Back to Journals » Cancer Management and Research » Volume 13
Analysis of Curative Effect and Influencing Factors of N1 Stage Papillary Thyroid Micro-Carcinoma and Papillary Thyroid Non-Micro Carcinoma After Initial Radioactive Iodine Ablation Therapy
Authors Yun C , Wu M, Xiao J, Liu Y, Zhang W, Cao J
Received 18 November 2020
Accepted for publication 19 January 2021
Published 12 February 2021 Volume 2021:13 Pages 1427—1434
DOI https://doi.org/10.2147/CMAR.S292395
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Canhua Yun,1 Meiling Wu,1 Juan Xiao,2 Yong Liu,1 Wei Zhang,1 Jingjia Cao1
1Department of Nuclear Medicine, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250033, People’s Republic of China; 2Center of Evidence-Based Medicine, Institute of Medical Sciences, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250033, People’s Republic of China
Correspondence: Jingjia Cao No. 247, Beiyuan Street, Jinan City, Shandong Province, People’s Republic of China
Tel +86 17660083825
Email [email protected]
Objective: To compare the efficacy and influencing factors of initial radioactive iodine (RAI) ablation therapy for postoperative N1 stage papillary thyroid micro-carcinoma (PTMC) and papillary thyroid non-micro carcinoma (PTC), and to explore the necessity of RAI for N1 stage PTMC.
Methods: A retrospective analysis of patients with N1 stage papillary thyroid cancer who underwent RAI in our department from January 2018 to June 2019. According to the tumor diameter, papillary thyroid carcinoma was divided into PTMC group (≤ 1.0cm) with 129 patients and PTC group (> 1.0 cm) with 214 patients. According to the 2015 ATA guidelines, the patient’s treatment response was evaluated 6– 8 months after discharge from the hospital: excellent response (ER), indeterminate response (IDR), biochemical incomplete response (BIR), and structural incomplete response (SIR). IDR, BIR, and SIR were classified into NER group. Chi-squared test, independent sample t-test, Mann–Whitney U test, and binary logistic regression analysis were used to compare the differences between PTMC and PTC patients.
Results: The ps-Tg of the PTMC group was significantly lower than that of the PTC group (P = 0.001), and the ER ratio of the PTMC group was higher (χ2 = 5.445, P < 0.05). The ER ratio of PTMC patients in the N1a group was significantly higher than that of PTC patients (80%, 66.7%, χ2 = 4.076, P < 0.05), while the ER ratio of PTMC in the N1b group was not significantly different from that of PTC. Gender, N stage, and ps-Tg were found to be independent factors of RAI treatment response.
Conclusion: The efficacy of the initial RAI of PTMC patients was significantly better than that of PTC patients. There was no significant difference in the efficacy of RAI between males with PTMC, N1b stage, ps-Tg ≥ 5.87ng/mL and PTC patients, which suggested that RAI is necessary for these patients.
Keywords: papillary thyroid micro-carcinoma, papillary thyroid non-micro carcinoma, curative effect, RAI
Background
Recently, the incidence rate of papillary thyroid carcinoma has been increasing. Among it, the papillary thyroid micro-carcinoma (PTMC) is the fastest growing type. However, its diagnosis and treatment remain controversial.1 Radioactive iodine (RAI) ablation is an important adjuvant therapy for papillary thyroid carcinoma patients postoperation. It can significantly reduce the risk of recurrence and death of papillary thyroid carcinoma patients.2 Compared with papillary thyroid non-micro carcinoma (PTC), PTMC lesions are more limited and relatively inactive, and have better clinical prognosis. However, PTMC is still aggressive and can cause local recurrence and cervical lymph node metastasis. PTMC is not equivalent to low-risk cancer.3 At present, it is considered that RAI treatment is not necessary for PTMC in N0M0 stage. But there is still disagreement about whether patients with N1a-N1b stage need RAI treatment or not.2,4 According to the 2015 edition of the American Thyroid Association (ATA) guidelines, the clinical outcomes of patients after initial RAI can be divided into excellent response (ER), indeterminate response (IDR), biochemical incomplete response (BIR), and structural incomplete response (SIR).4 The recurrence rate of ER patients is only 1% - 4% and the risk of disease-specific death is less than 1%. 15% – 20% of IDR patients will have structural disease identified during follow-ups. The probability of it developing into structural disease in BIR patients is about 20%. However, 50% – 85% of patients continue to have persistent disease with SIR despite additional therapy. For patients with SIR, the risk of disease-specific death rates was as high as 11% with loco-regional metastases and structurally distant metastases which were found in 50% of patients.4 At present, few studies compared the therapeutic efficacy of RAI between PTC and PTMC. The aim of this study was to compare the efficacy of RAI between PTC and PTMC in stage N1 through retrospectively analyzing the data in our hospital.
Data and Methods
Research Population
Patient data extracted from the clinical electronic medical record system were de-identified so that all private information of patients was not included. The Institutional Review Board of the Second Hospital, Cheeloo College of Medicine, Shandong University approved the use of medical records and allowed us to obtain written consent from each patient (KYLL-2018[LW]013). All procedures complied with the Declaration of Helsinki for research involving human subjects. All the patients signed informed consent before RAI.
The data of patients with papillary thyroid carcinoma treated with initial RAI were retrospectively collected from January 2018 to June 2019. Patients with total thyroidectomy/near-total thyroidectomy combined with lymph node dissection were included. The patients with one of the following: incomplete clinical data; inadequate information of follow-up; no cervical lymph node metastasis; distant metastasis (examined by ultrasonography, chest CT, 131I whole body imaging, whole body bone imaging or magnetic resonance imaging, etc.); PTMC with only one tumor lesion; bad subtypes (such as: tall cell variant, diffuse sclerosing variant, columnar cell variant, etc.); positive anti-thyroglobulin antibody (TgAb) > 4000U/L) or preablative-stimulated TSH (ps-TSH) < 30 uIU/mL were excluded.
Grouping and Information of Collected Data
According to the maximum postoperative tumor diameter, the patients were divided into PTC group (tumor diameter > 1.0cm) and PTMC group (tumor diameter ≤ 1.0cm). According to the eighth edition of AJCC, patients were divided into N1a and N1b stage.
The data including gender, age, pathological information (extrathyroidal extension (ETE), Hashimoto’s thyroiditis (HT), single and double lobes), treatment time (TT) from postoperation to the initial RAI treatment, urinary iodine level, ps-TSH, preablative-stimulated thyroglobulin (ps-Tg), lymph node metastasis, and other data were collected.
Initial RAI Therapy
Before admission, the patients were ordered to stop taking levothyroxine sodium tablets and have a low iodine diet for 4 weeks. The implementation was self-managed by the patients. On the day of admission, fasting urine and blood samples of patients were collected. Urinary iodine level and serological indexes (ps-Tg, TgAb, ps-TSH, free triiodothyronine, free thyroxine, triiodothyronine, thyroxine) were recorded. If no contraindications, all patients included had ablation with 3.70GBq (100 mCi) 131I administered orally. A whole body post-treatment scan (Rx-131I-WBS) was performed 3 days after RAI treatment. 6–8 months after discharge, patients were monitored for serological indicators (Tg, TgAb, TSH, free triiodothyronine, free thyroxine, triiodothyronine, and thyroxine), neck ultrasonography, and diagnostic whole body 131I imaging (Dx-131I-WBS).
Laboratory Detection Methods
Urinary iodine level was determined by arsenic-cerium catalytic spectrophotometry with mild acid digestion, and the determination range was 30–1500 ug/L. Tg and TgAb were determined by electrochemiluminescence immunoassay in the range of 0–464 ng/mL and 0–4000 U/L, respectively. TSH was determined by the chemiluminescence method in the range of 0–100.0 uIU/mL. Ps-TSH > 100 uIU/mL was recorded as 100 uIU/mL.
Definitions of Response to RAI
Based on imaging findings and stimulated/suppressed Tg levels of the initial follow-up examination, patients were classified into four response-to-therapy categories recommended by the ATA guidelines as shown in Table 1. According to ATA guidelines, considering follow-up Tg level, Dx-WBS examination, neck ultrasonography, and other imaging examinations, patients were divided into two groups: ER group and NER group. IDR, BIR, and SIR were classified into NER group. Three nuclear medicine physicians independently assessed the outcome of follow-up examinations after treatment and reached agreement through consultation when discordance appeared.
 |
Table 1 Clinical Implications of Response to Therapy Reclassification in Patients with Differentiated Thyroid Cancer Treated with Total Thyroidectomy and Radioiodine Remnant Ablation |
Statistical Analysis
SPSS 20.0 statistical software was used for analysis. The categorical data were described as cases and percentages. Continuous data with normal distribution were expressed as means ± standard deviation (SD). Continuous data with skewed distribution were expressed as median (quartile). χ2 test, independent sample t-test, and Mann–Whitney U test were used to compare the differences between two groups of patients. The binary logistic regression model was established, and the factors with P < 0.10 in univariate analysis were included in the model to further explore the independent factors affecting the prognosis. In addition, receiver operating characteristic (ROC) curve was performed to determine the cut-off value of ps-Tg for ER. P< 0.05 was considered statistically significant.
Result
Basic Characteristics of Patients in PTC and PTMC Groups
129 PTMC patients (38 males and 91 females, mean age 42.78 ± 10.52 years) and 214 PTC patients (61 males and 153 females, mean age 41.71 ± 11.75 years) were included. There were no significant differences in gender, age, presence or absence of ETE, HT, single and double lobes, TT, urinary iodine level, ps-TSH between the two groups (all P > 0.05). The ps-Tg of the PTMC group was significantly lower than that of the PTC group, and the difference was statistically significant (P = 0.001). After the patients were further divided into N1a and N1b groups, the ps-Tg between the two groups was also significantly different (P values were 0.001 and 0.006, respectively) (Table 2).
 |
Table 2 General Data of PTC and PTMC and the Relationship Between Lymph Node Metastasis and Treatment Response |
Relationship Between Lymph Node Metastasis and Treatment Response in PTC and PTMC Groups
Patients were divided into ER group and NER group according to the response after RAI. The ER ratio of the PTMC group was significantly higher than that of the PTC group (P = 0.02). After the patients were further divided into N1a and N1b groups, the ER ratio of the PTMC group in the N1a group was significantly higher than that of the PTC group, while the ER ratio of the PTMC group in the N1b group was not significantly different compared with that of PTC group (Table 2).
Comparing the number of lymph node metastases and response to RAI between the N1a group and the N1b group, no significant difference in treatment response was found between the PTC group and the PTMC group (P> 0.05). However, with the stage from N1a to N1b and the number of lymph node metastases increasing, the percentage of ER gradually decreased and the percentage of NER gradually increased (Figure 1).
 |
Figure 1 With the stage from N1a to N1b and the number of lymph node metastases increasing, the percentage of ER gradually decreased and the percentage of NER gradually increased. |
Comparison of PTC and PTMC According to RAI Response (ER and NER)
In the PTC group, female gender, single lobe, and N1a were protective factors for treatment response, and ps-Tg in the ER group was significantly lower than that in the NER group (P< 0.05). The median ps-Tg of the ER group was 5.39 ng/mL (4.33, 8.84), which was significantly lower than that of the NER group (15.46 ng/mL (7.67, 34.68), (P = 0.001)) (Table 3). Gender, single and double lobes, N stage, and ps-Tg level were further included into the binary logistic regression model (NER group was regarded as the reference group). The result showed that gender, N stage, and ps-Tg were independent factors for treatment response (Table 4). The ROC curve of the relationship between ps-Tg and ER is shown in Figure 2. The area under the curve (AUC) was 0.806 and the cut-off value was ps-Tg = 10.61. The sensitivity and specificity were 0.753 and 0.744, respectively, which suggested that the model had good performance.
 |
Table 3 Comparison of PTC and PTMC After Being Divided into ER and NER Groups |
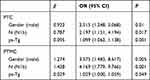 |
Table 4 Multivariate Logistic Regression Analysis on the RAI Treatment Response in PTC and PTMC |
 |
Figure 2 ROC curve of the relationship between ps-Tg and RAI response in patients with PTC and PTMC. |
In the PTMC group, female gender, maximum diameter < 0.5 cm tumor, and N1a stage were protective factors for treatment response. Ps-Tg in ER group was significantly lower than that in the NER group (P< 0.05) (Table 3). Gender, tumor maximum diameter, N stage, and ps-Tg level were further included in the binary logistic regression model (NER group was regarded as the reference group), and the results showed that gender, N stage, and ps-Tg were independent factors for RAI treatment response (Table 4). The median ps-Tg of ER group was 3.1 ng/mL (1.21, 9.7), which was significantly lower than that of the NER group, 4.275 ng/mL (2.28, 17.07) (P = 0.007). The ROC curve of the relationship between ps-Tg and ER was shown in Figure 2. The AUC was 0.690, the cut-off value was ps-Tg = 5.87. The sensitivity and specificity were 0.452 and 0.831, respectively. Using ps-Tg to predict the RAI treatment response, the effect in PTMC was not as good as that in the PTC group.
Discussion
The incidence rate of thyroid cancer is increasing year by year. Studies have reported that PTMC accounts for nearly 50% of newly found papillary thyroid carcinoma.5 Although PTMC progresses slowly and has a good prognosis,6 there is still a local recurrence rate of 2%-6%, and distant metastasis occurs in 1%-2% of patients.7 At present, the best treatment for papillary thyroid carcinoma is surgery + RAI + TSH suppression therapy. RAI can remove the residual thyroid tissue for papillary thyroid carcinoma patients postoperation and clear metastatic lesions, which can effectively reduce the recurrence rate.2 Chinese experts reached consensus on diagnosis and treatment of papillary thyroid carcinoma (2016 Edition): for PTMC patients with metastasis (especially distant metastasis), RAI can help to eliminate residual lesions or metastases that cannot be removed by surgery, which aids to alleviate the disease and reduce the risk of recurrence of PTMC.1 However, PTMC may have different degrees of cervical lymph node metastasis. AJCC 8th Edition of TNM stage changed the Ⅶ area of lymph node metastasis from lateral lymph node metastasis (N1b) to central lymph node metastasis (N1a).8 Therefore, in order to alleviate the condition, reduce the recurrence rate, and the possibility of distant metastasis, and to facilitate postoperative Tg stratification, re-staging and follow-up, increase the sensitivity of early diagnosis of recurrence, and reduce the patient’s psychological and medical cost burden, it is of great significance to compare the efficacy and influencing factors of initial RAI between the PTMC group and the PTC group and to explore the necessity of RAI for PTMC in N1 stage.
In this study, 67.4% of patients in thePTMC group achieved ER status, which was significantly higher than that in the PTC group with 54.7%. The result was consistent with that of Feng et al.3 The ps-Tg of the PTC group was significantly higher than that of the PTMC group. After being further divided into N1a group and N1b group, the ER ratio of PTMC in the N1a group was significantly higher than that of the PTC group, and ps-Tg in the N1b group was also significantly higher than that in the N1a group, while the ER ratio of PTMC patients in the N1b group was not significantly different from that in the PTC group. In the further multivariate analysis, N stage was also an independent influencing factor of RAI treatment response. Compared with the N1a group, the OR value of PTMC in the N1b group was 4.169, which was significantly higher than the 2.197 of PTC. This suggests the necessity of RAI for PTMC in the N1b group compared with the N1a group. Comparing the number of lymph node metastases (≥ 3) in the N1a group and N1b group, there was no significant difference in RAI treatment response between the PTC group and the PTMC group. The percentages of ER and NER in each group of N1a, N1a, and lymph node metastasis ≥ 3, N1b, N1b, and lymph node metastasis ≥ 3 were calculated. It was found that with the stage from N1a to N1b and the number of lymph node metastases increasing, the percentage of ER gradually decreased and the percentage of NER gradually increased. This implies that PTMC may be an early lesion of PTC,9 it also reflects that the central lymph node is the cervical lymph node metastasis pathway.10
In this study, the male to female ratios of PTMC and PTC were 1:2.39 and 1:2.51, respectively. Gender was an independent factor of RAI response in multivariate analysis, which was consistent with the results of Long et al,11 indicating that both PTMC and PTC are more likely to occur in female patients. Regarding age (≥ 55 years old) as an independent factor affecting TNM staging, there was no statistical difference in this study (P> 0.05). This may be related to the selection of stage N1 and fewer patients ≥ 55 years old in this study. As one of the factors that affect RAI treatment response, TT was inconsistent in previous literature.12,13 Li et al considered that among the low and medium risk patients postoperation for differentiated thyroid carcinoma (DTC), the risk of poor prognosis for patients who delayed RAI treatment for more than 3 months was 3.77 times higher than that for patients who received RAI treatment within 3 months.12 Wang et al suggested that RAI treatment within 6 months postoperation may have no significant effect on prognosis.13 In this study, the median TT of ER and NER in the PTMC group were 75 days and 79 days, respectively, and the median TT of ER and NER in the PTC group were 74 days and 66 days, respectively, which had no statistical difference. It indicated that TT around 3 months had no significant effect on the efficacy of RAI in PTMC and PTC patients.
TSH can stimulate the expression of NIS on the cell membrane to enhance the 131I absorbed by residual thyroid tissue or DTC lesions, thereby its increase can improve the therapeutic efficacy of RAI. Ps-TSH>30uIU/mL is required for RAI therapy, which was recommended by the ATA guideline. Xin et al believe that for patients with ps-TSH of 90~120 uIU/mL, the efficacy of RAI is significantly higher than for those with other ps-TSH levels.14 However, no statistical difference of ps-TSH levels was found between the ER and NER groups among PTC and PTMC patients in this study, which may be associated with the fact that the upper limit of ps-TSH was 100 uIU/mL in this study. It is widely agreed that ps-Tg levels influence RAI response.15,16 Kim et al considered that the ps-Tg level can effectively predict the efficacy of RAI, and elevated ps-Tg levels may indicate treatment failure. The results of this study were consistent with those studies. It was found that the lower the level of ps-Tg before RAI, the easier it was to obtain the best therapeutic response after the initial RAI, whether for PTC or PTMC. Ps-Tg was also an independent influencing factor of RAI efficacy in multivariate analysis. Through ROC curve analysis, the optimal cut-off value of ps-Tg was 10.61 ng/mL with the AUC of 0.806 in the PTC group, which was close to the analytical limit of Webb et al (ps-Tg< 10 ng/mL).17 The optimal cut-off value of ps-Tg was 5.87 ng/mL with the AUC of 0.690 in the PTMC group. The use of ps-Tg to predict the therapeutic effect of RAI has lower sensitivity in PTMC, which may be related to the lower ps-Tg of PTMC compared with PTC.
Liu et al believed that the tumor size was related to the aggressiveness and prognosis of papillary thyroid carcinoma, and that it could independently predict the treatment response of RAI.18 In this study, because tumors were divided into PTMC and PTC groups according to the largest diameter of the tumor, there was no statistical difference in tumor size in the RAI treatment response of the PTMC and PTC groups. However, the overall RAI treatment response of the PTMC group was significantly better than that of the PTC group, which also reflects the correlation between tumor size and prognosis. After further dividing PTMC into two groups according to the maximum diameter: > 0.5 cm and ≤ 0.5 cm, the group with ≤ 0.5 cm had better treatment response, which was consistent with the results reported by Lim et al.19 But it was excluded from the multivariate analysis, which means that the maximum diameter ≤ 0.5cm was not an independent protective factor for PTMC RAI treatment response. Former studies have shown that PTMC was an early lesion of PTC, and no significant difference was found between them at the gene level.9 This study found that the proportion of ER gradually decreased with the maximum diameter of tumors from ≤ 0.5 cm, 0.5–1.0 cm, 1.0 −2.0 cm to >2.0 cm, which was consistent with the study by Liu et al18 which showed that the risk of NER increased with the increase in tumor diameter. It also showed that PTMC is the early stage of PTC. The guidelines proposed by the World Health Organization for early detection, early diagnosis, early treatment and improvement of cure rate of tumors also stated similar opinions.20
In previous literature,12,18,21 the presence of ETE and HT was often mentioned as influencing factors for RAI treatment of postoperative DTC patients. There was no significant effect of ETE and HT on the efficacy of PTC and PTMC in this study, which may be related to the exclusion of distant metastasis and TgAb-positive patients.
Conclusion
The efficacy of initial RAI in PTMC patients was significantly better than that in PTC patients. Gender, N stage, and ps-Tg were independent influencing factors of the initial RAI response of PTC and PTMC. There was no significant difference in the initial RAI efficacy between PTMC (male, N1b stage and ps-Tg> 5.87 ng/mL) and PTC patients, suggesting the necessity of RAI in such PTMC patients. For patients with stage N1a PTMC, individualized treatment should be carried out to avoid excessive and insufficient diagnosis and treatment, so as to maximize the benefits for patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chinese Association of Thyroid Oncology. Chinese Expert Consensus on Diagnosis and Treatment of Papillary Thyroid Microcarcinoma. Chin J Clin Oncol. 2016;43(10):405–411. doi:10.3969/j.issn.1000-8179.2016.10.001
2. Chinese Society of Nuclear Medicine. Clinical guidelines for 131I therapy of differentiated thyroid cancer [J]. China J Nucl Med Mol Imaging. 2014;34(4):264–278.
3. Feng S, Tan J, et al. Clinical characteristics and 131I efficacy of papillary thyroid microcarcinoma and papillary thyroid non-micro carcinoma [J]. Int J Radiat Med Nucl Med. 2018;42:2. doi:10.3760/cma.j.issn.1673-4114.2018.02.003
4. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer[J]. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
5. Domínguez JM, Nilo F, Martínez MT, et al. Papillary thyroid microcarcinoma: characteristics at presentation, and evaluation of clinical and histological features associated with a worse prognosis in a Latin American cohort [J]. Arch Endocrinol Metab. 2018;62(1):6–13. doi:10.20945/2359-3997000000013
6. Noguchi S, Yamashita H, Uchino S, et al. Papillary microcarcinoma[J]. World J Surg. 2008;32(5):747–753. doi:10.1007/s00268-007-9453-0
7. Hay ID. Management of Patients with Low-Risk Papillary Thyroid Carcinoma [J]. Endocr Pract. 2007;13(5):521–533. doi:10.4158/EP.13.5.521
8. M B Amin, Edge S, Greene F, et al. 2017 AJCC Cancer Staging Manual [M].
9. Youn Y-K, Kim H, Park W-Y, et al. Comparative analysis of gene expression profiles of papillary thyroid microcarcinoma and papillary thyroid carcinoma [J]. J Cancer Re Therapeutics. 2010;6(4):452–457. doi:10.4103/0973-1482.77103
10. Roh J-L, Kim J-M, Park CI. Central cervical nodal metastasis from papillary thyroid microcarcinoma: pattern and factors predictive of nodal metastasis [J]. Ann Surg Oncol. 2008;15(9):2482–2486. doi:10.1245/s10434-008-0044-6
11. Long Y, Caixia A, Zhang W. Characteristics of 185 thyroid micropapillary carcinoma patients undergoing 131I treatment [J]. China J Nucl Med Mol Imaging. 2019;39:9. doi:10.3760/cma.j.issn.2095-2848.2019.09.005
12. Li H, Zhang Y-Q, Wang C, et al. Delayed initial radioiodine therapy related to incomplete response in low- to intermediate-risk differentiated thyroid cancer. Clin Endocrinol (Oxf). 2018;88(4):601–606. doi:10.1111/cen.13551
13. Wang X, Song Q, Dongdong X. Effects of Timing of Initial Postoperative Radioactive Iodine Therapy on the Outcome of Patients with Differentiated Thyroid Cancer [J]. J China Med Univ. 2019;48(4):359–369. doi:10.12007/j.issn.0258-4646.2019.04.016
14. Xin L, Lin Y. Change of pre-ablative thyroid-stimulating hormone after thyroid hormone withdrawal and its response to 131I therapy in patients with low to intermediate risk differentiated thyroid cancer [J]. China J Nucl Med Mol Imaging. 2016;35(6):389–393. doi:10.3760/cma.j.issn.2095-2848.2016.05.003
15. Gonzalez C, Aulinas A, Colom C, et al. Thyroglobulin as early prognostic marker to predict remission at 18–24 months in differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2014;80(2):301–306. doi:10.1111/cen.12282
16. Kim H, Kim S-J, Kim I-J, et al. Limited clinical value of periablative changes of serum markers in the prediction of biochemical remission in patients with papillary thyroid cancer [J]. Nucl Med Mol Imaging. 2013;47(4):268–272. doi:10.1007/s13139-013-0220-x
17. Webb RC, Howard RS, Stojadinovic A, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012;97(8):2754–2763. doi:10.1210/jc.2012-1533
18. Liu J, Liang J, Lin Y. Relationship between preablative stimulated thyroglobulin and the excellent response in differentiated thyroid carcinoma [J]. China Oncology. 2019;29(2):125–130. doi:10.19401/j.cnki.1007-3639.2019.02.005
19. Lim YC, Choi EC, Yoon Y-H, et al. Central lymph node metastases in unilateral papillary thyroid microcarcinoma[J]. Br J Surg. 2009;96(3):253–257. doi:10.1002/bjs.6484
20. WHO. Guide to Cancer Early Diagnosis. ISBN: 978-92-4-151194-0. World Health Organization; 2017.
21. Du F, Hu S, Wu C, et al. [Analysis of the factors affecting the efficacy of (131)I remnant ablation in patients after thyroidectomy for papillary thyroid microcarcinoma].. Zhonghua Zhong Liu Za Zhi. 2018;40(8):610–613. doi:10.3760/cma.j.issn.0253-3766.2018.08.009
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
