2009;18(1):74–81. doi:10.1044/1058-0360(2008/07-0040)
32. Cho Y, Ryu W, Lee H, et al. Which factors affect the severity of dysphagia in lateral medullary infarction? Dysphagia. 2020;35(3):414–418.
33. Chun M, Kim D, Chang M. Comparison of dysphagia outcomes between rostral and caudal lateral medullary infarct patients. Int J Neurosci. 2017;127(11):965–970. doi:10.1080/00207454.2017.1282479
34. Tao L, Lin J, Zou M, et al. A comparative analysis of 375 patients with lateral and medial medullary infarction. Brain Behav. 2021;11(8):e2224. doi:10.1002/brb3.2224
35. De Stefano A, Dispenza F, Kulamarva G, et al. Predictive factors of severity and persistence of oropharyngeal dysphagia in sub-acute stroke. Eur Arch Otorhinolaryngol. 2021;278(3):741–748. doi:10.1007/s00405-020-06429-2
36. Kumar S, Doughty C, Doros G, et al. Recovery of swallowing after dysphagic stroke: an analysis of prognostic factors. J Stroke Cerebrovasc Dis. 2014;23(1):56–62. doi:10.1016/j.jstrokecerebrovasdis.2012.09.005
37. Wbarritt A, Gsmithard D. Role of cerebral cortex plasticity in the recovery of swallowing function following dysphagic stroke. Dysphagia. 2009;24(1):83–90. doi:10.1007/s00455-008-9162-3
38. Runde D. Calculated decisions: NIH stroke scale/score (NIHSS). Emerg Med Pract. 2020;22(7):D6–D7.
39. Bakhtiyari J, Sarraf P, Nakhostin-Ansari N, et al. Effects of early intervention of swallowing therapy on recovery from dysphagia following stroke. Iran J Neurol. 2015;14(3):119–124.
40. Zheng T, Zhu X, Liang H, et al. Impact of early enteral nutrition on short term prognosis after acute stroke. J Clin Neurosci. 2015;22(9):1473–1476. doi:10.1016/j.jocn.2015.03.028
41. Robbins J, Butler S, Daniels S, et al. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res. 2008;51(1):S276–S300. doi:10.1044/1092-4388(2008/021)
42. Coleman E, Moudgal R, Lang K, et al. Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep. 2017;19(12):59. doi:10.1007/s11883-017-0686-6
43. Zhang P, Zhang Q, Pu H, et al. Very early-initiated physical rehabilitation protects against ischemic brain injury. Front Biosci (Elite Ed). 2012;4:2476–2489. doi:10.2741/e559
44. Zhang Y, Zhang P, Shen X, et al. Early exercise protects the blood-brain barrier from ischemic brain injury via the regulation of MMP-9 and occludin in rats. Int J Mol Sci. 2013;14(6):11096–11112. doi:10.3390/ijms140611096
45. Zhang L, Hu X, Luo J, et al. Physical exercise improves functional recovery through mitigation of autophagy, attenuation of apoptosis and enhancement of neurogenesis after MCAO in rats. BMC Neurosci. 2013;14:46. doi:10.1186/1471-2202-14-46
46. Sugawara E, Saito A, Okamoto M, et al. [Central respiratory failure occurred in the subacute phase of unilateral Wallenberg’s syndrome: a case report]. Rinsho Shinkeigaku. 2014;54(4):303–307. Japanese.
47. Mishina M, Ohkubo S, Kamiya N, et al. Efficacy of tracheostomy for central alveolar hypoventilation syndrome caused by lateral medullary infarction. J Nippon Med Sch. 2014;81(4):276–284. doi:10.1272/jnms.81.276
48. Arai N, Obuchi M, Matsuhisa A, et al. [Two cases with unilateral lateral medullary infarction associated with central respiratory failure]. Rinsho Shinkeigaku. 2008;48(5):343–346. Japanese. doi:10.5692/clinicalneurol.48.343
49. Planjar-Prvan M, Krmpotic P, Jergovic I, et al. [Central sleep apnea (Ondine’s curse syndrome) in medullary infarction]. Acta Med Croatica. 2010;64(4):297–301. Swedish.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.