Back to Journals » OncoTargets and Therapy » Volume 7
Analysis of exfoliated gastric carcinoma cells attached on surgical supplies
Authors Yu X, Ma Y, Hu X, Zhang Q, Ye Z
Received 17 April 2014
Accepted for publication 28 June 2014
Published 10 October 2014 Volume 2014:7 Pages 1869—1873
DOI https://doi.org/10.2147/OTT.S66412
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Xiao-Fen Yu,1 Ying-Yu Ma,2 Xian-Qin Hu,1 Qin-Fang Zhang,1 Zai-Yuan Ye3
1Operating Theatre, Zhejiang Provincial People’s Hospital, 2Key Laboratory of Gastroenterology of Zhejiang Province, Zhejiang Provincial People’s Hospital, 3Department of Gastrointestinal Surgery, Zhejiang Provincial People’s Hospital, Hangzhou, People’s Republic of China
Abstract: Surgery is considered to have a leading role in the treatment of gastric carcinoma. Surgical supplies are used to cut, divide, and ligate during surgery, and are not only in close contact with normal tissues, but may also be contaminated by pathological tissues and cells. This study sought to determine the presence of exfoliated tumor cells on surgical supplies at different stages during the surgical procedure. We collected five types of surgical supplies from 90 patients who underwent D2 radical gastrectomy to find out if there was any cancer cells attached to them. Highest numbers of cancer cells were found on gauze used to clean the surgical instruments and on the gloves of scrub nurses. The likelihood of finding cancer cells increased with advancing clinical stage of disease, lower differentiation of cancer cells, increasing frequency of use of supplies and extent of contact, and was also associated with the characteristic of surgical supplies. Dissemination of tumor cells could be prevented by using a number of methods, depending on the type of surgical supply items.
Keywords: exfoliated tumor cells, surgical supplies, gastric carcinoma, metastasis, prevention
Introduction
Surgical resection is the treatment of choice for gastric cancer if there is no distant metastasis and/or contraindication for surgery. Surgical supplies are used for cutting, dividing, and ligating during the surgical procedure, and are not only exposed to normal tissues, but also to contaminated pathological tissues and cells.1 In this study, we observed and analyzed exfoliated tumor cells during different stages of the surgical procedure to provide a theoretical basis for developing a cancer-free technique.
Materials and methods
Gastric cancer samples
Ninety patients with gastric cancer treated in the department of general surgery at our hospital from April 2011 to February 2012 were enrolled in this study, which was approved by the medical ethics committee of Zhejiang Provincial People’s Hospital. The surgeries were performed by same surgical team, with rotating scrub nurses. All the patients underwent D2 radical gastrectomy, and five categories of surgical supplies were identified and collected for further examination. To make a definitive diagnosis, the following preoperative examinations were included: complete blood count, urinalysis, fecal analysis, prothrombin time, electrocardiogram, echocardiogram, chest X-ray, gastroscopy, pathologic analysis to make a definitive diagnosis, and an upper abdominal enhanced spiral computed tomography scan to rule out distant metastasis. All patients underwent general anesthesia.
Collection of exfoliated tumor cells
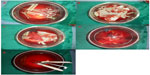
Five groups of surgical supplies were identified: surgical instruments, including eight hemostats, two scalpels, two pairs of scissors, two needle holders, four stitches, three retractors, two bowel clamps, and two Kocher clamps (group A); surgical gloves from the operator, first assistant, and second assistant (group B); gloves used by scrub nurses, gauze used to clean surgical equipment, and residual suture threads (group C); gauze used to clear the operative field and for hemostasis (group D); and stapler devices (group E, Figure 1).
The five different types of surgical supplies were completely submerged for 30 seconds in five separate sterile containers, each filled with 2,000 mL of saline at 37°C, and the joint of surgical instruments were kept open, and then scrubbed gently. The immersion fluid was centrifuged (at 2,000 rpm ×10 minutes, TDL-408) and the supernatant was discarded. The precipitates were resuspended in Roswell Park Memorial Institute 1640 medium containing 20% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and seeded in a cell culture plate, then maintained at 37°C in 5% CO2.
Detection of exfoliated tumor cells
Viable carcinoma cells seen to be growing in adherent cultures was defined as positive. If one group was positive, all surgical supplies were considered to be positive. Carcinoma cells were fixed in methyl alcohol, and stained with Wright and Giemsa. Cell morphology was then observed using an inverted microscope.
Results
Relationship between exfoliated tumor cells and TNM stage and differentiation
Tumor cells with a complete structure are shown in Figure 2. Ninety gastric cancers were included in the study. The proportions of exfoliated tumor cell-positive specimens were 50.0% (5/10) in TNM stage I, 73.5% (25/34) in TNM stage II, and 87.0% (40/46) in TNM stage III, and showed a significant difference (P=0.026, Table 1). The frequencies of exfoliated tumor cell positivity in patients with well differentiated gastric carcinoma (37.5%, 3/8) and moderately differentiated gastric carcinoma (70.8%, 17/24) were significantly lower than in those with poorly differentiated gastric carcinoma (84.5%, 49/58, P=0.011).
  | Figure 2 Tumor cells stained with Wright and Giemsa, and observed using an inverted microscope at ×1,000 magnification. |
Relationship between exfoliated tumor cells and surgical supplies
Variable amounts of cancer cells were seen in all five groups of surgical supplies, due to their different frequency of utilization and range of contact. Exfoliative tumor cells were detected in 14.4% (13/90) of specimens in group A, 10.0% (9/90) in group B, 30.0% (27/90) in group C, 18.9% (17/90) in group D, and 3.3% (3/90) in group E, again with a significant difference (P<0.001, Table 1).
Discussion
Gastric carcinoma is one of the most common malignant tumors in the 21st century.2 With advances in the development of surgical techniques and medical equipment, the radical resection rate in advanced gastric cancer continues to increase, along with significant improvement in the 5-year survival rate.3 However, approximately half of the patients who undergo radical gastric resection die as a result of peritoneal metastasis.4 Spreading of exfoliated cancer cells is necessary for peritoneal metastasis, and this occurs when cancer cells infiltrate the gastric wall and then travel to the peritoneal cavity. Cancer cells exfoliated from the specimen margin, or from blood and lymph when the blood vessel and lymph vessel were cut off, especially in conditions of cancer embolus in the blood and lymphatic vessel. In addition, improper operation in surgical procedure could free more cancer cells.5 As observed in recent research, exfoliation and migration of cancer cells is associated with expression of adhesion molecules on the surface of cancer cells. Decreased adhesion between tumor cells leads to easier exfoliation of cancer cells.6
Cancer cells are characterized by anoikis resistance, meaning that exfoliated cancer cells can migrate and survive for a long time.7 Moreover, coagulation of fibrin exudates forms a protective layer in the surgical field, preventing phagocytosis of exfoliated cancer cells by immune competent cells.8 Exfoliated cancer cells in the peritoneal cavity have been found to be the primary source of pollution of surgical supplies; contaminated surgical supplies could lead to dissemination of cancer cells and an increased risk of peritoneal metastasis.1
In our study, the cultivated exfoliated cancer cells were round, with an intact and clear cellular membrane and tight cellular junctions. The nuclei were large and evenly stained, the nucleocytoplasmic ratio was high, and the border of karyotheca was clear. An intact nucleus means that the cancer cell has the ability to invade, regenerate, and proliferate, indicating that surgical supplies are an important pathway for dissemination of cancer cells.
In our study, positive rates for cancer cells in TNM stages II and III were significantly higher than in TNM stage I, implying that advanced gastric carcinoma is accompanied by invasion and exfoliation of cancer cells into the peritoneal cavity. Positivity rates in the poorly differentiated cancer group were higher than in the other two groups, which could be because poorly differentiated cancers have a decreased capacity for intercellular adhesion, resulting in easier escape. Furthermore, poorly differentiated cancers were significantly different from moderately differentiated cancers (P<0.05). As earlier studies have reported, exfoliation of cancer cells during radical gastric resection is closely associated with serosal involvement, level of cancer differentiation, lymphatic metastasis, and clinical stage of disease.9 Similarly, we propose that exfoliation of cancer cells during radical gastric resection is linked to serosal involvement, cell differentiation, lymphatic metastasis, and clinical stage of disease.
Varying amounts of cancer cells were seen for the five types of surgical supplies due to different frequencies of use and range of contact. Positivity rates were ranked as follows:
- Gauze used in cleaning surgical instruments and gloves used by scrub nurses – because of the high frequency of use and extensive contact. Therefore, we should conduct different methods according to the characteristic of the surgical supplies to ensure cancer-free operation. In clinical application, scrub nurses should always clean instruments as the figure shows (Figure 3).
- Gauze used for keeping the operative field clear and for pressure hemostasis – because of the mesh structure preventing the cancer cells from exfoliating.
- Surgical instruments – these are utilized to divide, cut, and ligate during the surgical procedure, and because of their smooth surface, few cancer cells can be retained.
- Gloves used by surgeons – although these are in close contact with pathologic tissue, they are not as sharp as other surgical instruments, so the positivity rate appears smaller than for the above groups.
- According to the guideline for treatment of gastric cancer, a tumor resection margin of 6 cm is required; cancer cells should not be detected on the anastomat stapler, but in the process of changing the stapler cartridge, gloves containing cancer cells may contaminate this site, and accounted for two positive cases in our 90 patients.
There is still a limitation in the current basic experimental research. We merely set out to determine the likelihood of exfoliated tumor cells attaching to surgical supplies used in patients with gastric cancer, and to provide a foundation from which clinical care could benefit in the future. Further studies, such as treatment of exfoliated tumor cells, are still required for the purpose of clinical application.
In summary, surgical supplies may be contaminated by cancer cells during radical gastrectomy. The rate of detectable cancer cell positivity increased with advancing clinical stage of disease, a lower level of cancer cell differentiation, increased frequency of use and range of contact, and the nature of the surgical supplies used. However, it is difficult to switch surgical instruments, especially precise instruments that are used with high frequency. Xu et al recommend that potentially contaminated surgical instruments should be immersed in distilled water for 5 minutes to inactivate by tumor cells.10 As the surgery was a consecutive process, prolonged handling of surgical instruments led to a prolonged operative duration, and even affected the choice of instruments used by surgeons. Efficient methods of inactivating tumor cells have yet to be identified.
Acknowledgment
This work was supported by the Medicine and Health Research Foundation of Zhejiang Province (grant 2013KYA011).
Disclosure
The authors report no conflicts of interest in this work.
References
Atkin G, Chopada A, Mitchell I. Colorectal cancer metastasis: in the surgeon’s hands? Int Semin Surg Oncol. 2005;2(1):5. | |
Archie V, Kauh J, Jones DV Jr, Cruz V, Karpeh MS Jr, Thomas CR Jr. Gastric cancer: standards for the 21st century. Crit Rev Oncol Hematol. 2006;57(2):123–131. | |
Yu W, Whang I, Suh I, Averbach A, Chang D, Sugarbaker PH. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg. 1998;228(3):347–354. | |
Wang Z, Zhang X, Xu H, Zhou X, Jiang L, Lu C. Detection of peritoneal micrometastasis by reverse transcriptase-polymerase chain reaction for heparanase mRNA and cytology in peritoneal wash samples. J Surg Oncol. 2005;90(2):59–65. | |
Marutsuka T, Shimada S, Shiomori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003;9(2):678–685. | |
Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24(2):73–76. | |
Shanmugathasan M, Jothy S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int. 2000;50(4):273–279. | |
Kodera Y, Yamamura Y, Shimizu Y, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999;72(2):60–64. | |
Benevolo M, Mottolese M, Cosimelli M, et al. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol. 1998;16(10):3406–3411. | |
Xu L, Chen X, Lv B, et al. [Comparison between effects of soaking surgical instruments in distilled water and normal saline on killing tumor cells adhered to the instruments]. Chinese Journal of Nursing. 2005;40:810–811. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.